Abstract
DAPK1 can induce apoptosis in several cells; to determine the effect of DAPK1 would provide a new potential therapeutic strategy for treating pancreatic cancer. The aim of the present study was to investigate the effect of DAPK1 gene on proliferation, migration, and invasion of carcinoma of pancreas BxPC-3 cell line and explore the possible mechanisms. In our study, DAPK1 over-expressed cells were established by using the lentiviral transfection method, and DAPK1 obviously increased in BxPC-3 cells after transient transfection. Cell Counting Kit-8 (CCK-8) assay was used to determine the BxPC-3 cells proliferation after transfection. Apoptosis of the BxPC-3 cells was determined by using flow cytometry analysis. In addition, cell adhesion assay and in vitro invasion assay were performed. Western blotting was used to determine the protein expressions of caspase-3, DAPK1, VEGF, PEDF, MMP2, AKT, P-AKT, P-ERK, Bcl2, and Bax. Our results demonstrated that DAPK1 gene over-expression can suppress the proliferation, migration, and invasion of carcinoma of pancreas BxPC-3 cell line, and the possible mechanisms may be correlated to induction of mitochondria-mediated apoptosis, down-regulations of MMP-2 and VEGF, up-regulations of PEDF, through the PI3K/Akt and ERK pathways.
Keywords: DAPK1, carcinoma of pancreas, BxPC-3 cell, proliferation, migration, invasion
Introduction
Pancreatic cancer is one of malignant tumors in both men and women worldwide which has one of the poorest prognoses among all malignancies of human, leading to a very high mortality with a nearly 95% mortality rate [1-3]. It’s reported that the median survival duration of pancreatic cancer is only approximately 6 months following diagnosis, and the 5 years survival rate is less than 5% [1,4,5]. In the early stage of pancreatic cancer, there are few symptoms; however, the common symptoms of pancreatic cancer (including abdominal or back pain, obstructive jaundice, and weight loss) usually appear in the later stage [2,6]. In addition, it is well known that pancreatic cancer is associated with a substantial risk of invasion and metastasis and tissue invasion and metastasis is one of the primary reasons of the mortality of pancreatic cancer patients [2,4]. The majority of pancreatic cancer patients are diagnosed at the late stage in the disease development and the vast majority of pancreatic cancer patients thus present with advanced inoperable, metastatic disease [4,5]. Thus, there is a critical need to discover new and reliable therapeutic strategies to treat of pancreatic cancer.
Death-associated protein kinase1 (DAPK1), belongs to a newly identified and classified family of calcium/calmodulin (Ca2+/CaM)-regulated Ser/Thr kinase, has been considered as a multi domain protein [7,8]. Besides the kinase and CaM regulatory domain, DAPK1 was reported to contain a C-terminal death domain, which can regulate cells’ death, survival, and proliferation [8,9]. Previous investigations have revealed that DAPK1 was down-regulated or not observed in many malignant tumors [8,10,11]; whereas over-expression of DAPK1 can induce apoptosis in several cell lines [8,12]. In our present investigation, after our aim is to determine the effect of DAPK1 gene over-expression on proliferation, migration, and invasion of carcinoma of pancreas BxPC-3 cell line, which would provide a new potential therapeutic strategy for treating pancreatic cancer.
Materials and methods
Reagents and cell lines
BxPC-3 and 293T cell line was purchased from the American Type Culture Collection (MD, USA) (we obtained all these cells from lawful method and all tests did not involve ethical consideration in this research). DMEM medium was purchased from Gibco. Ltd. Co. (USA); fetal bovine serum (FBS) was purchased from Sijiqing Biotech. Co. (Hangzhou, China); DH5α cells and High Pure dNTPs were purchased from Transgen Biotech. (Beijing, China); Taq polymerase and DNA marker were purchased from Takara (Japan); plasmid extraction kit was purchased from Beijing Solarbio science & technology (Beijing, China); 250 bp DNA ladder plus was purchased from Generay Bio. (Shanghai, China); Liposome transfection Kit was purchased from Invitrogen Bio. (Shanghai, China); PLKO.1, PLKO.1 vector containing EGFP, and PCMV-U6 were purchased from Addgene (USA); Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Biochem (Shanghai, China); Annexin V/FITC kit and matrigel were purchased from BD Biosciences (Shanghai, China); Crystal Violet, GIMSA, trypsinase, acrylamide, sodium lauryl sulfate (SDS), and Phosphate Buffered Saline (PBS) were purchased from JRDun Biotech. (Shanghai, China); Transwell well culture chambers were purchased from Corning (New York, USA). BCA protein assay kit was purchased from Thermo Fisher Scientific. (Shanghai, China); Nitrocellulose filter membrane was purchased from Millipore Biotech. (USA); Caspase-3, DAPK1, VEGF, PEDF, MMP2 monoclonal antibody was purchased from Abcam Biotech. (Shanghai, China); AKT, P-AKT, P-ERK, and GAPDH monoclonal antibody was purchased from CST Biotech. (Shanghai, China); Bcl2, and Bax monoclonal antibody was purchased from Santa Biotech. (Japan); goat-anti-rabbit (HRP) was purchased from Beyotime (Jiangsu, China).
