Abstract
A 60-year-old man complained of nausea, vomiting, decreased appetite, and a feeling of abdominal fullness in August 2013. Based on biopsy findings from an upper gastrointestinal endoscopy examination, a diagnosis of non-Hodgkin’s lymphoma (NHL), diffuse large B-cell lymphoma (DLBCL), non-GC type, was made. F18-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) revealed abnormal accumulations solely in the gastric wall (SUVmax = 14.5), the left adrenal gland (SUVmax = 14.3), and the right adrenal gland (SUVmax = 8.5). The clinical stage (Ann Arbor) was IVA, the serum LDH level was within the reference range, and the International Prognostic Index (IPI) was low-intermediate. The serum soluble IL-2 receptor level was within the reference range, and there was no evidence of HIV, EB virus, or autoimmune disease. After the completion of 4 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) and 2 parallel cycles of prophylactic intrathecal (I.T.), an upper gastrointestinal endoscopy and a FDG-PET/CT examination showed complete remission (CR). The patient received 8 cycles of ritsuximab therapy, 6 cycles of CHOP, and 3 cycles of I.T. The patient has maintained a CR for about 14 months. A literature search revealed that malignant lymphoma with involvement confined to the adrenal gland and gastrointestinal tract is exceedingly rare, and only 3 cases of malignant lymphoma have been reported, with involvement of the stomach in 2 cases and the duodenum in 1 case. All of the cases were diagnosed as DLBCL. The case described herein represents the third case with involvement of the stomach.
Keywords: Diffuse large B-cell lymphoma (DLBCL), adrenal gland, stomach
Introduction
Tumors of the adrenal gland may be either primary or secondary neoplasms. Primary adrenal tumors mainly occur as adenomas or adrenal carcinomas [1], while primary adrenal lymphomas are rare [2]. Among cases of secondary adrenal tumors, 90% were carcinomas and 49% were bilateral tumors [3]. Lymphomatous invasion of the adrenal gland was discovered by computed tomography (CT) examination in 5% of cases [4] and by a morbid anatomy in 25%-35% of cases [5,6]. Secondary adrenal lymphoma is considered to be not uncommon. Almost all secondary adrenal lymphomas have been documented to arise in the retroperitoneal lymph node or the ipsilateral kidney [7], and lymphoma with the involvement of both adrenal glands and the stomach alone, as seen in the present case, is thought to be uncommon. No more than 4 case reports, including the present case, have been described in which tumor invasion of the adrenal gland and the digestive tract was evident [8-10]. All these cases consisted of diffuse large B-cell lymphoma (DLBCL). A literature review has suggested that the prognosis may be favorable insofar as the malignant involvement is confined to the gastrointestinal tract and adrenal glands. While reports documenting a poor prognosis of patients with primary adrenal lymphoma are relatively common [11-13], the prognosis and clinical features of secondary adrenal lymphoma remain unclear. Further exploration and assessments of accumulated cases are needed.
Case report
The patient was a 60-year-old man whose chief complaints were nausea, vomiting, decreased appetite, and a sense of abdominal fullness; he had been diagnosed as having hypertension at the age of 58 years. His family history was non-contributory. Regarding the present illness, the patient had sought medical advice at a local clinic because of his chief complaints, which had developed in early August 2013. A suspicion of Borrmann type III gastric carcinoma was entertained based on the results of an upper gastrointestinal endoscopy (U-GIS); therefore, he was referred to the Department of Surgery of this hospital in late August. A re-examination using U-GIS also roused a suspicion of gastric cancer (Figure 1A, 1B), but hematoxylin-eosin (HE)-stained biopsy specimens showed the sheet-like growth of large atypical cells with prominent nucleoli (Figure 2A, 2B) and immunostaining revealed these atypical cells to be positive for cluster of differentiation (CD) 20, B-cell lymphoma (BCL) 2, and multiple myeloma oncogene (MUM) 1 (Figure 2C-E). The cells were negative for BCL-6, CD5, and CD10 (Figure 2F-H). As for the MIB-1 labeling index, 90% of the cells were found to be positive (Figure 2I). The patient was therefore diagnosed as having non-Hodgkin’s lymphoma, DLBCL, non-GC type, and the patient was examined in the Department of Hematology in early September. F18-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) revealed abnormal FDG accumulations in the gastric wall (SUVmax = 14.5), the left adrenal gland (SUVmax = 14.3), and the right adrenal gland (SUVmax = 8.5) (Figure 3A, 3B). Adrenal biopsies from the abnormal uptake sites were considered but could not be performed because of a lack of consent from the patient and his family members. No abnormalities were noted in a bone marrow examination or in a cerebrospinal fluid examination. The clinical stage was IVA according to the Ann Arbor staging system, and the International Prognostic Index (IPI) was low-intermediate (clinical stage and extranodal lesions). The patient was admitted to this hospital in mid-September.
