Abstract
The aim of the current research work was to study the chemical composition of the essential oil of Monarda punctata along with evaluating the essential oil and its major components for their antibacterial effects against some frequently encountered respiratory infection causing pathogens. Gas chromatographic mass spectrometric analysis revealed the presence of 13 chemical constituents with thymol (75.2%), p-cymene (6.7%), limonene (5.4), and carvacrol (3.5%) as the major constituents. The oil composition was dominated by the oxygenated monoterpenes. Antibacterial activity of the essential oil and its major constituents (thymol, p-cymene, limonene) was evaluated against Streptococcus pyogenes, methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus pneumoniae, Haemophilus influenzae and Escherichia coli. The study revealed that the essential oil and its constituents exhibited a broad spectrum and variable degree of antibacterial activity against different strains. Among the tested strains, Streptococcus pyogenes, Escherichia coli and Streptococcus pneumoniae were the most susceptible bacterial strain showing lowest MIC and MBC values. Methicillin-resistant Staphylococcus aureus was the most resistant bacterial strain to the essential oil treatment showing relatively higher MIC and MBC values. Scanning electron microscopy revealed that the essential oil induced potent and dose-dependent membrane damage in S. pyogenes and MRSA bacterial strains. The reactive oxygen species generated by the Monarda punctata essential oil were identified using 2’, 7’-dichlorofluorescein diacetate (DCFDA).This study indicated that the Monarda punctata essential oil to a great extent and thymol to a lower extent triggered a substantial increase in the ROS levels in S. pyogenes bacterial cultures which ultimately cause membrane damage as revealed by SEM results.
Keywords: Monarda punctata, essential oil, thymol, p-cymene, Streptococcus pyogenes, methicillin-resistant Staphylococcus aureus
Introduction
Upper respiratory tract infections (URTIs) are the ailments triggered by an acute infection involving nose, sinuses, pharynx, or larynx. The various URTIs include common cold, sinusitis, laryngitis, pharyngitis etc [1]. These infections are frequently caused by viruses like rhinovirus, corona virus, parainfluenza virus, adenovirus etc [2]. Many of the URTIs are also caused by bacteria like Streptococcus pyogenes, Streptococcus pneumoneae, Heamophilus influenzae, Corynebacterium diptheriae, Bordetela pertursis and Bacillus anthracis. Bronchitis, which is an inflammation of the mucous membranes of the bronchi, is also caused by certain types of bacteria. About 10% of the cases are caused by bacteria like Mycoplasma pneumoniae, Chlamydophila pneumoniae, Bordetella perturis, Streptococcus pneumoniae etc [3,4].
Essential oils (which are composed of an odoriferous mixture of monoterpenes and Sesquiterpenes) have a long history of use in combating infection and many are marketed for their antiseptic properties. Essential oils are nature’s antiseptics and their ability to kill microorganisms has been well documented over the centuries. Hippocrates, the founder of medicine, used aromatic plants as early as 500BC. For thousands of years, aromatic oils have been used to relieve a wide variety of human maladies including bronchitis, pneumonia, pharyngitis, diarrhoea, periodontal disease, wounds, and numerous other illnesses. Many traditions surrounding the use of these oils are buried in antiquity, passed down orally from master to student until the origin of specific treatments were lost to the ages. In antiquity, medicinal oils were derived from aromatic plants and resins by extraction into other fatty oils such as olive oil and used as a mixture [5-7]. Many of the scientists consider essential oils as alternative to antibiotics. There has been an increase in the wide spread development of drug resistant microorganisms such MRSA, multidrug resistant strain of Klebsiella pneumonia and P. Aeruginosa. The essential oils target bacterial cell wall and cytoplasmic membrane and cause membrane permeabilization, which is followed by leakage of essential ions, drop in membrane potential, breakdown of the proton pump and depletion of the ATP pool [8,9]. Tea tree oil, as an example, disrupts the cell membrane of the bacterium, causing a loss of potassium ions from the cell. The lipophilic (lipid loving) monoterpenes integrate with the phospholipids in the membrane and leakage occurs. Antimicrobial essential oils have also been known to cause inhibition of glucose dependant respiration in S. aureus, E. coli and C. albicans. Some clinical research in 2003 set out to compare tea tree oil with the standard topical antibiotic mupirocin. The purpose of the trial was to compare two methods of eliminating MRSA - from the nose.
