Abstract
Background: Signal transducer of activator of transcription 3 (STAT3) and cyclinD1 are overexpressed in various human cancers, and their overexpression positively correlates to tumor progression and poor prognosis. However, the clinical significance of dual high expression of these two proteins in esophageal squamous cell carcinoma (ESCC) has yet to be determined. Methods: The expression of STAT3 and cyclinD1 was analyzed in tissue microarrays containing tumor and adjacent tissue samples from 82 patients who had undergone curative resection for histologically proven ESCC. Kaplan-Meier plots and Cox proportional hazards regression model were used to analyze the prognostic value of STAT3 and cyclinD1 expression. Results: We discovered that expressions of STAT3 and cyclinD1 in cancer tissues were significantly higher than that in adjacent tissues. High expression of STAT3 and cyclinD1 was associated with malignant behaviors. Moreover, the expression of STAT3 was positively associated with the expression of cyclinD1. High STAT3 or cyclinD1 expression alone was associated with lower overall survival (OS) rates. Furthermore, dual high expression of STAT3 and cyclinD1 expression predict even worse survival outcome in both univariate and multivariate analysis. Conclusion: STAT3 and cyclinD1 correlate with more aggressive tumor behavior in ESCC. When STAT3 and cyclinD1 are considered together, they serve as effective prognostic markers in patients with surgically resected ESCC.
Keywords: Signal transducer of activator of transcription 3, cyclinD1, esophageal squamous cell carcinoma, immunohistochemistry, prognosis
Introduction
Recently, in the worldwide, especially China, there has been a dramatic rise in the incidence and mortality due to esophageal adenocarcinoma and squamous cell carcinoma [1,2] and esophageal squamous cell carcinoma (ESCC) occurs at a high frequency in China (13 per 100 000) [3]. As the dominant type of esophageal cancer in China, has a generally poor prognosis due to the lack of effective clinical methods for its early detection [4]. There is a pressing need for reliable biomarkers to identify the subset of patients with a high risk of poor survival outcomes and guide treatment.
STAT (signal transducers and activators of transcription) are important cellular mediators in response to various cytokines and growth factors which act as shuttle proteins of signals in the cytoplasm and as transcription activators in the nucleus [5]. Among various STAT, STAT3 was integrally involved in tumor initiation, progression and maintenance as an important role in maintaining cancer stem cells in vitro and in mouse tumor models [6]. In colorectal carcinoma, STAT3 may play important roles in the tumorigenesis and be indicative of a poor prognosis due to its correlation with distant metastases and a larger tumor size [7]. It has been reported in prostate cancer that STAT3 is a major factor promoting an immunosuppressive environment which allows tumor growth and metastasis [8].
As a cell cycle regulator, cyclinD1 is essential for progression through G1 phase and is a candidate of proto-oncogene [9]. It has been proposed cyclinD1 expression is an independent risk factor in metastasizing bladder cancer [10]. Moreover, the overexpression of cyclinD1 was an independent adverse prognostic indicator for overall survival and relapse-free survival in estrogen-negative breast cancer patients [11].
Gagin D et al provided the first evidence that constitutive activation of STAT3 played a causative role in overexpression of cyclinD1, and STAT3 proteins was implicated in resistance to apoptosis, potentially via their ability to modulate cyclinD1 expression in head and neck squamous cell carcinoma (HNSCC) [12]. Given that STAT3 and cyclinD1 were independent prognostic indicators in some kinds of cancers, and the close relationship between the two proteins, the combined impact of STAT3 and cyclinD1 on the survival time of ESCC patients have yet to be characterized with documented clinical and pathological information.
The purpose of this study was to determine the expressions of STAT3 and cyclinD1 proteins and to analyze their correlations with the clinicopathological parameters, including age, sex, histological grade, stages and tumor size in ESCC patients. Additionally, we assessed the influence of STAT3 and cyclinD1 expression on the overall survival of patients with ESCC.
Materials and methods
Patients and tissue specimens
The study cohort includes paraffin-embedded ESCC samples and adjacent noncancerous tissues from 82 patients who underwent curative surgery between June 2003 and June 2007 in Guangzhou Cancer Hospital, China. All cases were clinically diagnosed and confirmed pathologically as ESCC without distant metastasis at the time of diagnosis.