Cell culture
BxPC-3 cells were cultured in DMEM medium containing 15% (v/v) heat-inactivated FBS, 100 mg/ml streptomycin and 100 U/ml penicillin at 37°C in a humidified and 5% CO2 atmosphere.
Plasmid construction and transient transfection
The human DAPK1 gene was sub-cloned into a lentiviral vector (PLKO.1) to generate the lentiviral expression vector (PLKO.1-DAPK1). Then, the recombinant lentiviruses were produced by 293T cells following the co-transfection of PLKO.1-DAPK1. The resulting recombinant lentiviruses carrying DAPK1 were then used to infect BxPC-3 cells. The DAPK1 expression in untreated BxPC-3 cells, cells treated with control vector (Mock group), and DAPK1 gene over-expressed BxPC-3 cells (BxPC-3-DAPK1) were detected by using real-time fluorogenic PCR and Western blotting.
Real-time fluorogenic PCR assays of expression of DAPK1
BxPC-3 cells were harvested, and total RNA was extracted using Trizol reagent (Invitrogen, USA). Total RNA was used for cDNA synthesis of DAPK1 and GAPDH by reverse transcription using quantitative real-time PCR (ABI-7300, USA). All mRNA primers were designed by Premier 5.0 and synthesized by JRDun Biotech. (Shanghai, China). Primers used for the real-time PCR are showed in Table 1. Reverse transcription was performed according to the manufacturer’s recommendation of the quantitative real-time RT-PCR reaction kit 11 (SYBR Green, Thermo Fisher Scientific, Shanghai, China).
Table 1.
Primer used for the Real-time PCR
| Gene name | Primer sequence | Size | |
|---|---|---|---|
| DAPK1 | F: | 5’-CAAGACAGGCACGGCAATAC-3’ | 186 bps |
| R: | 5’-GGCTCCCATCAGACAGAGATAC-3’ | ||
| GAPDH | F: | 5’-CACCCACTCCTCCACCTTTG-3’ | 110 bps |
| R: | 5’-CCACCACCCTGTTGCTGTAG-3’ |
F: Forward primer, R: Reverse primer.
Western blot
BxPC-3 cells were harvested, and total proteins were extracted, and the protein concentration was determined using BCA Protein Assay Reagent. Then equal amounts of protein (25 μg) were separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE), blotted on nitrocellulose filter membrane and probed with caspase-3, DAPK1, VEGF, PEDF, MMP2, AKT, P-AKT, P-ERK, Bcl2, and Bax monoclonal antibody respectively, followed by incubation with a goat anti-rabbit/HRP conjugate and chemiluminescence detection. To normalize for protein loading, antibodies directed against GAPDH were used, and the proteins expression levels were expressed as a relative value to that of GAPDH.
CCK-8 assay
Cells (5 × 103/100 μl) were seeded in 96-well plates and cultured for 0, 12, 24, and 48 h at 37°C, respectively. After that, 100 μl serum free DMEM containing 10% CCK-8 reagents (v/v) was added in each well, and cells were cultured for 1 h at 37°C. Finally, optical density values (OD) were read at 450 nm using a 96-well plate reader (Multiscan MK3, Thermo, Finland) [13].
Apoptosis assay by flow cytometry analysis
BxPC-3 cells were harvested, then cells (5~10 × 104) were washed with PBS and stained using an Annexin V/FITC kit. The cell apoptosis was detected by flow cytometry (FCM) on a FACS calibur flow cytometer (BD Bioscience, USA). The percentage of cells in early apoptosis was calculated by Annexin V-positivity and PI-negativity, while the percentage of cells in late apoptosis was calculated by Annexin V-positivity and PI-positivity [14].
Cell adhesion assay
Cell adhesion assay was carried out in 12-well plates according to the method described previously [15]. The wells were precoated with fibronectin overnight at room temperature. BxPC-3 cells were harvested and re-suspended in RPMI 1640 containing 10% FBS; then, cells were added (1 × 105/well) to each well and incubated at 37°C for 1 h. The wells were washed twice with warm PBS to remove the unattached cells, and the attached cells were then stained with GIMSA for 10 min. Once stained, the cells were observed by using the optical microscope (Olympus, Japan).