Due to their multifunctionality and diverse chemical composition, essential oils and their components find a huge application in medicine and aroma therapy. Essential oils exhibit potent antimicrobial actions against a wide range of Gram-positive and Gram-negative bacteria [10]. Essential oils and their components have also been used traditionally for treating respiratory tract infections and are used these days as ethical medicines for colds [11-13]. Inhalation therapy using essential oils has been used to treat acute sinusitis and acute and chronic bronchitis. It has been reported that inhalation therapy using volatile essential oil vapours enhanced the respiratory tract fluid output [14], maintained the ventilation and drainage of the sinuses, reduced asthma and the inflammation of the trachea [15-17].
The objective of the present study was to study the chemical composition of the essential oil of Monarda punctata along with evaluating the essential oil and its major components for their antibacterial effects against some frequently encountered respiratory infection causing pathogens. Scanning Electron Microscopy (SEM) revealed that the essential oil of Monarda punctata induced substantial and dose-dependent morphological changes in the Streptococcus pyogenes bacterial strain killing more than 85% of the bacteria at higher concentration.
Materials and methods
Materials
The major essential oil components viz; thymol, p-cymene, limonene, and carvacrol were purchased from Sigma-Aldrich Chemical Company.
Plant material and essential oil isolation
The flowers of Monarda punctata were collected from a local region, China, during June-July 2013. The plant was identified by a well-known taxonomist. The extraction of the essential oil from the dried flowers was carried out by hydrodistillation for 4 hours using Clevenger apparatus as recommended in the European pharmacopoeia. The dried flower samples (500 gm) were subjected to hydrodistillation at separate times. The essential oil was collected and then dehydrated with anhydrous Na2SO4 (Sigma-Aldrich) and stored at -4°C before use.
GC-MS analysis and identification of components
GC-MS analysis was carried on a Varian Gas Chromatograph series 3800 fitted with a RTX-5 ms fused silica capillary column (30 m × 0.25 mm, film thickness 0.25 μm) using split/splitless injection, coupled with a 4000 series mass detector under the following conditions: injection volume 1.2 μl with split ratio 1:60, helium as carrier gas at 1.2 ml/min constant flow mode, injector temperature 240°C, oven temperature was programmed from 50 to 280°C at 5°C/min. Mass spectra: electron impact (EI+) mode, 70 ev and ion source temperature 250°C. Mass spectra were recorded over 50-500 a.m.u range. Identification of the essential oil constituents was done on the basis of Retention Index [RI, determined with respect to homologous series of n-alkanes (C5-C28, Polyscience Corp., Niles IL) under the same experimental conditions], co-injection with standards (Sigma Aldrich and standard isolates), MS Library search (NIST 05 and Wiley), by comparing with the MS literature data [18].
Microorganism strains and culture media
Streptococcus pyogenes (ATCC 12344), Methicillin-resistant Staphylococcus aureus (MRSA) (ATCC 43300), Streptococcus pneumoniae (ATCC 2730), Haemophilus influenzae (ATCC 33391), Escherichia coli (Clinical strain) were used in the present study. All these bacteria strains were obtained from the State Key Laboratory of Microbial Resources (SKLMR), the institute of microbiology, Chinese academy of Sciences, China. Bacterial strains were grown on nutrient agar plates at 37°C and maintained on nutrient agar slants. Cell suspension of microorganisms in 0.5% NaCl was adjusted at 0.5 Mcfarland to obtain approximately 105 cfu/ml.
Determination of MIC and MBC values
The MIC (minimal inhibitory concentration) and MBC (minimal bactericidal concentration) tests were carried out by the broth microdilution method [19]. The essential oil and the major components were dissolved in sterilized physiological saline solution (0.9%) supplemented with Tween-80 (Sigma) at final concentration of 0.5% (v/v). Serial doubling dilutions of the essential oils were prepared in a 96-well microtiter plate ranging from 10% to 0.125%. Each dilution (100 μL) was dispensed into the wells, then inoculated with (100 μL) of the bacterial suspension. The final concentration of each strain was adjusted to 105-106 CFU/mL. The MIC is defined as the lowest concentration of the essential oils, at which the microorganism does not show visible growth. The MBC is the lowest concentration at which the microorganism is killed. All experiments were performed in triplicate.
