Abstract
The phosphoinositide 3-kinases (PI3Ks) are a critical family of signaling enzymes that participate in many cellular processes that promote the transformation of a normal cell into a cancer cell. These processes include cancer cell proliferation, migration, and invasion. However, the correlation between PI3Ks and multidrug resistance (MDR) remains unclear. The prognostic value of PI3Ks has not been previously evaluated. Thus, this study aims to evaluate the association between PIK3CA and PIK3CB expression and the MDR gene in colorectal cancer (CRC) patients. Immunohistochemistry was employed to detect the expressions of PIK3CA, PIK3CB, MDR-1, LRP, GST-π, and Topo II in 316 CRC specimens. Patients were followed-up annually by telephone or at an outpatient clinic. Results revealed that PIK3CA and PIK3CB expression was correlated with the degree of tumor differentiation and lymph node metastasis (P < 0.05). The overexpression of MDR-1, LRP, Topo II, and GST-π was found to be 72.78%, 70.89%, 77.53%, and 76.58% of CRC, respectively. Correlation analysis showed that PIK3CA and PIK3CB expression exhibits a positive correlation with MDR-1, LRP, and GST-π with correlation coefficients of 0.288, 0.128, and 0.197, respectively (P < 0.05). Kaplan-Meier analysis revealed that the five-year survival rate of patients without lymph node metastasis, positive expression of PIK3CA and PIK3CB, and negative expression of GST-π and MDR-1 was higher than those with these characteristics. Multivariate analysis revealed that GST-π, MDR-1 expression, and lymph node metastasis could serve as independent predictive factors of overall survival. The expression of both PIK3CA and PIK3CB is increased and related to the development and progress of colorectal carcinoma and MDR. The combined detection of PIK3CA andPIK3CB is important for patients with colorectal carcinoma in prognosis and optimal therapy.
Keywords: Colorectal cancer, MDR, PIK3CA, PIK3CB, prognosis
Introduction
Colorectal cancer (CRC) is the third most common tumor and the second most common cause of cancer-related deaths worldwide [1]. Chemotherapy is used as adjuvant therapy for resectable CRC patients or as palliative therapy for advanced/metastatic CRC patients to prevent the recurrence or to improve survival or quality of life. However, the major disadvantage of chemotherapy is the development of multidrug resistance (MDR), which refers to the desensitization of tumor cells to multiple chemotherapeutic drugs after the repeated use of a single chemotherapeutic drug [2]. Several novel factors acting downstream of the initial drug-induced insult have recently been shown to be associated with the development of MDR; these factors include enhanced DNA repair activity, defective apoptosis pathway, and altered signaling pathways [3,4]. However, a well-recognized mechanism that leads to MDR involves the abnormal changes in signaling pathways. The phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway serves a major function not only in tumor development but also in the potential response of the tumor to treatment. Akt is a key mediator of PI3K signaling and is located at an intersection of multiple pathways that are implicated in cell proliferation, survival, transcription, and metabolic processes [5]. Considerable evidence has recently suggested that the PI3K/Akt signaling pathway is frequently activated in human CRCs [6].
Previous studies have shown that PIK3CA and PIK3CB serve an important function in the development of CRC [7,8]. However, the correlation of PIK3CA and PIK3CB with drug resistance remains unclear. In addition, most studies involving class I PI3Ks focus only on activating mutations in PIK3CA [9]. Therefore, we investigated whether PIK3CA and PIK3CB affect drug resistance and prognosis in CRC.
Previous studies have shown that the PI3K signaling pathway contributes to development of CRC. However, the effect of PIK3CA and PIK3CB on drug resistance remains unclear.
Materials and methods
Clinical specimens and patient data
A total of 316 CRC tissue samples at different stages were collected from the Department of Pathology of Binzhou Medical University Hospital between January 2005 and May 2009. Tissue samples were routinely fixed in 10% buffered neutral formalin. Cancer tissues were cut into wedge shapes, whereas normal tissues were cut at least 5 cm away from the tumor margin. All CRC patients were clinically and pathologically proven to have not received preoperative chemotherapy or radiotherapy. All specimens were collected with the informed consent of the patients, and the Ethical Committee of Binzhou Medical University Hospital approved the protocols used in this study. Clinicopathologic classification was performed according to the National Comprehensive Cancer Network classification parameters. Demographic and clinicopathological parameters were prospectively recorded using a chart review. Patients were followed-up annually by telephone or at outpatient clinics until May 2014 or death.