In vitro invasion assay
The invasive activities of cells were determined by using the Transwell cell culture chamber which described previously [16]. Cell invasion assays were performed using Transwell well culture chambers which were pre-coated with 80 μl of Matrigel. The coated filters were washed thoroughly in PBS and dried immediately before use. 0.75 ml DMEM containing 10% FBS was placed in the lower chamber, and 0.5 ml cells (1 × 105/ml) in DMEM containing 1% FBS were placed in the upper chamber and incubated for 48 h at 37°C in 5% CO2. The number of the invaded cells through Matrigel-coated PVPF filter was measured by counting cells stained with 0.5% crystal violet solution. Once stained, the cells were observed by using the optical microscope (Olympus, Japan).
Statistical analysis
Data are presented as mean ± standard deviation, and statistical analyses were performed using the two-tailed Student’s t test with a significance level of p < 0.05.
Results
Expression of DAPK1 in BxPC-3 cells after transient transfection
As can be seen from Figure 1, after transient transfection, the DAPK1 genes were significantly up-regulated in the BxPC-3 cells (P < 0.01), compared to the both untreated-BxPC-3 group and MOCK group (Figure 1A). Furthermore, the results of our western blotting experiment also demonstrated that DAPK1 obviously increased after transient transfection (Figure 1B).
Figure 1.
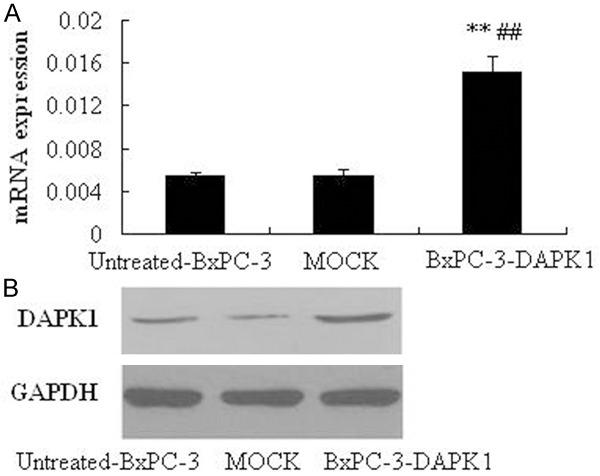
Expression of DAPK1 in BxPC-3 cells after transient transfection. A. mRNA expressions of DAPK-1 determined by real time PCR (RT-PCR). B. Protein expressions of DAPK-1 determined by western blotting. MOCK means the cells treated with control vector, and BxPC-3-DAPK1 means DAPK1 gene over-expressed BxPC-3 cells, **P < 0.01, compared to Untreated-BxPC-3 group; ##P < 0.01, compared to MOCK group.
Effects of DAPK1 over-expression on BxPC-3 cell proliferation
BxPC-3 cell proliferation was detected by the CCK-8 assay after transient transfection. As shown in Table 2, there was no significant difference between untreated-BxPC-3 group and MOCK group (P > 0.05). However, the proliferation of BxPC-3 cells in BxPC-3-DAPK1 group was significantly inhibited at 12, 24, and 48 h (P < 0.05, P < 0.05, P < 0.01, respectively), compared to both the untreated-BxPC-3 group and MOCK group. Our present study indicated that the over-expression of DAPK1 could significantly inhibit proliferation of BxPC-3 cells.
Table 2.
Effect of DAPK1 over-expression on proliferation of BxPC-3 cell line
| 0 h | 12 h | 24 h | 48 h | |
|---|---|---|---|---|
| Untreated-BxPC-3 | 0.258±0.0026 | 0.31±0.0046 | 0.386±0.0046 | 0.606±0.0032 |
| MOCK | 0.254±0.0045 | 0.31±0.0031 | 0.385±0.0032 | 0.604±0.0042 |
| BxPC-3-DAPK1 | 0.246±0.0035 | 0.285±0.0040*,# | 0.334±0.0038*,# | 0.387±0.0026**,## |
MOCK means the cells treated with control vector, and BxPC-3-DAPK1 means DAPK1 gene over-expressed BxPC-3 cells.
P < 0.05, compared to Untreated-BxPC-3 group;
P < 0.01, compared to Untreated-BxPC-3 group;
P < 0.05, compared to MOCK group;
P < 0.01, compared to MOCK group.
Apoptosis of BxPC-3 cells by flow cytometry analysis
To confirm whether the anti-proliferative effect of DAPK1 over-expression on BxPC-3 cell was related to induction of apoptosis, flow cytometry analysis was performed. From our present investigation, the apoptosis rate BxPC-3-DAPK1 group was 37.5%, significantly higher than in untreated-BxPC-3 group (7.3%) and MOCK group (9.2%) (Figure 2). Our results revealed that DAPK1 over-expression could significantly increase cells’ apoptosis, compared to both the untreated-BxPC-3 group and MOCK group.
Figure 2.