Time-kill dynamic curves
Time-kill dynamic were performed as described already [20] with slight alterations. The final concentration of suspension of the strain was adjusted to 105-106 CFU/mL. MICs, 2 MICs and MBCs of Monarda punctata essential oil and its main components were chosen for use in the time-kill dynamic protocols. After incubating for 0, 1, 2, 4, 8, 12, 24 and 30 h with the broth microdilution method, liquids (50 μL) were removed from the test solution for ten-fold serial dilution. Thereafter, a 50 μL liquid from each dilution was spread on the surface of the agar plates and incubated at 37°C for 24 h, and the number of CFU/mL was counted. The solution with no essential oil was used as a control. Time-kill curves were created by plotting the log number of CFU/Ml against time (h).
Scanning electron microscopy (SEM) study of the bacteria after oil exposure
The SEM studies of the bacterial cultures were carried out as under. The overnight culture of Streptococcus pyogenes grown on MH agar at 37°C was put in a saline solution comprising of 0.2% Tween-80. Three different concentrations of the essential oil (30, 60, 60 and 90 μg/ml) were prepared and added into this suspension and was incubated at room temperature. After 24 hours, the bacterial cells were centrifuged at 8000 × g for 15 minutes. The bacterial cells were then washed with 0.1 mol/l Tris-acetate buffer (PH 7.1), fixed in tris-acetate buffer containing 1.5% glutaraldehyde, and then freeze-dried. Each of the bacterial culture was observed by SEM (Hitachi, Japan) at magnifications of 10,000 ×. The bacterial cell suspension in saline with no essential oil treatment served as a negative positive control.
Detection of reactive oxygen species (ROS) generated by essential oil
The reactive oxygen species generated by the Monarda punctata essential oil was identified using 2’, 7’-dichlorofluorescein diacetate (DCFDA) [21]. The concentration of essential oil, thymol, limonene and p-cymene used was 90 μg/ml each and the number of bacterial cells used was adjusted to 105 CFU/ml. After all cultures were incubated at 37°C for 4 h, they were centrifuged at 4°C for 20 min at 300 × g, and then each supernatant was treated with 50 μM DCFDA for 1.5 h. The ROS formed in the sample were detected using Fluorescence Multi-Detection Reader (BIOTEK, U.S.A.) at 485 nm of fluorescence excitation wavelength, and 528 nm of emission wavelength.
Results
Chemical composition of the essential oil of Monarda punctata
The chemical compounds of the essential oil of Monarda punctata identified by GC-MS are given in (Table 1) and the GC-MS total ion chromatogram (TIC) is shown in (Figure 1). The yield of the essential oil obtained from the flowers was about 0.9% w/v. A total of 13 components were identified constituting 97.2% of the total oil composition. Thymol, p-cymene, limonene and carvacrol were found to be the major components. The oil composition was dominated by the presence of oxygenated monoterpenes constituting 83.2% of the total oil composition.
Table 1.
Chemical composition of the essential oil from the flowers of Monarda punctata
| S. No | RT | Compound | % Peak area | Methods of identification |
|---|---|---|---|---|
| 1 | α-Thujene | 1.0 | MS, RI | |
| 2 | α-Pinene | 0.2 | MS, RI | |
| 3 | β-Pinene | 0.9 | MS, RI | |
| 4 | α-Phellandrene | 0.1 | MS, RI | |
| 5 | α-Terpinene | 1.5 | MS, RI | |
| 6 | P-Cymene | 6.7 | MS, RI, Std | |
| 7 | Carene | 0.2 | MS, RI | |
| 8 | Limonene | 5.4 | MS, RI, Std | |
| 9 | 4-Terpineol | 0.1 | MS, RI | |
| 10 | Methyl carvacrol | 0.4 | MS, RI | |
| 11 | Thymol | 75.2 | MS, RI, Std | |
| 12 | Carvacrol | 3.5 | MS, RI, Std | |
| 13 | β-Caryophyllene | 0.1 | MS, RI | |
| Total (%) | 95.3 | |||
| Monoterpene hydrocarbons | 16.0 | |||
| Oxygenated monoterpenes | 79.2 | |||
| Sesquiterpene hydrocarbons | 0.1 | |||
| Oxygenated sesquiterpenes | nil | |||
Figure 1.