Immunohistochemistry and scoring
Before staining, paraffin-embedded tissue blocks were cut with thicknesses of 4 μm. Sections were deparaffinized in an oven at 60°C for 2 h and then rehydrated with two and three changes of xylene and ethanol, respectively. Antigen retrieval was performed using the microwave retrieval method. Endogenous peroxidase activity was quenched through incubation with 3% hydrogen peroxide for 10 min at room temperature. Nonspecific binding was blocked by incubating sections with 10% normal goat serum in PBS for 30 min at room temperature. Without washing, sections were incubated with rabbit monoclonal antibody against human PIK3CA (1:150; Abcam, Cambridge, MA, USA), PIK3CB (1:250; Abcam, Cambridge, MA, USA), MDR-1 (Fuzhou Maixin Biotech Co.), LRP (Fuzhou Maixin Biotech Co.), GST-π (Fuzhou Maixin Biotech Co.), and Top II (Fuzhou Maixin Biotech Co.) at 4°C overnight. The sections were then incubated with horseradish peroxidase-conjugated secondary goat anti-rabbit antibody (Abcam, San Francisco, USA) for 1 h at room temperature. Sections were then washed with PBS and treated with the Metal-enhanced DAB Substrate Kit (Thermo Scientific, USA) to visualize the antigen-antibody complex. Two researchers who were unaware of clinicopathological status of the specimens scored each section separately. The percentage of stained cells on each section was scored as: 0 (less than 5%), 1 (5%-25%), 2 (26%-50%), and 3 (> 50%). Staining intensity was scored as: 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). The final score of each specimen was calculated by the multiplying stained cell score with the staining intensity score. Thus, the final score ranged from 0 to 9. Low expression was defined as a final score of < 4, whereas a score ≥ 4 was determined to be high expression.
Statistical analysis
All statistical analyses were conducted with the SPSS 18.0 software (SPSS Inc., Chicago, USA). The correlation of PIK3CA and PIK3CB expression with clinical parameters was analyzed using Χ and Fisher’s exact tests. Overall survival (OS) was plotted using the Kaplan-Meier method. The analyses of prognostic factors for OS were determined using multivariate Cox proportional hazards regression method. P value ≤ 0.05 was considered statistically significant.
Results
Correlation of PIK3CA and PIK3CB with different clinicopathologic parameters
We analyzed the correlation between the PIK3CA and PIK3CB expression levels of CRC samples and a set of clinicopathologic parameters, including age, gender, histological type and tumor site (Table 1). High PIK3CA and PIK3CB expression was found to be significantly correlated with histological grade (P=0.015) and lymph node metastasis (P=0.005). Other characteristics, such as age (P=0.076), gender (P=0.252), large size (P=0.114), tumor site (P=0.317), and histological type (P=0.256), were not associated with PIK3CA and PIK3CB expression levels.
Table 1.
Correlation of PIK3CA andPIK3CB with different clinicopathologic parameters
| Parameters | PIK3CA (+) PIK3CB (+) | PIK3CA (+) PIK3CB (-) | PIK3CA (-) PIK3CB (+) | PIK3CA (-) PIK3CB (-) | X2 value | P value | r value |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| < 60 | 56 | 16 | 64 | 28 | 6.867 | 0.076 | 0.018 |
| ≥ 60 | 92 | 12 | 28 | 20 | |||
| Gender | |||||||
| Male | 80 | 10 | 50 | 22 | 4.088 | 0.252 | 0.035 |
| Female | 68 | 18 | 42 | 26 | |||
| Tumor sizes(cm) | |||||||
| < 5 | 60 | 13 | 48 | 28 | 6.037 | 0.114 | -0.137 |
| ≥ 5 | 88 | 15 | 44 | 20 | |||
| Tumor site | |||||||
| Colon | 72 | 16 | 52 | 20 | 3.528 | 0.317 | 0.001 |
| Rectal | 76 | 12 | 40 | 28 | |||
| Histologic grade | |||||||
| Grade 1 | 82 | 13 | 55 | 11 | 19.699 | 0.015※ | 0.136 |
| Grade 2 | 50 | 11 | 26 | 27 | |||
| Grade 3 | 16 | 4 | 11 | 10 | |||
| Histology | |||||||
| Tubular | 134 | 22 | 82 | 43 | 14.052 | 0.021※ | 0.129 |
| Mucinous | 14 | 6 | 10 | 5 | |||
| Depth of invasion | |||||||
| Mucosa and submucosa | 8 | 0 | 5 | 5 | 11.646 | 0.147 | -0.082 |
| Myometrium | 27 | 12 | 20 | 12 | |||
| Epicardial | 113 | 16 | 67 | 31 | |||
| Lymph node metastasis | |||||||
| Yes | 98 | 22 | 50 | 22 | 12.987 | 0.005※ | 1.168 |
| No | 50 | 6 | 42 | 26 |
indicate statistical significant (p < 0.05).