Written informed consent was obtained from all ESCC patients and this study was approved by the research Ethics Committee of Guangzhou Cancer Hospital. This investigation conformed to the principles outlined in the Declaration of Helsinki. Demographic and clinicopathological data of ESCC patients were collected from medical records. None of the patients received radiotherapy or chemotherapy prior to curative surgery. The following clinicopathological parameters were recorded: age, sex, histological grade, tumor size, depth of invasion, and clinical stage. The histopathological diagnosis was determined according to the World Health Organization criteria [13]: Grade I (there is a high proportion of large, differentiated, keratinocyte-like squamous cells and a low proportion of small basal-type cells); Grade II (between the Grade I and Grade III); Grade III (predominantly consist of basal-type cells, which usually exhibit a high mitotic rate). Tumor staging was based on the 6th edition of the tumor-node-metastasis (TNM) classification of the International Union against Cancer.
Tissue microarray construction
All H&E stained slides from the ESCC patients were reviewed by three histopathologists (Xueyun Zhong, Jiwei Ma, Yong Zhang) and representative areas were located away from necrotic and hemorrhagic materials and were premarked in the corresponding paraffin blocks. A hollow needle was used to punch and remove bipartite cylinders tissue core (1.0 mm in diameter) from selected donor tissue regions. Then, the punched tissue cores were inserted into a recipient paraffin block with a precisely spaced array pattern, using an automatic tissue arraying instrument (Beecher Instruments, Silver Spring, Maryland, USA). Two core biopsies (1 mm in diameter) were taken from each representative tumor tissue and peritumoral tissue to construct the tissue microarray (TMA). In accordance with the provisions of UltrasensitiveTM S-P Kit (Fuzhou Maixin Biotechnology Development Co., Ltd), we disposed the TMA in proper order. The TMA we made was neat, complete and without loss and the histological structure target sites were well-preserved.
Immunohistochemical staining
Consecutive sections measuring 4 μm from TMA were placed on pathological slides for immunohistochemistry (IHC) staining. IHC staining was performed following a standard streptavidinbiotin-peroxidase complex method. Tissue sections were heated at 65°C for 60 minutes, deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol to water. Antigen retrieval was achieved by boiling samples at 100°C in citrate buffer solution (pH = 6.0) for 10 minutes. The endogenous peroxidase activity was blocked by incubating sections with 10% normal goat serum for 1 hour at room temperature. Then the sections were incubated overnight at 4°C in a moist chamber, then purified rabbit antihuman STAT3 polyclonal antibody (1:250, Santa Cruz Biotech, CA, USA) or mouse anti-human cyclinD1 monoclonal antibody (1:50, Beijing Sequoia Jinqiao Biotech Corporation). After washing with PBS, sections were incubated with biotin-labeled rabbit anti-goat secondary antibody at room temperature for 10 min followed by incubating with streptavidin-peroxidase for 10 min. Diaminobenzidine was used as the final chromogen, and hematoxylin was used for counter-staining. Staining with PBS instead of primary antibody against STAT3 and cyclinD1 were used as negative control.
Immunohistochemical scoring
The protein expression level of STAT3 and cyclinD1 was evaluated by microscopic examination of stained tissue sections. STAT3 and cyclinD1 expression level was determined by integrating the percentage of positive tumor cells and the intensity of positive staining. The intensity of staining was scored as follows: negative (score 0), bordering (score 1), weak (score 2), moderate (score 3), and strong (score 4). The extent of staining was scored according to the percentage of positive stained tumor cells in the field: negative (score 0), 0-25% (score 1), 26-50% (score 2), 51-75% (score 3), and 76-100% (score 4).
The product of the intensity and extent score was considered as the overall IHC score (values: from 0 to 16). The staining was observed and assessed by two independent pathologists (Haiying Li and Jiwei Ma) without knowing the identity of the samples. If there was a discrepancy in individual evaluations, then the two pathologists reevaluated the sections together to reach a consensus.
Statistical analysis
The data were analyzed with SPSS software version 19.0. The association between STAT3/cyclinD1 protein expression and clinicopathological features was analyzed by Chi-square test. The time to death was defined as the period between curative surgery and death due to any reason. Kaplan-Meier and log-rank tests were used to compare overall survival rates, while Cox regression analysis was used for the multivariate analysis. In all statistical analyses, a P value <0.05 was considered statistically significant, and all P values were two-sided.