Figure 1.
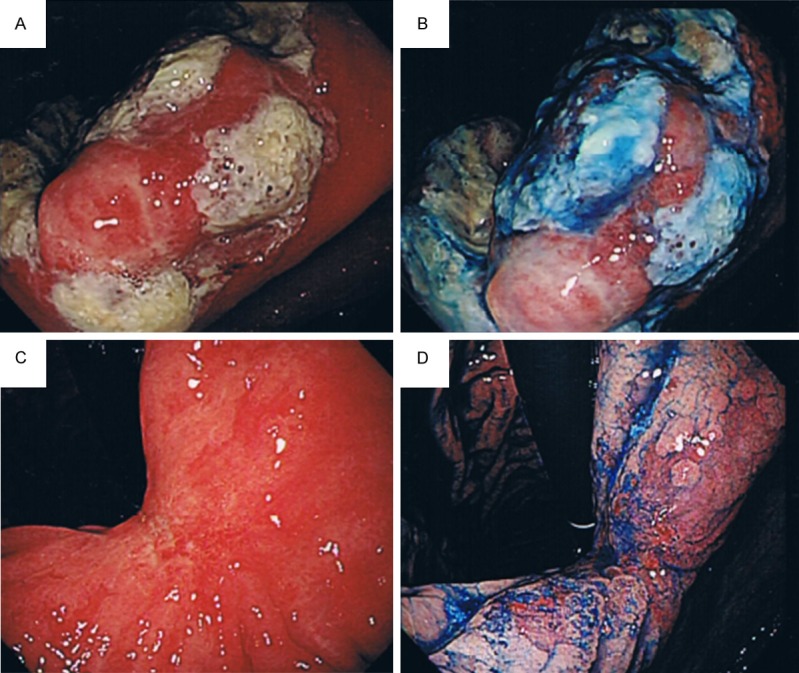
Upper gastrointestinal endoscopy findings. A. Conventional. B. Indigocarmine chromoendoscopy: a discrete ulcer with marginal elevation was noted on the lesser curvature at the angulus before the treatment. C. Conventional. D. Indigocarmine chromoendoscopy: after the treatment, the lesion had almost completely disappeared, with only a scar left on the lesser curvature at the angulus.
Figure 2.
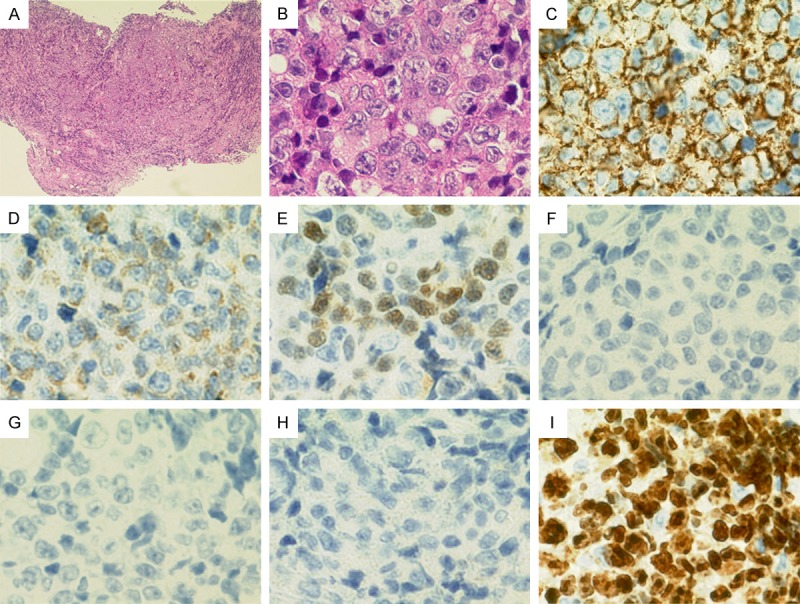
Pathologic findings of gastric biopsy specimens. A. (HE, × 40): Sheet-like growth of large atypical cells with prominent nucleoli. B. (HE, × 600): Sheet-like growth of large atypical cells with prominent nucleoli. C. (CD20, × 600): Positive. D. (bcl-2, × 600): Weakly positive. E. (MUM-1, × 600): Positive. F. (bcl-6, × 600): Negative. G. (CD5, × 600): Negative. H. (CD10, × 600): Negative. I. (MIB-1 index, × 600): 90% positive.