PIK3CB and PIK3CB protein expression is positively correlated with MDR-1, LRP, and GST-π
In colorectal carcinoma, the high expression rates of MDR-1, LRP, GST-π, and Topo II were 72.78%, 70.89%, 77.53%, and 76.58%, respectively. As is shown in Table 2, PIK3CA and PIK3CB expression was found to be positively correlated with MDR-1, LRP, and GST-π (r=0.288, P=0.001, r=0.128, P=0.023, and r=0.197, P=0.001), whereas no correlation was found between PIK3CA and PIK3CB expression and Topo II (P > 0.05) (Figure 1).
Table 2.
Correlation of PIK3CA andPIK3CB with MDR
| MDR | PIK3CA (+) PIK3CB (+) | PIK3CA (+) PIK3CB (-) | PIK3CA (-) PIK3CB (+) | PIK3CA (-) PIK3CB (-) | X2 value | P value | r value |
|---|---|---|---|---|---|---|---|
| MDR-1 (+) | 122 | 22 | 68 | 18 | 37.656 | 0.001※ | 0.288 |
| (-) | 26 | 6 | 24 | 30 | |||
| LRP (+) | 110 | 18 | 75 | 21 | 23.608 | 0.023※ | 0.128 |
| (-) | 38 | 10 | 17 | 27 | |||
| TopII (+) | 112 | 13 | 86 | 34 | 30.508 | 0.204 | -0.072 |
| (-) | 36 | 15 | 6 | 14 | |||
| GST-π (+) | 120 | 22 | 80 | 20 | 39.882 | 0.001※ | 0.197 |
| (-) | 28 | 6 | 12 | 28 |
indicate statistical significant (p < 0.05).
Figure 1.
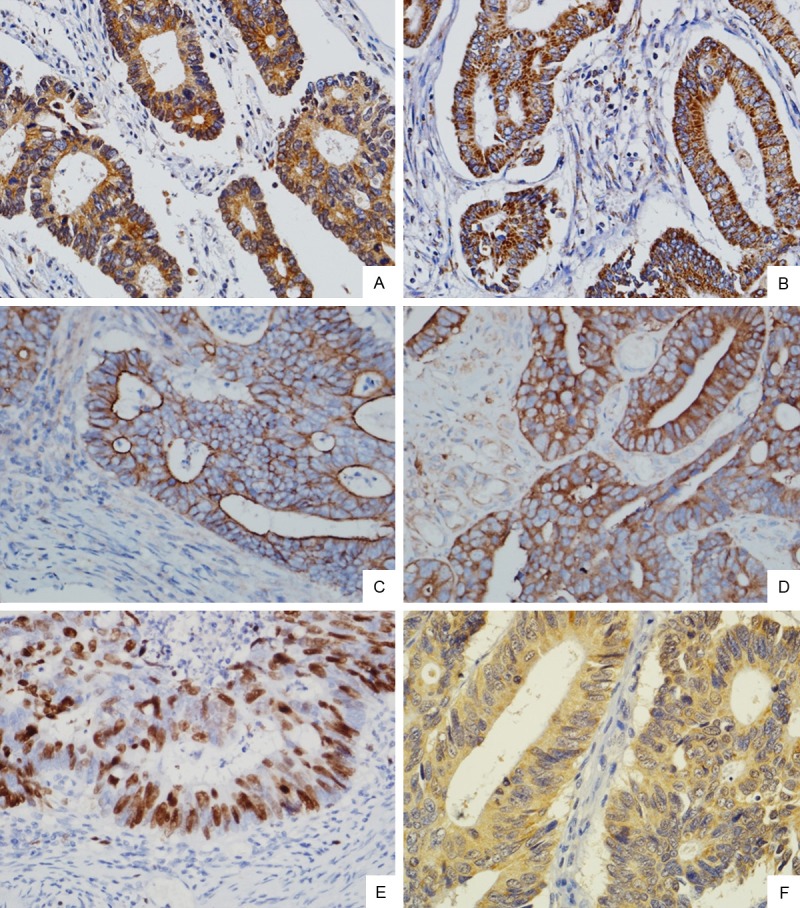
Immunohistochemical staining of PIK3CA, PIK3CB, MDR-1, LRP, TOPII and GST-π protein expressions in CRC tissues. High-power view (Original magnification ×400) shows strong staining for PIK3CA, PIK3CB, MDR-1, LRP, and GST-π in the cytoplasm of cancer cells (A. PIK3CA; B. PIK3CB; D. LRP; F. GST-π), MDR-1 in the membrane and cytoplasm of cancer cells (C), Topo II in the nucleus of cancer cells (E).