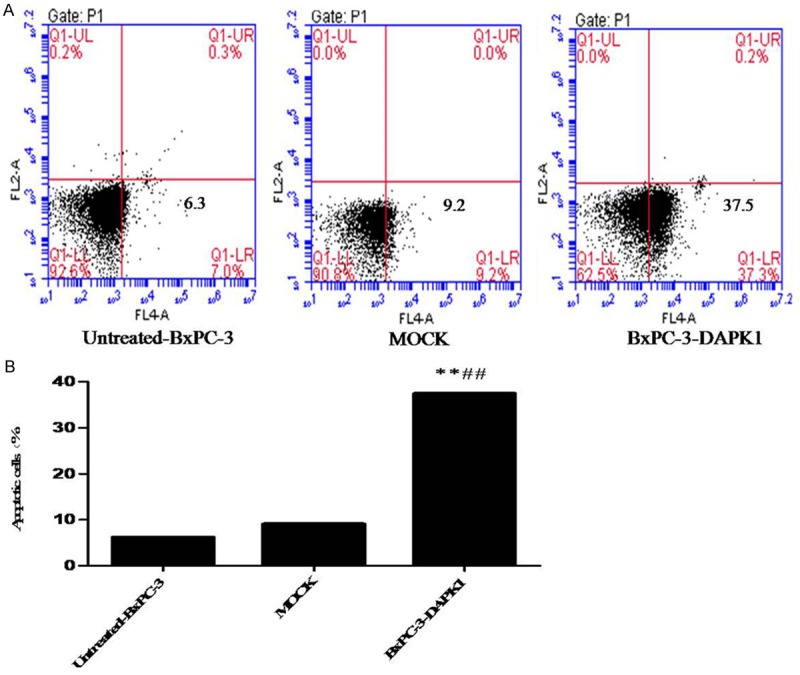
Results of apoptosis assay by flow cytometry analysis. MOCK means the cells treated with control vector, and BxPC-3-DAPK1 means DAPK1 gene over-expressed BxPC-3 cells. A. A representative assay was shown. B. Data show mean ± SD, n=3, **P < 0.01, compared to Untreated-BxPC-3 group; ##P < 0.01, compared to MOCK group.
Changes of cell adhesion
As shown in Figure 3, there was no obviously difference between untreated-BxPC-3 group and MOCK group. However, in the BxPC-3-DAPK1 group, the cells’ adhesion was significantly inhibited, compared to both the untreated-BxPC-3 group and MOCK group. Our present study indicated that the over-expression of DAPK1 could significantly suppress adhesion of BxPC-3 cells.
Figure 3.
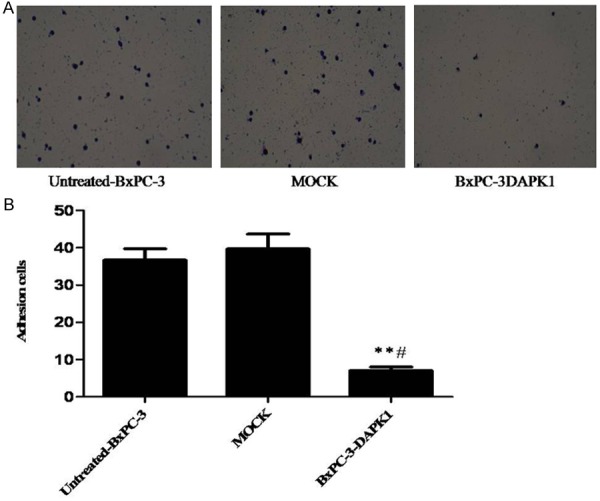
Results of cell adhesion assay. MOCK means the cells treated with control vector, and BxPC-3-DAPK1 means DAPK1 gene over-expressed BxPC-3 cells. A. A representative assay was shown. B. Data show mean ± SD, n=3, **P < 0.01, compared to Untreated-BxPC-3 group; #P < 0.05, compared to MOCK group.
In vitro invasion of BxPC-3 cells
In the results of our present study (Figure 4), similar to the results of cell adhesion assay, over-expression of DAPK1 effectively inhibited the cell invasion of BxPC-3 cells, which indicated that over-expression of DAPK1 could significantly suppress invasion of BxPC-3 cells.
Figure 4.