Figure 3.
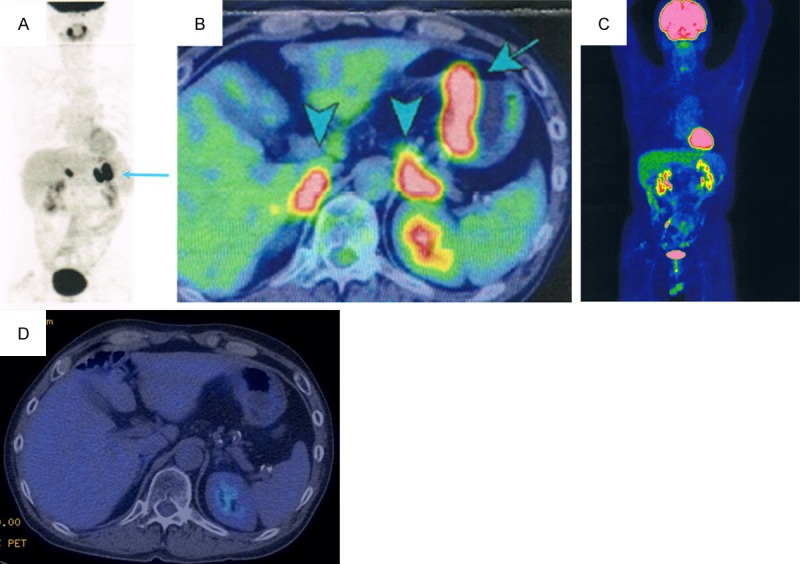
FDG-PET/CT findings. A, B. Abnormal accumulations are visible in the gastric wall (SUVmax = 14.5), the left adrenal gland (SUVmax = 14.3), and the right adrenal gland (SUVmax = 8.5). C, D. No abnormal accumulation is noted, indicating a CR.
The patient’s status upon admission was as follows: height, 164 cm; body weight, 63.0 kg; temperature, 36.4°C; blood pressure, 132/78 mmHg; pulse rate, 74 beats/min (regular); clear consciousness; palpebral conjunctiva, slightly anemic; bulbar conjunctiva, no sign of jaundice; oral cavity, no abnormalities; both lungs, clear; heart sounds, clear; and liver and spleen, impalpable. No neurological abnormalities were observed. No superficial lymph nodes were palpable.
The patient’s laboratory findings upon admission were as follows (see Table 1): negative for anti-human immunodeficiency virus (HIV) antibodies, and lactate dehydrogenase (LDH) and soluble interleukin (IL) -2 receptor levels and adrenal function tests were within the respective reference ranges.
Table 1.