Results
Clinical characteristics of ESCC patients
Table 1 lists the characteristics of recruited patients (n = 82). The gender ratio of male to female was 3.56: 1. The median age was 57 years (range: from 36 to75 years). The median tumor size (maximum diameter) was 4 cm (range: from 0.8 to 12.5 cm). The number of cases in Grade II and Grade III was 76 (92.7%) was and 6 (7.3%). The depth of invasion consists of two types: muscular layer, 16 cases, 19.5% and serosa layer, 66 cases, 80.5%. Tumor stage was distributed as follows: I + II, 28 cases, 34.1%; III + IV, 54 cases, 65.9%. The follow-up information of 82 patients was collected within the range from 1 to 39 months after surgery.
Table 1.
Comparison of STAT3 and cyclinD1 protein expression in ESCC tissues and adjacent non-cancerous tissue
| Group | Cancer tissues | Non-cancerous tissues | χ2 | P value* |
|---|---|---|---|---|
| High expression of STAT3** | 54/82 (65.85%) | 14/82 (17.07%) | 40.20 | <0.01 |
| Low expression of STAT3 | 28/82 (34.15%) | 68/82 (82.93%) | ||
| High expression of cyclinD1 | 43/82 (52.44%) | 21/82 (25.61%) | 12.40 | <0.01 |
| Low expression of cyclinD1 | 39/82 (47.56%) | 61/82 (74.40%) |
Chi square test;
Signal transducers and activators of transcription 3.
STAT3 and cyclinD1 proteins expression is up-regulated in ESCC tissues compared to adjacent noncancerous tissues
According to IHC staining, the cancer cells were found to be relatively homogenous within a tumor (excluding necrotic, hemorrhagic, and fibrotic components). Representative cases of immunohistochemical staining are shown in Figure 1. Both the expression of STAT3 and CyclinD1 were found to be mainly located in cytoplasm/nucleus and nucleus, respectively. Brown cytoplasm and nucleus immunoreactivity for the STAT3 and cyclinD1 were recognized as positive staining.
Figure 1.
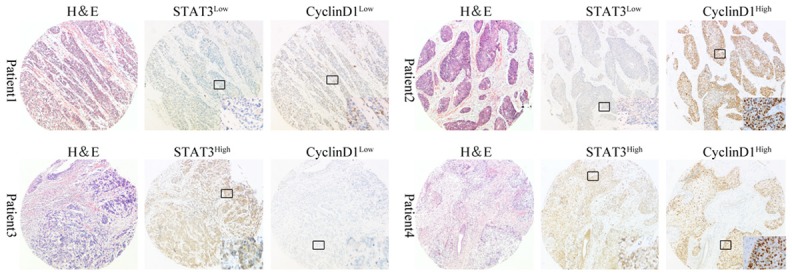
Expressions of STAT3 and cyclinD1 in different ESCC tissues (n = 82). Representative ESCC tumor samples showed the high or low expression of STAT3 and cyclinD1. All pictures were taken under 50X magnification. Inserts in each lower right corner of IHC staining in four representative patients show 400X magnification of a representative part of the tissue.
The median scores of STAT3 and cyclinD1 expression in the TMA were 8 and 4, respectively, according to the staining index as mentioned above. Thus, we defined overall positivity index 0-7 as low expression and 8-16 as high expression for STAT3 at protein level; and 0-3 and 4-16 for cyclinD1, correspondingly. As shown in Table 1, high expression of STAT3 and cyclinD1 were detected in 54 and 43 out of 82 TMA cancer tissues (65.9% and 52.4%) while the remaining cancer tissues and most of the adjacent non-cancerous tissues showed low expression of STAT3 and cyclinD1. Both STAT3 and cyclinD1 protein expression levels in the ESCC tissues were significantly higher than those in the adjacent non-cancerous tissues (Table 1).
Relationship between STAT3 and cyclinD1 proteins expression and ESCC patients’ clinicopathological variables
The association among STAT3, cyclinD1 expression in ESCC and clinicopathological variables was assessed and displayed in Table 2. High expression of STAT3 was found to significantly correlate with advanced tumor stage (P = 0.047) and high expression of cyclinD1 was found to significantly correlate with lager tumor size (P = 0.009) and advanced tumor stage (P = 0.012). No significant difference in STAT3 or cyclinD1 expression was observed with gender, age at surgery, histological grade, and depth of invasion (P > 0.05). Interestingly, the expression of STAT3 was positively associated with the expression of cyclinD1 (P = 0.025).
Table 2.