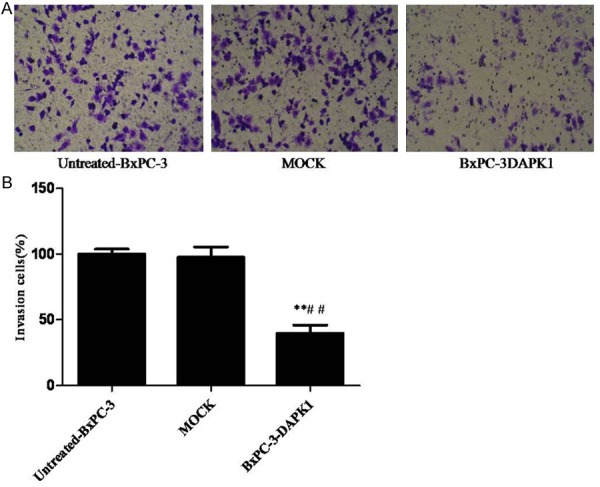
Results of cell in vitro invasion assay. MOCK means the cells treated with control vector, and BxPC-3-DAPK1 means DAPK1 gene over-expressed BxPC-3 cells. A. BxPC-3 cell invasion assay was performed. B. The data are presented as mean ± SD, n=3, **P < 0.01, compared to Untreated-BxPC-3 group; ##P < 0.01 compared to MOCK group.
Proteins expression of caspase-3, DAPK1, VEGF, PEDF, MMP2, AKT, P-AKT, P-ERK, Bcl2, and Bax by western blotting
In the results mentioned above, we can come to the conclusion that DAPK1 over-expression can inhibit the proliferation, migration, and invasion of carcinoma of pancreas BxPC-3 cell line. To investigate the possible mechanisms, many related proteins were determined by Western blotting. As shown in Figure 5, there was no obviously difference between untreated-BxPC-3 group and MOCK group for these proteins expression. Importantly, the expression of p-AKT, p-ERK1/2, VEGF, MMP-2, Bcl-2 was down-regulated in the BxPC-3-DAPK1 group compared to both the untreated-BxPC-3 group and MOCK group, whereas the expression of PEDF, caspase-3, and Bax was up-regulated.
Figure 5.
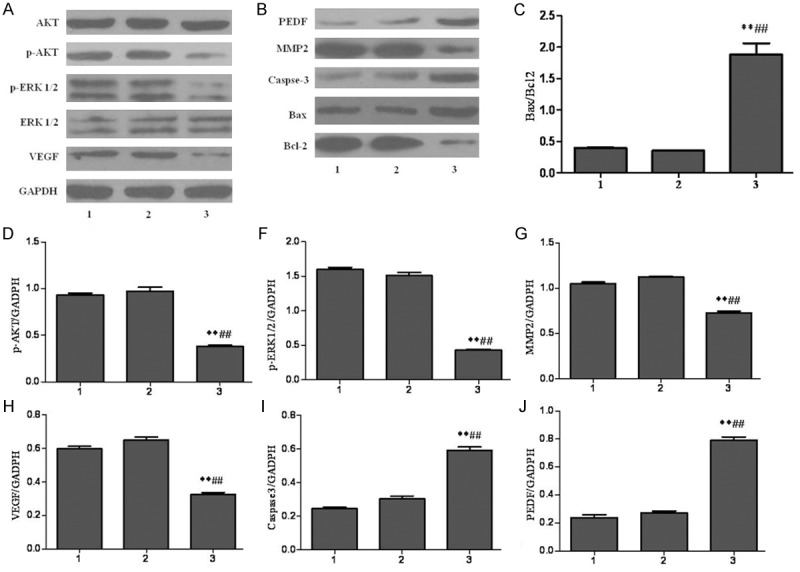
Results of the proteins expression by western blotting. 1-3 represented the proteins expressions in untreated BxPC-3 cells group, MOCK group, and BxPC-3-DAPK1 group, respectively. MOCK means the cells treated with control vector, and BxPC-3-DAPK1 means DAPK1 gene over-expressed BxPC-3 cells. GAPDH was detected as control for sample loading. The data are presented as mean ± SD, n=3, **P < 0.01 compared to Untreated-BxPC-3 group; ##P < 0.01 compared to MOCK group.
Discussion
To the best of our knowledge, our present study is the first work regarding inhibitory effects of DAPK1 over-expression on proliferation, migration, and invasion of carcinoma of pancreas BxPC-3 cell line. Results of our work also revealed that the mechanism may be related to regulations of p-AKT, p-ERK1/2, VEGF, PEDF, MMP-2, Bcl-2, caspase-3, and Bax proteins in BxPC-3 cells.
DAPK1 is a known cancer suppressor gene, and it can sensitize cancer cells to many of apoptotic signals generated by death receptors, matrix detachment, and cytokines [7,8,17]. Previous investigations reported that DAPK1 is down-regulated or loss in a large variety of human cancers [18], and up-regulation of DAPK1 is a apparently feasible approach to treatment of cancer. Lentiviral vector, which is a hotspot of vector study in gene therapy, can be able to effectively infect dividing or non-dividing cells, accept big fragment of exogenous target gene, and can be constantly expressed in cells [19,20]. In our study, we obtained the DAPK1 over-expressed cells by using the lentiviral transfection method, and DAPK1 obviously increased in BxPC-3 cells after transient transfection.