Laboratory findings upon admission
| Peripheral blood | WBC | 6100/μL |
| Neut | 70.8% ↑ | |
| Ly | 17.7% ↓ | |
| Mono | 6.8% | |
| Eo | 1.1% | |
| Ba | 0.4% | |
| RBC | 382 × 104/μL ↓ | |
| Hb | 11.7 g/dL ↓ | |
| Ht | 39.5% | |
| MCV | 103.3 fl ↑ | |
| MCH | 33.1 pg | |
| Plt | 19.9 × 104/μL | |
| Reti | 1.5% | |
| Blood coagulation | PT | 81% |
| APTT | 34.7 sec | |
| Urinalysis | No abnormality | |
| Biochemistry | T.P | 7.1 g/dL |
| Alb | 4.1 g/dL | |
| AST | 25 IU/L | |
| ALT | 28 IU/L | |
| LDH | 165 IU/L | |
| ALP | 296 IU/L | |
| γ-GTP | 57 IU/L ↑ | |
| T-Bill | 0.6 mg/dL | |
| BUN | 10 mg/dL | |
| Cr | 0.64 mg/dL | |
| Uric acid | 6.9 mg/dL | |
| CRP | 0.9 mg/dL ↑ | |
| Immuno-serological findings | Antinuclear antibody | Negative |
| IgG | 1056 mg/dL | |
| IgA | 333 mg/dL | |
| IgM | 107 mg/dL | |
| Anti-HIV antibody | Negative | |
| Anti-HTLV-1 antibody | Negative | |
| Soluble IL-2 receptor | 395 U/mL | |
| Adrenal function | ACTH (RI) | 23.8 pg/mL |
| Adrenalin | 14 pg/mL | |
| Noradrenalin | 218 pg/mL | |
| Dopamine | ≤ 5 pg/mL | |
| B aldosterone | 41.9 pg/mL | |
| Cortisol | 15.3 μg/mL | |
| Renin activity | 0.7 ng/mL/hr | |
| DHEA-S | 96 μg/dL |
indicates a found value elevated above the reference range;
indicates a found value lowered below the reference range.
Besides anemia, no abnormalities in the coagulation test, urinalysis, immuno-serologic tests, or adrenal function tests were observed. WBC, white blood cell; Neut, neutrocyte; Ly, lymphocyte; Mono, monocyte; Eo, eosinophil; Ba, basophil; RBC, red blood cell; Hb, hemoglobin; Ht, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; Plt, platelet; Reti, reticulocyte; PT, prothrombin activity; APTT, activated partial thromboplastin time; T.P, total protein; Alb, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GTP, γ-guanosine triphosphate; T-Bil, total bilirubin; BUN, blood urea nitrogen; Cr, creatinine; CRP, C-reactive protein; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; HTVL-1, human T-cell leukemia virus-1; ACTH, adrenocorticotropic hormone; RI, radioisotope; DHEA-S, sulfate salt of dehydroepiandrosterone.
The patient’s clinical course is shown in Figure 4. The patient received an initial cycle of ritsuximab therapy along with concurrent CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy (R-CHOP) and was discharged from hospital in early October. After the completion of 4 cycles of R-CHOP and 2 parallel cycles of prophylactic intrathecal (I.T.) chemotherapy, a complete remission (CR) was noted to have been attained based on the results of U-GIS (Figure 1C, 1D) and PET-CT (Figure 3C, 3D) examinations. In total, the patient had received 8 cycles of ritsuximab therapy, 6 cycles of CHOP therapy, and 3 cycles of prophylactic I.T. chemotherapy as of the completion of his treatment in February 2014. The patient has maintained a state of complete remission as of October 2014.
Figure 4.
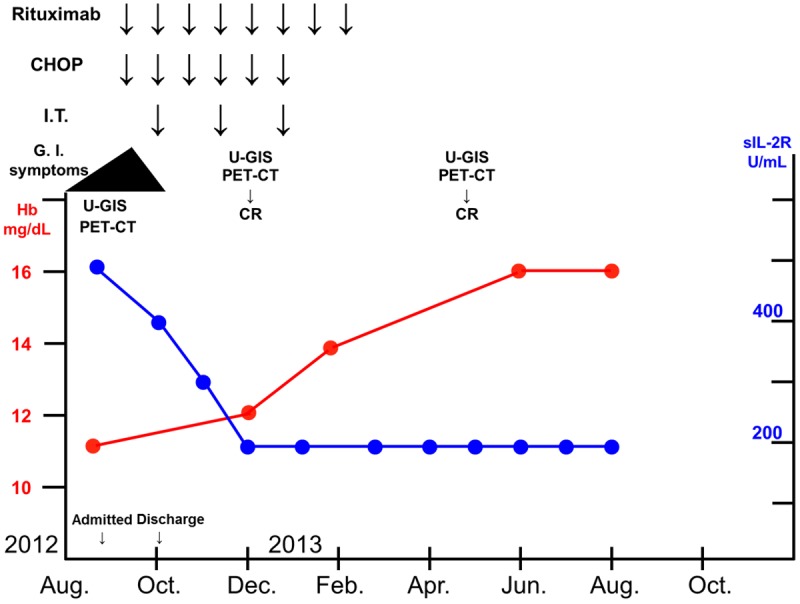
Clinical course. The patient received 8 cycles of ritsuximab therapy, 6 cycles of CHOP therapy, and 3 cycles of prophylactic I.T. chemotherapy. He has maintained a state of CR for about 14 months. Hb, hemoglobin; sIL-2R, soluble interleukin-2 receptor.