Correlation between PIK3CA, PIK3CB, and MDR expression levels and patient OS
The median survival time of 316 cases patients was 60 months, and the five-year survival rate of patients was 47.5%. The five-year survival rate of patients with PIK3CA-positive and PIK3CB-positive expression was 35.1%. The five-year survival rate of patients with PIK3CA-positive and PIK3CB-negative expression was 53.6%. The five-year survival rate of patients with PIK3CA-negative and PIK3CB-positive expression was 52.2%. The five-year survival rate of patients with PIK3CA-negaitive and PIK3CB-negaitive expression was 59.4%, which unlike that of patients with PIK3CA-positive and PIK3CB-positive expression. Kaplan-Meier analysis revealed that patients with PIK3CA-positive and PIK3CB-positive expression had a short OS, whereas those with PIK3CA-negative and PIK3CB-negaitive expression had a longer OS than the other three groups (P ≤ 0.05). Kaplan-Meier analysis showed that CRC patients with negative expressions of MDR-1 and GST-π and no lymph node metastasis have long survival time (Figure 2B and 2C). Cox regression analysis showed that PIK3CA, PIK3CB, MDR-1, GST-π, and lymph node metastasis were independent prognostic factors of CRC (Table 3).
Figure 2.
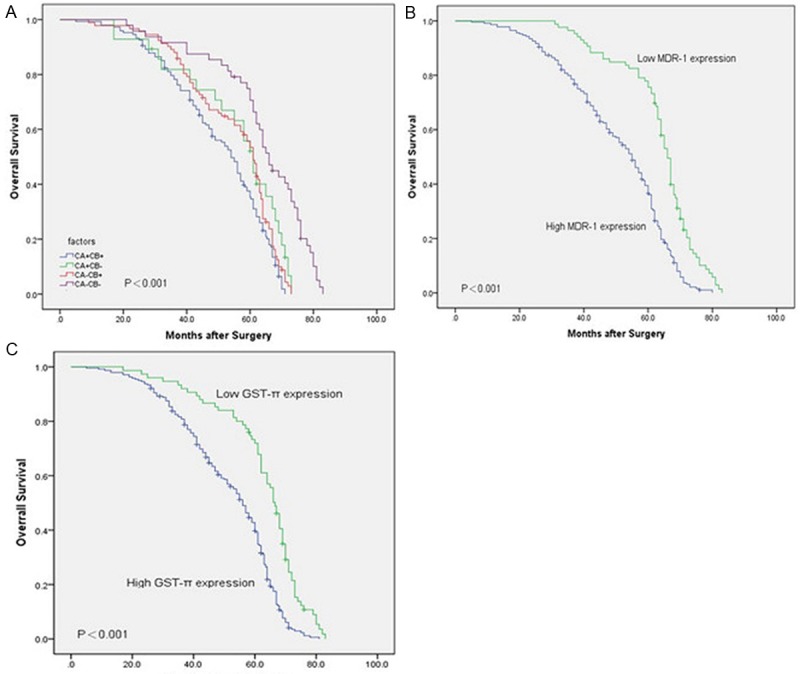
Kaplan-Meier survival curves for PIK3CA/PIK3CB (A), MDR-1 (B) and GST-π (C) expression in CRC. In Kaplan-Meier analysis, high PIK3CA/PIK3CB, MDR-1, and GST-π expressions correlated with a shorter OS in CRC patients than the corresponding controls.