In the following experiments, CCK-8 assay was used to determine the BxPC-3 cells proliferation. CCK-8 method is a one-step cyto-toxicity assay by using WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate), and is considered to be a more better alternative method to MTT assay [13]. In our present study, the results indicated that the over-expression of DAPK1 could significantly suppress BxPC-3 cells proliferation. In addition, the apoptosis assay by flow cytometry analysis also indicated that over-expression of DAPK1 can induce apoptosis of BxPC-3 cells which is a key reason for the anti-proliferative effect of over-expression of DAPK1 on BxPC-3 cells. Cancer cells invasion to extracellular matrix (ECM) is a crucial step in tumor metastasis process [16,21]. Furthermore, tumor cells adhesion to ECM is another key stage in formation of metastasis tumor [21]. Results of our present study demonstrated that over-expression of DAPK1 obviously inhibited the invasion of BxPC-3 cells in vitro; moreover, our study also indicated that over-expression of DAPK1 significantly inhibited the adhesion of BxPC-3 cells to the fibronectin.
To investigate the possible mechanisms, western blotting assay was performed to determine the proteins expressions of Caspase-3, DAPK1, VEGF, PEDF, MMP2, AKT, P-AKT, P-ERK, Bcl2, and Bax. Apoptosis is crucial for multi-cellular organisms’ development and health, and is the cell-intrinsic programmed suicide mechanism for damaged cells, and mitochondria-mediated apoptosis is the major apoptotic pathways [22,23]. Caspase proteins, cysteine-aspartic acid proteases, are the key mediators of apoptosis, and caspase-3, one of the key activated death proteases, is considered as a marker for cells undergoing apoptosis [24]. In addition, Bcl-2 family proteins also play important role in mitochondria-mediated apoptosis. Bax is a well-known pro-apoptotic protein, whereas Bcl-2 is anti-apoptotic protein; the ratio of Bax/Bcl-2 has a key role in induction of apoptosis, and an increased ratio can be activate mitochondria-mediated apoptosis [23,25,26]. In our study, our results indicated that over-expression of DAPK1 can down-regulate Bcl-2, whereas up-regulate the caspase-3 and Bax. Therefore, we can come to the conclusion that DAPK1 can induce apoptosis via mitochondria-mediated way. Matrix metalloproteinase’s (MMPs), belong to the family of zinc and calcium dependant endopeptidases, responsible for the first steps in ECM degradation during tumor genesis [27]. MMP2, also named gelatinase A, can digest collagen, and is commonly over-expressed in different type of malignancies tissue [28,29]. Our results revealed that over-expression of DAPK1 can down-regulate the expressions of MMP-2, which is closely related to the inhibitive effects of DAPK1 on invasion and metastasis of BxPC-3 cells. Vascular endothelial growth factor (VEGF) plays important role in angiogenesis, and it can directly induce endothelial cell migration, proliferation and tube formation; furthermore, angiogenesis is important for tumor progression, growth and metastasis [30,31]. Pigment epithelium-derived factor (PEDF), belongs to the superfamily of serine protease inhibitors, is considered to be a highly effective inhibitor of angiogenesis in cell culture, and it can reduce expressions and biological actions of VEGF [32,33]. In our results, over-expression of DAPK1 can down-regulate VEGF, whereas up-regulate the PEDF, which indicated that DAPK1 can inhibit the VEGF/PEDF pathway. The PI3K/Akt and ERK pathways play the key roles in transmission of cell signals to cell nucleus, and they can affect the genes expressions that regulate important cellular processes including cell growth, proliferation and apoptosis [34-36]. Our results showed that over-expression of DAPK1 can down-regulate both the expressions of phosphor-Akt (p-Akt) and phosphor- ERK1/2 (p-ERK1/2), which indicated that DAPK1 can inhibit the proliferation, migration, and invasion of carcinoma of pancreas BxPC-3 cell line partly via PI3K/Akt and ERK pathways.
In conclusion, our present investigation demonstrated that DAPK1 gene over-expression can suppress the proliferation, migration, and invasion of carcinoma of pancreas BxPC-3 cell line, and the mechanisms may be related to induction of mitochondria-mediated apoptosis, down-regulations of MMP-2 and VEGF, up-regulations of PEDF, and affect the PI3K/Akt and ERK pathways.
Acknowledgements
This work was supported by the project of science and technology of lishui city public welfare (2012jyzb13).
Disclosure of conflict of interest
None.