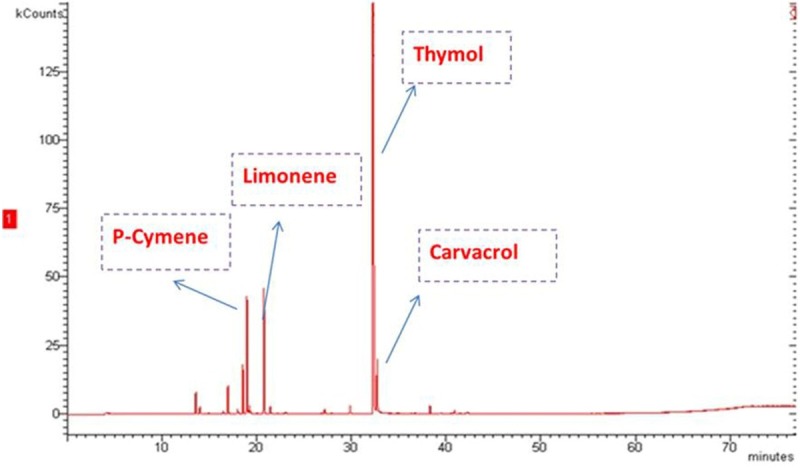
GC-MS Total Ion Chromatogram (TIC) of the essential oil of Monarda punctata.
Antibacterial activity
The antibacterial activity of the Monarda punctata essential oil and its major constituents (Thymol, p-cymene, limonene and carvacrol) was assessed against some respiratory infection causing bacterial strains including Streptococcus pyogenes, methicillin-resistant Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae and Escherichia coli. The effectiveness of the Monarda punctata essential oil and the chemical constituents was assessed by determining inhibition zones, MIC and MBC values. The results are given in (Figure 2), (MIC and MBC values are given). Streptococcus pyogenes, Escherichia coli and Streptococcus pneumonia were the most susceptible bacterial strain showing lowest MIC and MBC values. Methicillin-resistant Staphylococcus aureus was the most resistant bacterial strain to the essential oil treatment showing relatively higher MIC and MBC values. With the aim to identify which of the chemical compounds of the Monarda punctata essential oil were active against these respiratory bacteria, further in-vitro experiment was carried out evaluating the antibacterial effects of the major constituents (thymol, p-cymene and limonene). Almost all the tested essential oil constituents exhibited reasonable to potent antibacterial effect against various bacterial strains. The major compounds of the essential oil viz., thymol, p-cymene and limonene exhibited higher values of MIC and MBC indicating that the whole essential oil is much more potent antibacterial agent as compared to the individual constituents. Figure 3 shows the MIC and MBC values of thymol, p-cymene and limonene which constitute the bulk of the essential oil of Monarda punctata.
Figure 2.
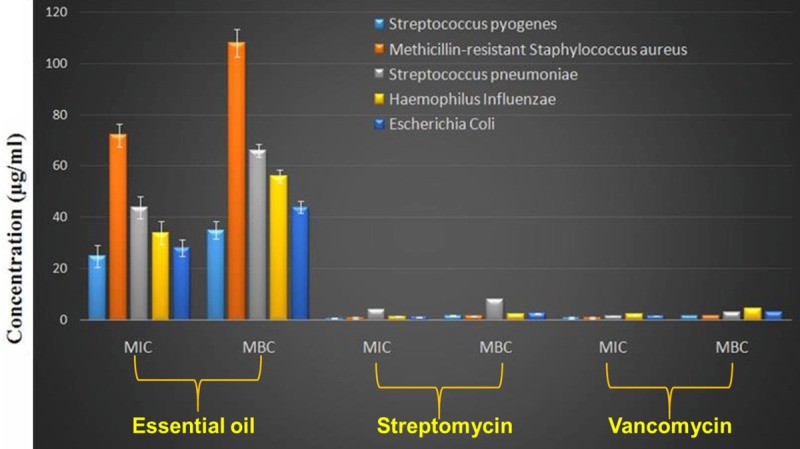
MIC and MBC values of the essential oil and two known antibiotics against different respiratory pathogens.
Figure 3.