Table 3.
Univariate and multivariate analysis of clinicopathologicparameters with OS by Cox proportional hazards regression
| Variable | RFS hazard ratio (95% Cl) | P value |
|---|---|---|
| Age (< 60, ≥ 60) | 1.015 (0.791-1.302) | 0.907 |
| Gender (male, female) | 1.017 (0.795-1.300) | 0.894 |
| Tumor sizes (< 5, ≥ 5) | 1.162 (0.907-1.488) | 0.235 |
| Tumor site (colon, rectal) | 0.985 (0.775-1.252) | 0.903 |
| Histologic grade (Grade 1, Grade 2, Grade 3) | 0.990 (0.685-1.432) | 0.958 |
| Histology (Tubular, Mucinous) | 0.730 (0.500-1.604) | 0.102 |
| Depth of invasion (Mucosa and submucosa, myometrium, epicardial) | 1.403 (0.826-2.383) | 0.210 |
| Lymph node metastasis (yes, no) | 1.309 (1.018-1.684) | 0.036※ |
| MDR-1 | 2.048 (1.523-2.753) | 0.000※ |
| LRP | 1.273 (0.952-1.705) | 0.105 |
| TopII | 1.272 (0.933-1.732) | 0.128 |
| GST-π | 1.431 (1.048-1.955) | 0.024※ |
indicate statistical significant (p < 0.05).
Discussion
The PI3K/Akt signaling pathway serves an important function in the proliferation, cell growth, cycle progression, apoptosis, migration, and survival of cancer cells [10,11]. Moreover, a variety of human tumors that are closely related to the development of abnormal activation in promoting normal cell development contribute to cancer cell transformation [9,12]. PI3Ks are lipid kinases that consist of three different classes (class I, II, and III) based on structures and activation mechanisms. Class I PI3Ks are the best-characterized enzymes and include class IA (p110α, p110β, and p110δ) and class IB (p110γ). Thus, recent studies have reported that different PI3K members have distinct, nonredundant cellular functions in cell signaling and tumorigenesis, e.g., p110α is required to sustain the proliferation of PIK3CA-mutant tumors [13], whereas p110β, which the PIK3CB gene encodes, is implicated in PTEN deficient tumorigenesis [14,15]. Although p110γ and p110δ expression is predominantly restricted to blood diseases, different human cancer cell lines have also been shown to express these PI3K isoforms, and the aberrant expression of p110δ has been found to contribute to neuroblastoma cell growth and survival [16]. Meanwhile, PIK3CA and PIK3CB are extensively expressed in tumors and are closely related to tumorigenesis, thus drawing considerable attention. Studies have shown that the mutations of PIK3CA and PIK3CB serve an important function in breast cancer, ovarian cancer, nasopharyngeal carcinoma, osteosarcoma, and other human malignancies in tumorigenesis and development [17-20]. In this study, we investigated both PIK3CA and PIK3CB mutation effects on drug resistance. Our results revealed that both PIK3CA and PIK3CB have a positive expression rate of 46.84%, significantly higher than that of the other three groups. We also found that high PIK3CA and PIK3CB expression is associated with cell differentiation and lymph node metastasis of CRC (P ≤ 0.05). This result suggests that PIK3CA and PIK3CB expression increases in CRC and may thus contribute to the tumorigenesis and progression of CRC. At later clinical stages, the pathological grade of low PI3K protein expression in cancer tissues is highly positive rate, and the corresponding expression is correlated with clinicopathological factors relative to lymph node metastasis and distant metastasis. These findings suggest that PI3Ks is associated with proliferation, metastasis, and infiltration in CRC. Therefore, the inhibition both of PIK3CA and PIK3CB can serve as a potentially useful approach for gastric cancer treatment.