Discussion
Whether the stomach or either of the adrenal glands was the site of the primary lesion could not be determined in the present case because the extent of the FDG accumulation at these sites on the FDG-PET images was essentially comparable. In other words, whether a primary adrenal lymphoma had infiltrated the gastric region or a primary gastric lymphoma had infiltrated the adrenal glands could not be determined. Although a biopsy of the adrenal tumors was not performed, the extent of the FDG accumulation in the adrenal glands as visualized using FDG-PET was practically the same as that in the stomach (SUVmax = 14.3), and the uptake accumulation disappeared after treatment; therefore, the case was diagnosed as DLBCL.
Tumors of the adrenal gland may be either primary or secondary neoplasms. Primary adrenal tumors mainly consist of adenomas, adrenal carcinomas, adrenal cysts, pheochromocytomas, ganglioneuromas, and myelolipomas [1], although less than 200 cases of primary adrenal lymphoma have actually been reported according to our extensive literature search [2]. The term “primary” as used in these papers refers to primary adrenal lymphomas; nevertheless, this term was rather vaguely defined in many cases, and several of these cases could have actually been secondary adrenal lymphomas. Stricter definitions of the terms “primary” and “secondary” are needed when reporting such cases in the future.
A report reviewing a total of 464 cases of secondary adrenal tumors experienced during a 30-year period described that 90% of these cases were carcinomas, 56% were adenocarcinomas, and 49% were bilateral tumors; no cases of lymphoma were included in the report. Furthermore, the site of the primary lesion was the lung in 35% of the cases, the stomach in 14%, the esophagus in 12%, and the hepatobiliary system in 10% [3].
However, lymphomatous involvement of the adrenal gland has been discovered based on CT findings in 5% of lymphoma cases [4] and by a morbid anatomy in 25%-35% [5,6]. Secondary adrenal lymphoma is considered to be not uncommon. Inasmuch as most secondary adrenal lymphomas have been documented to arise in the retroperitoneal lymph node or the ipsilateral kidney [7], lymphoma with involvement of both adrenal glands and the stomach alone, as seen in the present case, is thought to be extremely rare. No more than 4 cases have been reported, including the present case, in which the adrenal gland and the digestive tract were involved (Table 2). All of these cases were diagnosed as DLBCL and were reported in Japan, with the lesions confined to the gastrointestinal tract and the adrenal gland in 3 cases and with multiple organ involvement in the remaining case. The single case of multiple organ involvement had a fatal outcome [9], and it seems likely that the prognosis for this condition may be favorable insofar as the malignant involvement is confined to the gastrointestinal tract and adrenal glands.
Table 2.
Reported cases of lymphoma with involvement confined to the gastrointestinal tract and adrenal glands
| Case | Age & Sex | Symptoms | Histological features | Adrenal lesions | G.I. lesions | Other lesions | Treatment | Therapeutic response | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53/F | Upper abdominal pain, weight loss | DLBCL | Left | Elevation of posterior wall adjacent to antral pyloric ring | None | 4 cycles of CHOP therapy, gastrectomy, left adrenal-ectomy | CR | Alive | [8] |
| 2 | 93/F | Decreased appetite, disturbance of consciousness | DLBCL | Details unknown | Intragastric multiple masses | Spleen, jejunum, liver, pancreas, lumbar vertebrae | Parenteral fluid infusion, diuretics, elcitonin | PD | Died | [9] |
| 3 | 73/M | General malaise, high fever, skin pigmentation | DLBCL | Bilateral | Hemorrhage in duodenal mucosa | None | Hydrocortisone, 1 cycle of R-THPCOP therapy | PR | Alive | [10] |
| 4 | 60/M | Decreased appetite, vomiting, feeling of abdominal fullness | DLBCL | Bilateral | Ulcer on lesser curvature at angulus | None | 8 cycles of R, 6 cycles of CHOP therapy, 3 cycles of I.T. therapy | CR | Alive | Present case |
F, female; M, man; DLBCL, diffuse large B cell lymphoma; R-THPCOP, rituximab, pirarubicin, cyclophosphamide, vincristine, and prednisone; PD, progression disease; PR, partial remission.