Correlation analysis between clinicopathological parameters and expression of STAT3 and cyclinD1
| Variable | N | STAT3 expression | P value* | CyclinD1 expression | P value* | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| High | Low | High | Low | ||||
| Gender | 0.452 | 0.348 | |||||
| Male | 64 | 40 (62.50%) | 24 (37.50%) | 31 (48.40%) | 33 (51.60%) | ||
| Female | 18 | 13 (72.20%) | 5 (27.80%) | 11 (61.10%) | 7 (38.90%) | ||
| Age at surgery** | 0.398 | 0.824 | |||||
| <57 | 40 | 24 (60.00%) | 16 (40.00%) | 21 (52.50%) | 19 (47.50%) | ||
| ≥57 | 42 | 29 (69.00%) | 13 (31.00%) | 21 (50.00%) | 21 (50.00%) | ||
| Histological grade*** | 0.326 | 0.438 | |||||
| II | 76 | 48 (63.50%) | 28 (36.80%) | 38 (50.00%) | 38 (50.00%) | ||
| III | 6 | 5 (83.30%) | 1 (16.70%) | 4 (66.70%) | 2 (33.30%) | ||
| Tumor size** | 0.582 | 0.009 | |||||
| <4 cm | 28 | 19 (67.90%) | 9 (32.10%) | 9 (32.10%) | 19 (67.90%) | ||
| ≥4 cm | 44 | 27 (61.40%) | 17 (38.60%) | 28 (63.60%) | 16 (36.40%) | ||
| Depth of invasion | 0.052 | 0.077 | |||||
| Muscular layer | 16 | 7 (43.80%) | 9 (56.20%) | 5 (31.20%) | 11 (68.80%) | ||
| Serosa layer | 66 | 46 (69.70%) | 20 (30.30%) | 37 (56.10%) | 29 (43.90%) | ||
| Tumor stage | 0.047 | 0.012 | |||||
| I-II | 28 | 14 (50.00%) | 14 (50.00%) | 9 (32.10%) | 19 (67.90%) | ||
| III-IV | 54 | 39 (72.20%) | 15 (27.80%) | 33 (61.10%) | 21 (38.90%) | ||
Chi square test;
Median age and median tumor size;
Histological grade I of patients were missing.
Relationship between clinicopathological variables, STAT3 or cyclinD1 proteins expression, and overall survival by univariate analysis
In univariate survival analyses, Kaplan-Meier survival curves were employed and the statistics were carried out by log-rank method (Table 3). Kaplan-Meier analysis demonstrated that tumor stage had a significant impact on overall survival as a well-known clinicopathological prognostic factor (P = 0.016). What is more, overall survival in patients with high expression of STAT3/cyclinD1 was significantly shortened than in patients with low expression of STAT3/cyclinD1 (Figure 2). The mean value of overall survival time was 27.67 months or 23.09 months in patients with low expression of STAT3 or cyclinD1 compared with 17.48 months and 21.81 months in patients with high expression of STAT3 or cyclinD1, respectively (P = 0.044, 0.032).
Table 3.
Correlation of clinicopathological features with expressions of STAT3 and cyclinD1 protein by univariate survival analysis
| Patients features | N | Mean ± SE | Median ± SE | P value |
|---|---|---|---|---|
| Gender | 0.163 | |||
| Male | 64 | 24.43±2.28 | 21.00±3.62 | |
| Female | 18 | 15.72±2.46 | 15.00±4.25 | |
| Age at surgery | 0.095 | |||
| <57 | 40 | 27.19±3.11 | - | |
| ≥57 | 42 | 17.91±1.79 | 17.00±3.43 | |
| Tumor size | 0.749 | |||
| <4 cm | 28 | 20.48±2.47 | 22.00±3.73 | |
| ≥4 cm | 44 | 21.28±2.89 | 18.00±4.26 | |
| Histological grade | 0.155 | |||
| II | 76 | 22.42±1.97 | 20.00±2.76 | |
| III | 6 | 12.63±7.47 | 27.67±3.38 | |
| Depth of invasion | 0.245 | |||
| Muscular layer | 16 | 24.43±3.28 | - | |
| Serosa layer | 66 | 20.69±2.05 | 18.00±3.42 | |
| Tumor stage | 0.016 | |||
| I-II | 28 | 24.02±1.88 | 25.00±4.29 | |
| III-IV | 54 | 17.72±2.41 | 14.00±2.27 | |
| STAT3 protein expression | 0.023 | |||
| Low | 28 | 27.67±3.38 | - | |
| High | 54 | 17.48±1.58 | 17.00±1.80 | |
| CyclinD1 protein expression | 0.032 | |||
| Low | 39 | 23.09±1.78 | 25.00±3.06 | |
| High | 43 | 21.81±1.90 | 20.00±2.84 | |
| STAT3 and cyclinD1 protein expression | 0.008 | |||
| Dual low expression | 20 | 24.02±2.16 | - | 0.013# |
| Single high expression | 31 | 24.59±2.36 | 25.00±2.18 | 0.045## |
| Dual high expression | 31 | 13.51±2.29 | 9.00±2.50 | 0.980### |
P value of overall survival among patients with dual low expression of STAT3 and cyclinD1 (n = 20), high expression of STAT3 and low expression of cyclinD1 (n = 20) and dual high expression of STAT3 and cyclinD1 (n = 31);
P value of overall survival among patients with dual low expression of STAT3 and cyclinD1 (n = 20), high expression of cyclinD1 and low expression of STAT3 (n = 11) and dual high expression of STAT3 and cyclinD1 (n = 31);
P value of overall survival between patients with high expression of STAT3 and low expression of cyclinD1 (n = 20) and high expression of cyclinD1 and low expression of STAT3 (n = 11).