The major treatment for CRC modalities includes surgery and chemotherapy. However, the therapeutic effectiveness of these treatments is disappointing because of MDR and high-dose chemotherapy-induced toxicity to normal tissues. The simultaneous detection of multiple MDR gene products is necessary in clinical trials to determine the drug resistance of tumor cells to chemotherapy drugs objectively. The commonly used clinical drug resistance genes are MDR-1, LRP, GST-π, and Topo II, which can also indirectly reflect the condition of MDR in tumor tissues. Our results show that the positive rates of MDR-1, LRP, GST-π, and Topo II expressions are 72.78%, 70.89%, 77.53%, and 76.58%, respectively, indicating the presence of MDR in colorectal carcinoma.
Studies have suggested the potential involvement of elevated DNA repair activities, defective apoptosis pathways, altering metabolisms [21], and PI3K/Akt signaling pathways [22,23] in the MDR of human cancers, which includes CRC. The downregulation of PIK3CA or PIK3CB has recently been described in some tumor MDR cell lines and tissues. Guerreiro et al. found that isoform-specific p110α inhibitors impaired medulloblastoma cell proliferation and sensitized the cells to chemotherapy [24]. Jeong et al. showed that the specific inhibition of PI3K isoform p110β can resensitize PTX-resistant cancer cells to PTX in vitro and in vivo [25]. Currently, the well-recognized mechanism that leads to MDR is the overexpression of the MDR1 gene product, P-glycoprotein (P-gp). P-gp, a drug-transporting protein, can cause MDR in tumor cells by decreasing intracellular drug levels [26]. Thus, circumventing P-gp-mediated MDR is still the main goal of MDR-reversion in clinical chemotherapy. Previous studies have indicated that the activation of the PI3K/Akt signaling pathway is closely associated with the upregulation of MDR1 expression [27,28]. García MG et al. found that PI3K/Akt inhibition correlates with the downregulation of NF-κB activity and the inhibition of P-gp function [29]. Furthermore, PI3K/Akt serves a vital function in mediating survival signals, as well as in contributing to the inhibition of apoptosis and therapeutic resistance through multiple mechanisms [30,31]. Therefore, the inhibition of the PI3K/Akt pathway may promote chemotherapeutic drug-induced apoptosis and consequently enhance the chemosensitivity of various types of cancer.
In this study, we evaluated the correlation of the PI3K/Akt signaling pathway with MDR. Our results indicate that PIK3CA and PIK3CB expression correlates directly with MDR-1 (r=0.288), LRP (r=0.128), and GST-π (r=0.197), which in turn supports the findings that PIK3CA and PIK3CB expression is closely related to MDR. We found that the activity of the downstream factor NF-κB is enhanced and the expression of P-gp increases when PIK3CA and PIK3CB protein is highly expressed in colorectal carcinoma tissues. The chemotherapeutic drugs are leached out of cancer cells through the energy of ATP hydrolysis, resulting in low intracellular drug concentration and a decline in drug efficacy. The results indicate that CRC patients with overexpressed PI3KCA and PI3KCB can produce MDR when induced by LRP-related drugs, such as vincristine, adriamycin, and epirubicin, or GST-π related drugs, such as alkylating agents and mitomycin. Meanwhile, this phenomenon cannot be detected under Topo II-related chemotherapeutics.
Targeted cancer therapy is the future of cancer treatment. A number of targeted therapy drugs used in clinical tests exhibit good therapeutic effects. One of the most common treatments is the epidermal growth factor receptor (EGFR) targeting therapy. The main drugs used are gefitinib and cetuximab. Preclinical trials have confirmed the prolonged progression-free survival of advanced CRC patients with the use of these drugs, especially cetuximab. However, phase I/II clinical studies in patients with advanced CRC showed that despite gefitinib demonstrating objective responses in a minority of patients, the majority displayed no significant effects, which suggests a high level of intrinsic or acquired resistance to such treatment. Moreover, EGFR overexpression is clearly not the sole determinant of gefitinib therapy response. Recently published data indicate that a number of patients with mCRC that are unlikely to respond to anti-EGFR therapies can be identified when expression of PTEN and mutations of PIK3CA are concomitantly ascertained. In addition, clinical studies showed that patients with EGFR overexpression and PIK3CA mutation had been treated with cetuximab, and chemotherapy failed in some of these patients. The present study reveals that both PIK3CA and PIK3CB serve an important function in CRC and are related to the MDR and prognosis of CRC. Therefore, we speculate that aside from the mutation of PI3KCA, many other molecular gene mutations may occur in the PI3K/Akt signaling pathway and may this lead to treatment failure or drug resistance relative to cetuximab chemotherapy. Another important molecular in the PI3K/Akt pathway is PI3KCB. However, the effect of P13KCB mutation on cetuximab-targeted therapy requires further investigation.