While reports documenting a poor outcome of patients with primary adrenal lymphoma have been relatively common [11-13], the prognosis and clinical features of secondary adrenal lymphoma remain unclear. Consequently, further exploration and assessments of accumulated cases are needed. In the case described herein, a primary adrenal lymphoma might have invaded the stomach, potentially with a grave prognosis, urging a cautious follow-up.
Disclosure of conflict of interest
None.
References
- 1.Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309–40. doi: 10.1210/er.2002-0031. [DOI] [PubMed] [Google Scholar]
- 2.Rashidi A, Fisher SI. Primary adrenal lymphoma: a systematic review. Ann Hematol. 2013;92:1583–93. doi: 10.1007/s00277-013-1812-3. [DOI] [PubMed] [Google Scholar]
- 3.Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 2002;56:95–101. doi: 10.1046/j.0300-0664.2001.01435.x. [DOI] [PubMed] [Google Scholar]
- 4.Paling MR, Williamson BR. Adrenal involvement in non-Hodgkin lymphoma. AJR Am J Roentgenol. 1983;141:303–5. doi: 10.2214/ajr.141.2.303. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Diamond HD, Jaslowitz B, Craver LF. Lymphosarcoma: a review of 1269 cases. Medicine (Baltimore) 1961;40:31–84. doi: 10.1097/00005792-196102000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Straus DJ, Filippa DA, Lieberman PH, Koziner B, Thaler HT, Clarkson BD. The non-Hodgkin’s lymphomas. I. A retrospective clinical and pathologic analysis of 499 cases diagnosed between 1958 and 1969. Cancer. 1983;51:101–9. doi: 10.1002/1097-0142(19830101)51:1<101::aid-cncr2820510121>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Glazer HS, Lee JK, Balfe DM, Mauro MA, Griffith R, Sagel SS. Non-Hodgkin lymphoma: computed tomographic demonstration of unusual extranodal involvement. Radiology. 1983;149:211–7. doi: 10.1148/radiology.149.1.6225145. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda T, Okihama Y, Egami K, Wada M, Yoshioka M, Maeda S, Onda M. Complete cure of malignant lymphoma of the stomach with a huge adrenal lesion achieved by preoperative chemotherapy and surgery: report of a case. Surg Today. 2001;31:62–7. doi: 10.1007/s005950170223. [DOI] [PubMed] [Google Scholar]
- 9.Ota H, Azuma K, Horiuchi T, Kazama H, Araki A, Hosoi T, Sawabe M, Amizuka N, Orimo H. [An elderly case of non-Hodgkin’s lymphoma (NHL) with hypercalcemia] . Nippon Ronen Igakkai Zasshi. 2003;40:167–71. doi: 10.3143/geriatrics.40.167. [DOI] [PubMed] [Google Scholar]
- 10.Nishiuchi T, Imachi H, Fujiwara M, Murao K, Onishi H, Kiguchi T, Takimoto H, Kushida Y, Haba R, Ishida T. A case of non-Hodgkin’s lymphoma primary arising in both adrenal glands associated with adrenal failure. Endocrine. 2009;35:34–7. doi: 10.1007/s12020-008-9125-3. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H, Tomita N, Yokoyama M, Tsunoda S, Yano T, Murayama K, Hashimoto C, Tamura K, Sato K, Ishigatsubo Y. Prognostic impact of extranodal involvement in diffuse large B-cell lymphoma in the rituximab era. Cancer. 2012;118:4166–72. doi: 10.1002/cncr.27381. [DOI] [PubMed] [Google Scholar]
- 12.Ezer A, Parlakgümüş A, Kocer NE, Colakoğlu T, Nursal GN, Yildirim S. Primary adrenal non-Hodgkin’s lymphoma: report of two cases. Turk J Gastroenterol. 2011;22:643–7. doi: 10.4318/tjg.2011.0279. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Li Q, Pan Y. Bilateral primary adrenal lymphoma. Br J Haematol. 2010;150:250. doi: 10.1111/j.1365-2141.2010.08205.x. [DOI] [PubMed] [Google Scholar]