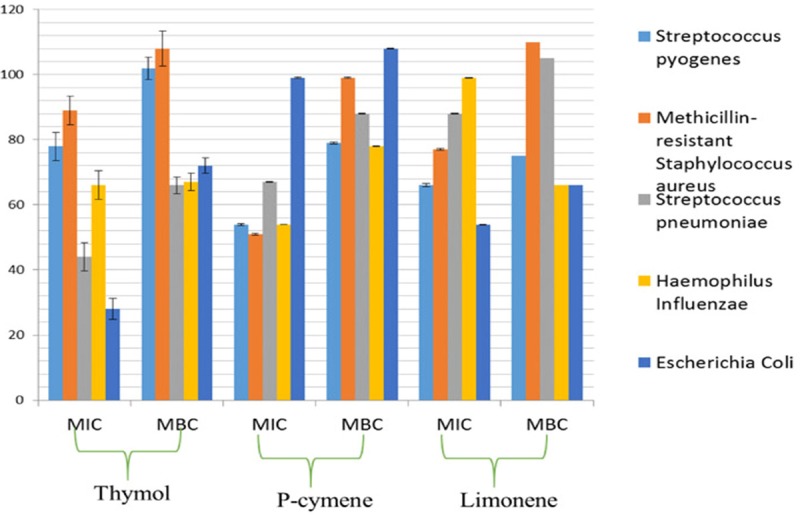
MIC and MBC values of thymol, p-cymene and limonene against different respiratory pathogens.
Time kill curve studies
The time-kill curve of Monarda punctata essential oil, towards Streptococcus pyogenes is shown in (Figure 4). MIC had a bacteriostatic effect on the bacterial population of Streptococcus pyogenes within the first 6 h, while MBC had a deadly effect on bacteria within 12 h by Monarda punctata essential oil. Time kill curve studies give an idea whether the essential oil has bacteriostatic or bactericidal effects with regard to the time span the microbes are exposed to the treatment of the drug. By this study, it can be concluded that within 12 h duration, the essential oil of Monarda punctata kills almost all the Streptococcus pyogenes bacterial strains.
Figure 4.
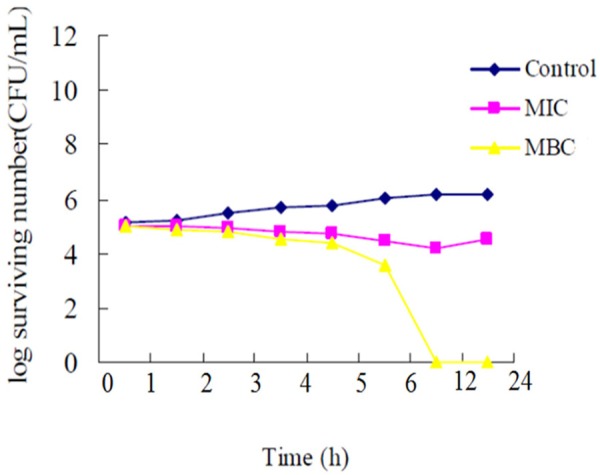
Time-kill curves of Monarda punctata essential oil against Streptococcus pyogenes. The control did not contain essential oil.
Scanning electron microscopy (SEM) studies of Monarda punctata essential oil
In order to determine whether the essential oil of Monarda punctata essential oil induced serious morphological/structural changes in S. pyogenes, scanning electron microscopy was used which revealed that the essential oil induced a remarkable serious morphological change in the bacterial architecture of S. pyogenes and Methicillin-resistant Staphylococcus aureus (MRSA). Figures 5 and 6 show the effect of the essential oil of Monarda punctata on the morphology of S. pyogenes and MRSA respectively. The bacterial cells of S. aureus were swollen after 12 hours of essential oil treatment. Three concentrations (30, 60, and 90 μg/ml) of the essential oil were used. SEM results showed that the essential oil induced dose-dependent cell morphological changes in the S. pyogenes and MRSA bacterial cells. It was observed that at a concentration of 90 μg/ml of the essential oil treatment to S. pyogenes and MRSA bacterial cells for 12 hours killed more than 95% of the bacterial cells indicating that the Monarda punctata essential oil produces bactericidal effects on these microbes. Alternatively, the untreated control cells did not show any changes in the cell morphology (Figures 5 and 6).
Figure 5.