Grille et al. [32] reported that Akt activation in cancer cells increases the motility required for tissue invasion and metastases and is consequently associated with poor prognosis in many cancers. This study suggests that the prognosis of CRC is associated with some clinical pathologic factors, such as differentiation degree and lymph node metastasis. In addition, the protein expression level of PIK3CA and PIK3CB is closely related with the prognosis of patients with CRC, especially when the expressions of both proteins simultaneously increase, thus resulting in high mortality rate and poor prognosis. Therefore, we hypothesized that PI3KCA and PI3KCB may be used as independent indexes of molecular biology to test colorectal malignant degree and prognosis. Furthermore, we observed that the prognosis of CRC has a strong relationship with the expression of the drug resistance genes MDR-1 and GST-π. The multivariate Cox proportional hazard regression analysis indicates that lymphatic metastasis and the expression of MDR-1 and GST-π can be independent factors for predicting the prognosis of CRC. All these results suggest that MDR-1 and GST-π expression can be an important factor in analyzing the prognosis of CRC. Combining these expressions with the traditional clinical pathologic factors can improve prognosis accuracy for patients with CRC.
In summary, our study proves that both PIK3CA and PIK3CB serve important functions in CRC carcinogenesis, drug resistance, and prognosis, which further suggests that PIK3CA and PIK3CB may be used as potential drug targets for CRC.
Acknowledgements
This work was supported by Scientific and Technological Project of Shandong Province.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Szakacs G, paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Nieto S, Zhivotovsky B. Role of alterations in the apoptotic machinery in sensitivity of cancer cells to treatment. Curr Pharm Des. 2006;12:4411–4425. doi: 10.2174/138161206779010495. [DOI] [PubMed] [Google Scholar]
- 4.Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL-2 in human gastric cancer cell. Int J Cancer. 2008;123:372–79. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 5.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Roberts TM, Shivdasani RA. Targeting PI3K signaling as a therapeutic approach for colorectal cancer. Gastroenterology. 2011;141:50–61. doi: 10.1053/j.gastro.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Binbin Cui, Ji Tao, Yanmei Y. Studies on the Expression Patterns of Class I PI3K Catalytic Subunits and Its Prognostic Significance in Colorectal Cancer. Cell Biochem Biophys. 2012;62:47–54. doi: 10.1007/s12013-011-9257-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhu YF, Yu BH, Li DL, Ke HL, Guo XZ, Xiao XY. PI3K expression and PIK3CA mutations are related to colorectal cancer metastases. World J Gastroenterol. 2012;18:3745–3751. doi: 10.3748/wjg.v18.i28.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nature Review Drug Discovery. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 11.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 12.Crowell JA, Steele VE, Fay JR. Targeting the AKT protein kinase for cancer chemoprevention. Mol Cancer Ther. 2007;6:2139–2148. doi: 10.1158/1535-7163.MCT-07-0120. [DOI] [PubMed] [Google Scholar]
- 13.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 14.Torbett NE, Luna-Moran A, Knight ZA, Houk A, Moasser M, Weiss W, Shokat KM, Stokoe D. A chemical screen in diverse breast cancer cell lines reveals genetic enhancers and suppressors of sensitivity to PI3K isoform-selective inhibition. Biochem J. 2008;415:97–110. doi: 10.1042/BJ20080639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wee S, Wiederschain D, Maira SM, Loo A, Miller C, deBeaumont R, Stegmeier F, Yao YM, Lengauer C. PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci U S A. 2008;105:13057–13062. doi: 10.1073/pnas.0802655105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boller D, Schramm A, Doepfner KT, Shalaby T, von Bueren AO, Eggert A, Grotzer MA, Arcaro A. Targeting the phosphoinositide 3-kinase isoform p110delta impairs growth and survival in neuroblastoma cells. Clin Cancer Res. 2008;14:1172–1181. doi: 10.1158/1078-0432.CCR-07-0737. [DOI] [PubMed] [Google Scholar]