Figure 2.

Relationship between the tumor stages, the expressions of STAT3, cyclinD1 and survival rates by Kaplan-Meier survival curve. A. Later tumor stage had the relationship with poor prognosis (P = 0.016); B, C. High expression of STAT3 and cyclinD1 were significantly correlated with poor prognosis (P = 0.032, 0.023); D. The dual high expression of STAT3 and cyclinD1 was significantly correlated with poor prognosis (P = 0.008).
Relationship of dual high expression of STAT3 and cyclinD1 proteins with overall survival by univariate analysis
The mean value of overall survival time was 13.51 months with dual high expression compared with 24.02 months and 24.59 months in the patients who carried with dual low and single high expression of STAT3 and cyclinD1. Univariate analyses also indicated that the difference of survival time among the ESCC patients with dual and single high expression and dual low expression of STAT3 or cyclinD1 was significant (P = 0.008), suggesting that combining with STAT3 and cyclinD1 maybe more favourable for predicting the outcome of the patients with ESCC (Table 3). Furthermore, we compared the survival time of three groups of ESCC patients with dual low expression of STAT3 and cyclinD1 (n = 20), high expression of STAT3 and low expression (n = 20)/high expression of cyclinD1 and low expression of STAT3 (n = 11) and dual high expression of STAT3 and cyclinD1 (n = 31), respectively. And we found that the prognosis of patients with dual high expression of STAT3 and cyclinD1 was the poorest (P = 0.013, 0.045). There is no significance of survival time (P = 0.980) between the patients with high expression of STAT3 and low expression of cyclinD1 (n = 20) and high expression of cyclinD1 and low expression of STAT3 (n = 11).
Prognostic significance of STAT3 and cyclinD1 protein expression in ESCC in multivariate analysis
Since variables observed to have a prognostic influence by univariate analysis may covariate, the expression of STAT3 and CyclinD1 protein expression and those clinicopathological parameters that were significant in univariate analysis were further examined in multivariate analysis using Cox regression model (Table 4).
Table 4.
Prognostic value of tumor stage and STAT3/cyclinD1 expression for the overall survival by Cox regression
| Variable | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Tumor stage* | 2.499 | 1.030-6.053 | 0.043 |
| STAT3 protein expression** | 2.687 | 1.091-6.619 | 0.032 |
| CyclinD1 protein expression** | 2.115 | 1.029-4.347 | 0.042 |
| STAT3 and cyclinD1 protein expression*** | 2.887 | 1.397-5.976 | 0.004 |
Stages III-IV versus stages I-II;
High expression versus low expression;
Dual high expression of STAT3 and cyclinD1 versus single high expression of STAT3 or cyclinD1 and dual low expression of STAT3 and cyclinD1.
The results showed that high expression of STAT3 or cyclinD1 protein were both independent prognostic factors for overall survival (STAT3 hazard ratio: 2.687, 95% confidence interval: 1.091~6.619, P = 0.032; cyclinD1 hazard ratio: 2.115, 95% confidence interval: 1.029~4.347, P = 0.042). More importantly, dual high expression of STAT3 and cyclinD1 protein was found to be the most significant independent adverse prognostic factor for overall survival (hazard ratio: 3.223, 95% confidence interval: 1.417~7.330, P = 0.005). With regard to clinicopathological parameters, tumor stage remained to be an independent prognostic factor for overall survival (hazard ratio: 2.499, 95% confidence interval: 1.030~6.053, P = 0.043).