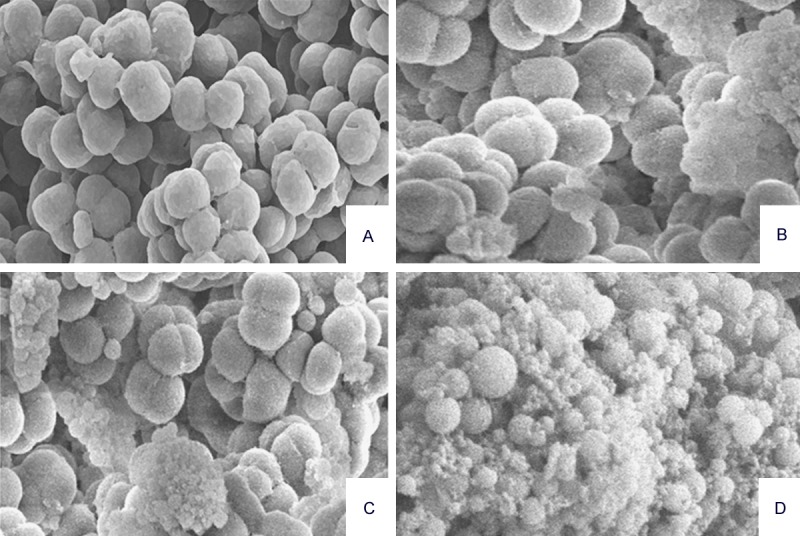
Electron micrographs of S. pyogenes exposed to different concentrations of the Monarda punctata essential oil. The images were taken at a magnification of 10,000 × (A, S. pyogenes 12 h in saline; B-D. scanning electron micrographs of S. Pyogenes treated for 12 h with 30, 60, and 90 μg/ml of the Monarda punctata essential oil.
Figure 6.
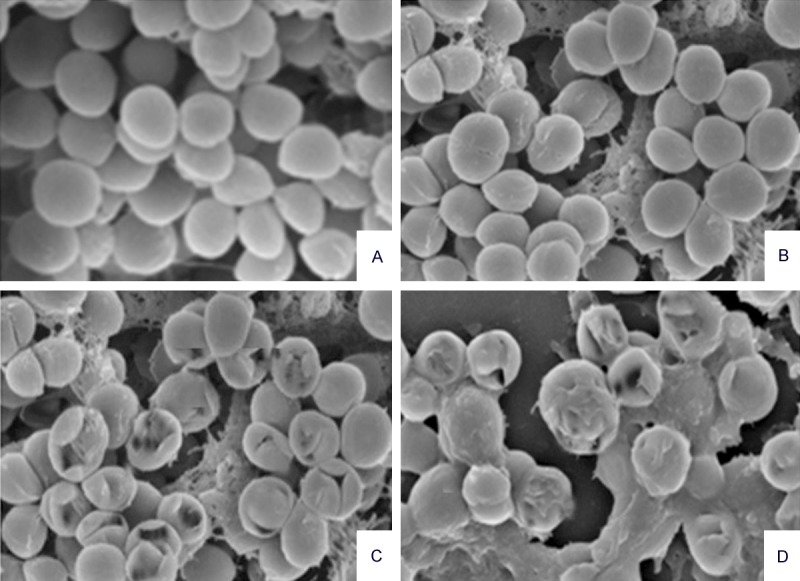
Electron micrographs of MRSA exposed to different concentrations of the Monarda punctata essential oil. The images were taken at a magnification of 10,000 × (A. MRSA12 h in saline; B-D. scanning electron micrographs of MRSA treated for 12 h with 30, 60 and 90 μg/ml of the Monarda punctata essential oil.
Generation of ROS from bacterial cells treated with Monarda punctata essential oil and three major constituents (thymol, p-cymene and limonene)
In several studies, it has been reported that various essential oils could trigger the formation of free radicals within bacterial cells, which eventually cause serious membrane damage. Increased levels of ROS could induce oxidative stress in bacterial cells which actually cause damage to cell membrane, vital proteins, DNA and bacterial respiratory system. In the current study, ROS was measured in S. pyogenes cultures using DCFDA. After 4 h incubation, elevated levels of ROS were detected and measured in the Monarda punctata essential oil treated bacterial group. Thymol treated group also showed moderate increase in the levels of ROS followed by p-cymene and limonene-treated groups (Figure 7).
Figure 7.