References
- 1.Dhillon H, Chikara S, Reindl KM. Piperlongumine induces pancreatic cancer cell death by enhancing reactive oxygen species and DNA damage. Toxicology Reports. 2014;1:309–318. doi: 10.1016/j.toxrep.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maginn EN, de Sousa CH, Wasan HS, Stronach EA. Opportunities for translation: Targeting DNA repair pathways in pancreatic cancer. Biochim Biophys Acta. 2014;1846:45–54. doi: 10.1016/j.bbcan.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Lou HZ, Weng XC, Pan HM, Pan Q, Sun P, Liu LL, Chen B. The novel mTORC1/2 dual inhibitor INK-128 suppresses survival and proliferation of primary and transformed human pancreatic cancer cells. Biochem Biophys Res Commun. 2014;450:973–8. doi: 10.1016/j.bbrc.2014.06.081. [DOI] [PubMed] [Google Scholar]
- 4.Oettle H. Progress in the knowledge and treatment of advanced pancreatic cancer: from benchside to bedside. Cancer Treat Rev. 2014;40:1039–47. doi: 10.1016/j.ctrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Hashim YM, Spitzer D, Vangveravong S, Hornick MC, Garg G, Hornick JR, Goedegebuure P, Mach RH, Hawkins WG. Targeted pancreatic cancer therapy with the small molecule drug conjugate SW IV-134. Mol Oncol. 2014;8:956–67. doi: 10.1016/j.molonc.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 7.Luo XJ, Li LL, Deng QP, Yu XF, Yang LF, Luo FJ, Xiao LB, Chen XY, Ye M, Liu JK. Grifolin, a potent antitumour natural product upregulates death-associated protein kinase 1 DAPK1 via p53 in nasopharyngeal carcinoma cells. Eur J Cancer. 2011;47:316–325. doi: 10.1016/j.ejca.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Wu B, Yao H, Wang S, Xu R. DAPK1 modulates a curcumin-induced G2/M arrest and apoptosis by regulating STAT3, NF-κB, and caspase-3 activation. Biochem Biophys Res Commun. 2013;434:75–80. doi: 10.1016/j.bbrc.2013.03.063. [DOI] [PubMed] [Google Scholar]
- 9.Cohen O, Feinstein E, Kimchi A. DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J. 1997;16:998–1008. doi: 10.1093/emboj/16.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raval A, Tanner SM, Byrd JC, Angerman EB, Perko JD, Chen SS, Hackanson B, Grever MR, Lucas DM, Matkovic JJ. Downregulation of Death-Associated Protein Kinase 1 (DAPK1) in Chronic Lymphocytic Leukemia. Cell. 2007;129:879–890. doi: 10.1016/j.cell.2007.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang J, Wang C, Fu F, Cheng L, Xu G. Relationship between the methylation of DAPK1 promoter and the clinical pathological characters in breast cancer. Int J Pathol Clin Med. 2010;4:002. [Google Scholar]
- 12.Wang WJ, Kuo JC, Yao CC, Chen RH. DAP-kinase induces apoptosis by suppressing integrin activity and disrupting matrix survival signals. J Cell Biol. 2002;159:169–179. doi: 10.1083/jcb.200204050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngamwongsatit P, Banada PP, Panbangred W, Bhunia AK. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J Microbiol Methods. 2008;73:211–215. doi: 10.1016/j.mimet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Wu JG, Peng W, Yi J, Wu YB, Chen TQ, Wong KH, Wu JZ. Chemical composition, antimicrobial activity against Staphylococcus aureus and a pro-apoptotic effect in SGC-7901 of the essential oil from Toona sinensis (A. Juss.) Roem. leaves. J Ethnopharmacol. 2014;154:198–205. doi: 10.1016/j.jep.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Z, Shulan Z. Inhibition effect of Guizhi-Fuling-decoction on the invasion of human cervical cancer. J Ethnopharmacol. 2008;120:25–35. doi: 10.1016/j.jep.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 16.Yang XK, Yang YD, Tang SQ, Xu L, Yang GH, Xu QY, Tang H, Wu JJ. Inhibitory Effect of Polysaccharides from Scutellaria barbata D. Don on Invasion and Metastasis of 95-D Cells Lines via Regulation of C-MET and E-CAD Expressions. Tropical Journal of Pharmaceutical Research. 2013;12:517–522. [Google Scholar]
- 17.Pelled D, Raveh T, Riebeling C, Fridkin M, Berissi H, Futerman AH, Kimchi A. Death-associated protein (DAP) kinase plays a central role in ceramide-induced apoptosis in cultured hippocampal neurons. J Biol Chem. 2002;277:1957–1961. doi: 10.1074/jbc.M104677200. [DOI] [PubMed] [Google Scholar]
- 18.Gozuacik D, Kimchi A. Review: Spotlight on Cancer DAPk Protein Family and Cancer. Autophagy. 2006;2:74–79. doi: 10.4161/auto.2.2.2459. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Peng X, Hu Z, Zhao Q, He J, Li J, Zhong X. Effects of over-expression of ANXA10 gene on proliferation and apoptosis of hepatocellular carcinoma cell line HepG2. J Huazhong Univ Sci Technolog Med Sci. 2012;32:669–674. doi: 10.1007/s11596-012-1015-5. [DOI] [PubMed] [Google Scholar]