Discussion
ESCC is one of the most malignant gastrointestinal cancers and occurs at a high frequency rate in China and other Asian countries [14]. Des the five-year survival rate remains low [15] and the prognosis has not been significantly improved [16,17]. The prediction of ESCC clinical prognosis still depends on conventional pathologic variables such as tumor size, tumor grade, lymph node and distal metastasis status [18,19]. Thus, it is of great clinical importance to find effective biomarkers for the prognosis of ESCC, as well as novel therapeutic strategies.
We determined that high expression of STAT3 in ESCC had a highly significant relationship with advanced tumor stage (P = 0.047) and poor prognosis (P = 0.023). The JAK-STAT pathway is an important oncogenic signaling cascade that consists of the Janus kinase (JAK) family of nonreceptor tyrosine kinases and the STAT family of transcription factors [5] which are activated by phosphorylation of tyrosine and serine residues by upstream kinases [20]. STAT3 was first described as a DNA-binding protein activated in interleukin 6-stimulated hepatocytes and selectively interacting with an enhancer element on promoters of acute-phase genes [21]. Aberrant signaling by STAT3 is found in many types of malignancies, including multiple myeloma, head and neck cancer, breast cancer, and prostate cancer [22-26]. Moreover, STAT3 also exerts tumor-extrinsic effects, supporting tumor survival and metastasis apart from its tumor-intrinsic effects [27]. We also added evidence that STAT3 expression was increased compared with the surrounding normal tissue in our study and to the prognostic value of STAT3 in archived ESCC cohort.
We also detected significant correlations between high cyclinD1 expression and lager tumor size (P = 0.009), advanced tumor stage (P = 0.012) and poor prognosis (P = 0.032). As we all know, cyclinD1 is the regulatory subunit of a dimeric holoenzyme including the cell cycledependent kinase CDK4, which promotes progression through the G1-S phase of the cell cycle. Except for regulating cell cycle, cyclinD1 also regulates angiogenesis, lipogenesis and mitochondrial function [28-31]. Compared with other two D-type cyclins, only overexpression of cyclinD1 is observed frequently in cancer and closely associated with malignant progression and metastatic disease [32]. As a result, it’s not surprising to find the prognostic significance of cyclinD1 in ESCC patients.
One of most notable findings of our study was the expression of STAT3 was positively associated with the expression of cyclinD1 (P = 0.025). Huang C et al. demonstrated that inhibiting SW1990 cells proliferation by RNAi against STAT3 can induce cell apoptosis and significantly reduced the levels of cyclinD1 when compared with parental and control vector-transfected cells, and significantly suppress tumor growth when it was directly injected into tumors [33]. Direct evidence of STAT3/cyclinD1 signaling axis came from CNE1 cells, breast cancer cells and hepatocellular carcinoma cells [34-36], showing that nuclear STAT3 could target the CYCLIN D1 promoter directly, in turn, upregulating the CYCLIN D1 promoter activity and transcription. To date, we report about an association between STAT3 and cyclinD1 expression in ESCC tissues for the first time, which warrants further investigation.
Another significant finding in our study was that high expression of STAT3 or cyclinD1 alone, as well as dual high expression of the combination of STAT3 and cyclinD1, was significantly correlated with OS of ESCC patients. Interestingly, patients with dual high expression of STAT3 and cyclinD1 had a significantly shorter survival time than the patients in the other 2 groups in both univariate and multivariate analyses after we made a direct comparison of prognosis between three subgroups (dual high expression, single high expression and dual low expression). It is plausible that JAK2/STAT3/cyclinD1 pathway activation would give birth to the observed aggressive behavior of ESCC and poor outcome of ESCC patients.
In this study, we determined the prognostic value of STAT3 and cyclinD1 in ESCC. To the best of our knowledge, this is the first report demonstrating combination of STAT3 and cyclinD1 enables us to more accurately predict the true prognosis of ESCC patients. The major limitation of the present work was its retrospective nature; further study is in progress to go to depth.
In summary, STAT3 and cyclinD1 correlate with more aggressive tumor behavior in ESCC. High STAT3 or cyclinD1 expression alone was associated with shorter survival time. Dual high expression of STAT3 and cyclinD1 expression predicted even worse survival outcome. When STAT3 and cyclinD1 are considered together, they serve as effective prognostic markers in patients with surgically resected ESCC. From the foregoing, STAT3 and cycinD1 may be the underlying molecular markers for the diagnosis and prognosis of ESCC.