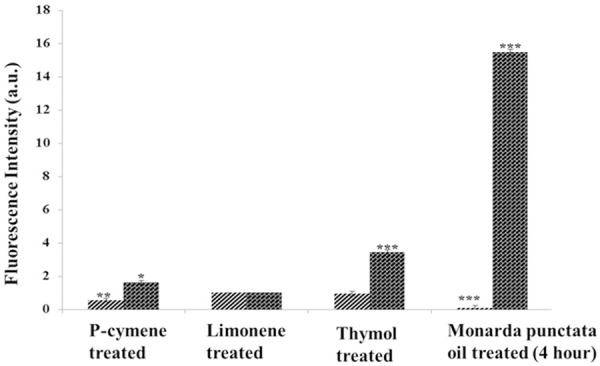
Formation of ROS in S. Pyogenes cells exposed to 90 μg/ml each of p-cymene, limonene, thymol and essential oil. Bacterial cultures were treated with 90 μg/ml each of these compounds and whole M.P essential oil for 4 h. ***Statistical significance at P < 0.01. **Statistical significance at P < 0.05.
Discussion
Since early times, essential oils have been used for the management of infections caused by different bacteria all over the world for centuries in the traditional medicine system. Today, essential oils have a wide range of applications and are used in alternative and holistic medicine for similar purposes. They are administered orally, topically or via aromatherapy procedures. An increasing number of scientific studies have initiated the process of explaining the specific mechanism (s) of action of essential oils and their components. Emerging evidence has shown that many essential oils have both non-specific and specific mechanisms of action which differs based on the comparative abundance and chemical composition of the components. Explanation of the mode of action of these essential oils/compounds may facilitate identification of new antibiotic targets and exploitation of novel biochemical pathways; pathways not currently targeted by existing antibiotics. Since antibiotic resistance is currently overtaking scientific research to find new drugs, medical scientists are confronting a return to the ‘pre-antibiotic era’. Essential oils or some of their components are used in perfumes and make-up products, in sanitary products, in dentistry, in agriculture, as food preservatives and additives, and as natural remedies [8]. In recent years, due to the new interest in natural products, plant essential oils have come more into the focus of phytomedicine [22,23]. It is important to develop a better understanding in basic research applications, especially, of the anti-microbial activity. The essential oils exhibit a broad spectrum of biological activity which has led to an increased interest among scientists [24].
In the current study, we evaluated the antibacterial potential of Monarda punctata essential oil as well as its three major constituents (thymol, p-cymene and limonene) against a panel of respiratory tract infection causing bacterial bugs including Streptococcus pyogenes, methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus pneumoniae, Haemophilus influenzae and Escherichia coli. The mode of action of the essential oil and its components was evaluated using scanning electron microscopy and ROS generation protocol in the bacterial cultures. Time kill curve studies were done to elucidate whether the essential oil has bacteriostatic or bactericidal effects. The results showed that the essential oil is very active against all tested microbes especially against S. pyogenes. S. pyogenes was further studied whether the essential oil induced morphological damage in it, which was finally confirmed by SEM results. The essential oil to a large extent and thymol to a lower extent triggered ROS generation in these bacterial cultures.
In conclusion, this study concludes that the essential oil of Monarda punctata is a potent source of antibacterial agents against various respiratory tract infection causing microbes. The antibacterial effects of the essential oil are facilitated through inducing significant morphological changes in bacterial cells along with generating reactive oxygen species within the bacterial cultures. The essential oil even caused serious morphological damage to the drug resistant Staphylococcus aureus which is very encouraging. These essential oils could be used as a safe and natural alternative to antibiotics with less incidence of showing multidrug resistance.
Disclosure of conflict of interest
None.