- 20.Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 21.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang TH, Bang JY, Kim MH, Kang IC, Kim HM, Jeong HJ. Atractylenolide III, a sesquiterpenoid, induces apoptosis in human lung carcinoma A549 cells via mitochondria-mediated death pathway. Food Chem Toxicol. 2011;49:514–519. doi: 10.1016/j.fct.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Zhu Y, Zhang T, Zhao Z, Zhao Y, Cheng P, Li H, Gao H, Su X. Anti-tumor effects of atractylenolide I isolated from Atractylodes macrocephala in human lung carcinoma cell lines. Molecules. 2013;18:13357–13368. doi: 10.3390/molecules181113357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma YS, Weng SW, Lin MW, Lu CC, Chiang JH, Yang JS, Lai KC, Lin JP, Tang NY, Lin JG. Antitumor effects of emodin on LS1034 human colon cancer cells in vitro and in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts model. Food Chem Toxicol. 2012;50:1271–1278. doi: 10.1016/j.fct.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 25.Antonsson B. Bax and other pro-apoptotic Bcl-2 family “killer-proteins” and their victim the mitochondrion. Cell Tissue Res. 2001;306:347–361. doi: 10.1007/s00441-001-0472-0. [DOI] [PubMed] [Google Scholar]
- 26.Strasser A, Huang D, Vaux DL. The role of the bcl-2/ced-9 gene family in cancer and general implications of defects in cell death control for tumourigenesis and resistance to chemotherapy. Biochim Biophys Acta. 1997;1333:F151–F178. doi: 10.1016/s0304-419x(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 27.Moroz A, Delella FK, Lacorte LM, Deffune E, Felisbino SL. Fibronectin induces MMP2 expression in human prostate cancer cells. Biochem Biophys Res Commun. 2013;430:1319–1321. doi: 10.1016/j.bbrc.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 28.Juuti A, Lundin J, Nordling S, Louhimo J, Haglund C. Epithelial MMP-2 expression correlates with worse prognosis in pancreatic cancer. Oncology. 2007;71:61–68. doi: 10.1159/000100988. [DOI] [PubMed] [Google Scholar]
- 29.Saffar H, Sanii S, Emami B, Heshmat R, Panah VH, Azimi S, Tavangar SM. Evaluation of MMP2 and Caspase-3 expression in 107 cases of papillary thyroid carcinoma and its association with prognostic factors. Pathol Res Pract. 2013;209:195–199. doi: 10.1016/j.prp.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69:11–16. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Cao G, Cen Y, Liu T, Peng W, Sun J, Li X, Zhou H. The radiosensitizing effect of CpG ODN107 on human glioma cells is tightly related to its antiangiogenic activity via suppression of HIF-1α/VEGF pathway. Int Immunopharmacol. 2013;17:237–244. doi: 10.1016/j.intimp.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Seki R, Yamagishi SI, Matsui T, Yoshida T, Torimura T, Ueno T, Sata M, Okamura T. Pigment epithelium-derived factor (PEDF) inhibits survival and proliferation of VEGF-exposed multiple myeloma cells through its anti-oxidative properties. Biochem Biophys Res Commun. 2013;431:693–697. doi: 10.1016/j.bbrc.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 33.Yamagishi SI, Matsui T, Nakamura K, Yoshida T, Shimizu K, Takegami Y, Shimizu T, Inoue H, Imaizumi T. Pigment-epithelium-derived factor (PEDF) inhibits angiotensin-II-induced vascular endothelial growth factor (VEGF) expression in MOLT-3 T cells through anti-oxidative properties. Microvascular Research. 2006;71:222–226. doi: 10.1016/j.mvr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Brzezianska E, Pastuszak-Lewandoska D. A minireview: the role of MAPK/ERK and PI3K/Akt pathways in thyroid follicular cell-derived neoplasm. Front Biosci (Landmark Ed) 2010;16:422–439. doi: 10.2741/3696. [DOI] [PubMed] [Google Scholar]
- 35.Liu C, Yang J, Fu W, Qi S, Wang C, Quan C, Yang K. Coactivation of the PI3K/Akt and ERK signaling pathways in PCB153-induced NF-κB activation and caspase inhibition. Toxicol Appl Pharmacol. 2014;277:270–278. doi: 10.1016/j.taap.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Wang H, Zhu J, Xu J, Ding K. Mollugin induces tumor cell apoptosis and autophagy via the PI3K/AKT/mTOR/p70S6K and ERK signaling pathways. Biochem Biophys Res Commun. 2014;450:247–54. doi: 10.1016/j.bbrc.2014.05.101. [DOI] [PubMed] [Google Scholar]