Acknowledgements
This work was sponsored by the National High Technology Research and Development Program of China (863 Program), No. 2012AA02A503, People’s Republic of China; the Science and Technology Innovation Key Project of Guangdong Higher Education Institutes, No. CXZD1110; Science and Technology Program of Guangzhou, No.2014J4100103, China.
Disclosure of conflict of interest
None.
References
- 1.Zeng R, Duan L, Kong Y, Liang Y, Wu X, Wei X, Yang K. Clinicopathological and prognostic role of MMP-9 in esophageal squamous cell carcinoma: a meta-analysis. Chin J Cancer Res. 2013;25:637–645. doi: 10.3978/j.issn.1000-9604.2013.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam TK, Freedman ND, Fan JH, Qiao YL, Dawsey SM, Taylor PR, Abnet CC. Prediagnostic plasma vitamin C and risk of gastric adenocarcinoma and esophageal squamous cell carcinoma in a Chinese population. Am J Clin Nutr. 2013;98:1289–1297. doi: 10.3945/ajcn.113.061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Chen JW, Xie JD, Ling YH, Li P, Yan SM, Xi SY, Luo RZ, Yun JP, Xie D, Cai MY. The prognostic effect of perineural invasion in esophageal squamous cell carcinoma. BMC Cancer. 2014;14:313. doi: 10.1186/1471-2407-14-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnell JE Jr. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 6.Xiong A, Yang Z, Shen Y, Zhou J, Shen Q. Transcription Factor STAT3 as a Novel Molecular Target for Cancer Prevention. Cancers (Basel) 2014;6:926–957. doi: 10.3390/cancers6020926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SW, Ahn YY, Kim YS, Kang SB, Nam SW, Lee DS, Jeong HY, Kim JM. The Immunohistochemical Expression of STAT3, Bcl-xL, and MMP-2 Proteins in Colon Adenoma and Adenocarcinoma. Gut Liver. 2012;6:45–51. doi: 10.5009/gnl.2012.6.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop JL, Thaper D, Zoubeidi A. The Multifaceted Roles of STAT3 Signaling in the Progression of Prostate Cancer. Cancers (Basel) 2014;6:829–859. doi: 10.3390/cancers6020829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Zhu JF, Liu YY, Han GP. An analysis of cyclin D1, cytokeratin 5/6 and cytokeratin 8/18 expression in breast papillomas and papillary carcinomas. Diagn Pathol. 2013;8:8. doi: 10.1186/1746-1596-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiler R, Thalmann GN, Rotzer D, Perren A, Fleischmann A. CCND1/CyclinD1 status in metastasizing bladder cancer: a prognosticator and predictor of chemotherapeutic response. Mod Pathol. 2014;27:87–95. doi: 10.1038/modpathol.2013.125. [DOI] [PubMed] [Google Scholar]
- 11.Umekita Y, Ohi Y, Sagara Y, Yoshida H. Overexpression of cyclinD1 predicts for poor prognosis in estrogen receptor-negative breast cancer patients. Int J Cancer. 2002;98:415–418. doi: 10.1002/ijc.10151. [DOI] [PubMed] [Google Scholar]
- 12.Gagarin D, Yang Z, Butler J, Wimmer M, Du B, Cahan P, McCaffrey TA. Genomic profiling of acquired resistance to apoptosis in cells derived from human atherosclerotic lesions: potential role of STATs, cyclinD1, BAD, and Bcl-XL. J Mo Cell Cardiol. 2005;39:453–465. doi: 10.1016/j.yjmcc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Kleihues P, Sobin LH. World Health Organization classification of tumors. Cancer. 2000;88:2887. doi: 10.1002/1097-0142(20000615)88:12<2887::aid-cncr32>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.Gao H, Wang L, Cui S, Wang M. Combination of meta-analysis and graph clustering to identify prognostic markers of ESCC. Genet Mol Biol. 2012;35:530–537. doi: 10.1590/S1415-47572012000300021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer. 2002;94:929–933. [PubMed] [Google Scholar]
- 16.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 17.Koshy M, Esiashvilli N, Landry JC, Thomas CR Jr, Matthews RH. Multiple management modalities in esophageal cancer: combined modality management approaches. Oncologist. 2004;9:147–159. doi: 10.1634/theoncologist.9-2-147. [DOI] [PubMed] [Google Scholar]
- 18.Lin DC, Du XL, Wang MR. Protein alterations in ESCC and clinical implications: a review. Dis Esophagus. 2009;22:9–20. doi: 10.1111/j.1442-2050.2008.00845.x. [DOI] [PubMed] [Google Scholar]