- 17.Paplomata E, O’Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol. 2014;6:154–66. doi: 10.1177/1758834014530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park MS, Dong SM, Kim BR, Seo SH, Kang S, Lee EJ, Lee SH, Rho SB. Thioridazine inhibits angiogenesis and tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian cancer xenografts. Oncotarget. 2014;5:4929–34. doi: 10.18632/oncotarget.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuen JW, Chung GT, Lun SW, Cheung CC, To KF, Lo KW. Epigenetic Inactivation of Inositol polyphosphate 4-phosphatase B (INPP4B), a Regulator of PI3K/AKT Signaling Pathway in EBV-Associated Nasopharyngeal Carcinoma. PLoS One. 2014;9:e105163. doi: 10.1371/journal.pone.0105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu LB, Jiang J, Zhu XP, Wang TF, Chen XY, Luo QF, Shu Y, Liu ZL, Huang SH. Knockdown of Aurora-B inhibits osteosarcoma cell invasion and migration via modulating PI3K/Akt/NF-κB signaling pathway. Int J Clin Exp Pathol. 2014;7:3984–91. [PMC free article] [PubMed] [Google Scholar]
- 21.Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL-2 in human gastric cancer cell. Int J Cancer. 2008;123:372–9. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 22.Xie X, Tang B, Zhou J, Gao Q, Zhang P. Inhibition of the PI3K/Akt pathway increases the chemosensitivity of gastric cancer to vincristine. Oncol Rep. 2013;30:773–82. doi: 10.3892/or.2013.2520. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, Han S, Liu J, Sun S, Han Z, Wu K, Fan D. Hypoxia-inducible factor-1a contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci. 2008;99:121–8. doi: 10.1111/j.1349-7006.2007.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerreiro AS, Fattet S, Fischer B, Shalaby T, Jackson SP, Schoenwaelder SM, Grotzer MA, Delattre O, Arcaro A. Targeting the PI3K p110 Isoform Inhibits Medulloblastoma Proliferation, Chemoresistance, and Migration. Clin Cancer Res. 2008;14:6761–6769. doi: 10.1158/1078-0432.CCR-08-0385. [DOI] [PubMed] [Google Scholar]
- 25.Jeong JY, Kim KS, Moon JS, Song JA, Choi SH, Kim KI, Kim TH, An HJ. Targeted inhibition of phosphatidyl inositol-3-kinase p110b, but not p110a, enhances apoptosis and sensitivity to paclitaxel in chemoresistant ovarian cancers. Apoptosis. 2013;18:509–20. doi: 10.1007/s10495-013-0807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Readi MZ, Hamdan D, Farrag N, El-Shazly A, Wink M. Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur J Pharmacol. 2010;626:139–145. doi: 10.1016/j.ejphar.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 27.Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe S, Mills GB, Unate H. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-κB signaling. Oncogene. 2002;21:1945–1954. doi: 10.1038/sj.onc.1205117. [DOI] [PubMed] [Google Scholar]
- 28.Barancík M, Bohácová V, Sedlák J, Sulová Z, Breier A. LY294, 002, a specific inhibitor of PI3K/Akt kinase pathway, antagonizes P-glycoprotein-mediated multidrug resistance. Eur J Pharm Sci. 2006;29:426–434. doi: 10.1016/j.ejps.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 29.García MG, Alaniz LD, Cordo Russo RI, Alvarez E, Hajos SE. PI3K/Akt inhibition modulates multidrug resistance and activates NF-kB in murine lymphoma cell lines. Leuk Res. 2009;33:288–96. doi: 10.1016/j.leukres.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 31.Nuutinen U, Postila V, Mättö M, Eeva J, Ropponen A, Eray M, Riikonen P, Pelkonen J. Inhibition of PI3-kinase-Akt pathway enhances dexamethasone-induced apoptosis in a human follicular lymphoma cell line. Exp Cell Res. 2006;312:322–30. doi: 10.1016/j.yexcr.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]