References
- 1.Eccles MP, Grimshaw JM, Johnston M, Steen N, Pitts NB, Thomas R, Glidewell E, Maclennan G, Bonetti D, Walker A. Applying psychological theories to evidence-based clinical practice: Identifying factors predictive of managing upper respiratory tract infections without antibiotics. Implement Sci. 2007;2:26. doi: 10.1186/1748-5908-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mäkelä MJ, Puhakka T, Ruuskanen O, Leinonen M, Saikku P, Kimpimäki M, Blomqvist S, Hyypiä T, Arstila P. Viruses and Bacteria in the Etiology of the Common Cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert RH. Diagnosis and treatment of acute bronchitis. Am Fam Physician. 2010;82:1345–1350. [PubMed] [Google Scholar]
- 4.Cohen J, Powderly W. Chapter 33: Bronchitis, Bronchiectasis, and Cystic Fibrosis. 2nd edition. Mosby (Elsevier); 2004. Infectious Diseases. ISBN 0323025730. [Google Scholar]
- 5.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Vollesen K, Hedberg I, Edwards S. Flora of Ethiopia. Addis Ababa, Ethiopia. National Herbarium. 1989;3:442–478. [Google Scholar]
- 7.Tucker AO. Frankincense and Myrrh. Econ Bot. 1986;40:425–433. [Google Scholar]
- 8.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils: a review. Food Chem Toxicol. 2008;46:446–75. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 9.Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 2007;21:308–23. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 10.Deans SG, Ritchie G. Antibacterial properties of plant essential oils. Internatl J Food Microbiol. 1987;5:165–180. [Google Scholar]
- 11.Rantzsch U, Vacca G, Dück R, Gillissen A. Anti-inflammatory effects of Myrtol standardized and other essential oils on alveolar macrophages from patients with chronic obstructive pulmonary disease. Eur J Med Res. 2009;7:205–9. doi: 10.1186/2047-783X-14-S4-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Federspil P, Wulkow R, Zimmermann T. Effects of standardized Myrtol in therapy of acute sinusitis-results of a double-blind, randomized multicenter study compared with placebo. Laryngorhinootologie. 1997;76:23–27. doi: 10.1055/s-2007-997381. [DOI] [PubMed] [Google Scholar]
- 13.Schindl R. Inhalation effect of volatile oils. Wien Med Wochenschr. 1972;122:591–593. [PubMed] [Google Scholar]
- 14.Boyd EM, Sheppard P. Nutmeg oil and camphene as inhaled expectorants. Arch Otolaryngol. 1970;92:372–37. doi: 10.1001/archotol.1970.04310040060011. [DOI] [PubMed] [Google Scholar]
- 15.Burrow A, Eccles R, Jones AS. The effects of camphor, eucalyptus and menthol vapours on nasal resistance to airflow and nasal sensation. Acta Otolaryngol. 1983;96:157–161. doi: 10.3109/00016488309132886. [DOI] [PubMed] [Google Scholar]
- 16.Shubina LP, Siurin SA, Savchenko VM. Inhalation of essential oils in the combined treatment of patients with chronic bronchitis. Vrach Delo. 1990;5:66–67. [PubMed] [Google Scholar]
- 17.Frohlich E. Lavender oil, review of clinical, pharmacological and bacteriological studies. Contribution to clarification of the mechanism of action. Wien Med Wochenschr. 1968;118:345–350. [PubMed] [Google Scholar]
- 18.Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream, Illinois, USA: Allured Publishing Corporation; 2007. [Google Scholar]
- 19.Yu J, Lei J, Yu H, Cai X, Zou G. Chemical composition and antimicrobial activity of the essential oil of Scutellaria barbata . Phytochemistry. 2004;65:881–884. doi: 10.1016/j.phytochem.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Avila JG, de Liverant JG, Martínez A, Martínez G, Muñoz JL, Arciniegas A, Romo de Vivar A. Mode of action of Buddleja cordata verbasco side against Staphylococcus aureus. J Ethnopharmacol. 1999;66:75–78. doi: 10.1016/s0378-8741(98)00203-7. [DOI] [PubMed] [Google Scholar]
- 21.Kye IS, Jeon YS, No JK, Kim YJ, Lee KH, Shin KH, Kim J, Yokozawa T, Chung HY. Reactive oxygen scavenging activity of green tea polyphenols. J Korea Gerontol. 1999;9:10–17. [Google Scholar]
- 22.Zu Y, Yu H, Liang L, Fu Y, Efferth T, Liu X, Wu N. Activities of Ten Essential Oils towards Propioni bacterium acnes and PC-3, A-549 and MCF-7 Cancer Cells. Molecules. 2010;15:3200–3210. doi: 10.3390/molecules15053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sylvestre M, Pichette A, Longtin A, Nagau F, Legualt J. Essential oil analysis and anticancer activity of leaf essential oil of croton flavens L. from Guadeloupe. J Ethnopharmacol. 2006;103:99–102. doi: 10.1016/j.jep.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Adorjan B, Buchbauer G. Biological properties of essential oils: An updated review. Flavour Fragr J. 2010;25:407–426. [Google Scholar]