- 19.Ashida A, Boku N, Aoyagi K, Sato H, Tsubosa Y, Minashi K, Muto M, Ohtsu A, Ochiai A, Yoshida T. Expression profiling of esophageal squamous cell carcinoma patients treated with definitive chemoradiotherapy: clinical implications. Int J Oncol. 2006;28:1345–1352. [PubMed] [Google Scholar]
- 20.Ihle JN. STATs and MAPKs: obligate or opportunistic partners in signaling. Bioessays. 1996;18:95–98. doi: 10.1002/bies.950180204. [DOI] [PubMed] [Google Scholar]
- 21.Ai T, Wang Z, Zhang M, Zhang L, Wang N, Li W, Song L. Expression and prognostic relevance of STAT3 and cyclin D1 in non-small cell lung cancer. Int J Biol Markers. 2012;27:e132–138. doi: 10.5301/JBM.2012.9146. [DOI] [PubMed] [Google Scholar]
- 22.Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS, Tweardy DJ. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor-mediated cell growth In vitro. J Clin Invest. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 24.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 25.Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang JM, Yang-Yen HF, Karras J. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barton BE, Murphy TF, Adem P, Watson RA, Irwin RJ, Huang HF. IL-6 signaling by STAT3 participates in the change from hyperplasia to neoplasia in NRP-152 and NRP-154 rat prostatic epithelial cells. BMC Cancer. 2001;1:19. doi: 10.1186/1471-2407-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bournazou E, Bromberg J. Targeting the tumor microenvironment: JAK-STAT3 signaling. JAKSTAT. 2013;2:e23828. doi: 10.4161/jkst.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Li Z, Lu Y, Du R, Katiyar S, Yang J, Fu M, Leader JE, Quong A, Novikoff PM. Cyclin D1 repression of nuclear respiratory factor 1 integrates nuclear DNA synthesis and mitochondrial function. Proc Natl Acad Sci U S A. 2006;103:11567–11572. doi: 10.1073/pnas.0603363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamaki T, Casimiro MC, Ju X, Quong AA, Katiyar S, Liu M, Jiao X, Li A, Zhang X, Lu Y. Cyclin D1 determines mitochondrial function in vivo. Mol Cell Biol. 2006;26:5449–5469. doi: 10.1128/MCB.02074-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C, Pattabiraman N, Zhou JN, Fu M, Sakamaki T, Albanese C, Li Z, Wu K, Hulit J, Neumeister P. Cyclin D1 repression of peroxisome proliferator-activated receptor gamma expression and transactivation. Mol Cell Biol. 2003;23:6159–6173. doi: 10.1128/MCB.23.17.6159-6173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasui M, Yamamoto H, Ngan CY, Damdinsuren B, Sugita Y, Fukunaga H, Gu J, Maeda M, Takemasa I, Ikeda M, Fujio Y, Sekimoto M, Matsuura N, Weinstein IB, Monden M. Antisense to cyclin D1 inhibits vascular endothelial growth factor-stimulated growth of vascular endothelial cells: implication of tumor vascularization. Clin Cancer Res. 2006;12:4720–4729. doi: 10.1158/1078-0432.CCR-05-1213. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Wang C, Prendergast GC, Pestell RG. Cyclin D1 functions in cell migration. Cell Cycle. 2006;5:2440–2442. doi: 10.4161/cc.5.21.3428. [DOI] [PubMed] [Google Scholar]
- 33.Huang C, Yang G, Jiang T, Cao J, Huang KJ, Qiu ZJ. Down-regulation of STAT3 expression by vector-based small interfering RNA inhibits pancreatic cancer growth. World J Gastroenterol. 2011;17:2992–3001. doi: 10.3748/wjg.v17.i25.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Shi Y, Yuan Q, Liu X, Yan B, Chen L, Tao Y, Cao Y. Epstein-Barr Virus encoded LMP1 regulates cyclin D1 promoter activity by nuclear EGFR and STAT3 in CNE1 cells. J Exp Clin Cancer Res. 2013;32:90. doi: 10.1186/1756-9966-32-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saxena NK, Vertino PM, Anania FA, Sharma D. Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem. 2007;282:13316–13325. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Won C, Lee CS, Lee JK, Kim TJ, Lee KH, Yang YM, Kim YN, Ye SK, Chung MH. CADPE suppresses cyclin D1 expression in hepatocellular carcinoma by blocking IL-6-induced STAT3 activation. Anticancer Res. 2010;30:481–488. [PubMed] [Google Scholar]


