Abstract
Objective: Our previous study demonstrated that α-naphthoflavone (α-NF) inhibits mouse 3T3-L1 pre-adipocytes differentiation via PPARγ, a key transcription factor in adipogenesis. Due to the critical role of inflammation in adipogenesis, we speculated that the suppression role of α-NF in adipogenesis might involve in modulation of cytokines secretion raised by adipocyte differentiation cocktail. Therefore, the present study aims to investigate the role of α-NF in modulating of inflammatory response during adipocytes differentiation and adipocyte-macrophage interaction. Methods: Conditioned medium from different doses of α-NF treated 10-day differentiated 3T3-L1 adipocytes were collected to culture RAW264.7 macrophages. Conditioned medium from activated macrophages and α-NF pre-treated macrophage were used to investigate the effects of α-NF in adipocytes differentiation. Cultured cells and medium were harvested for RT-PCR, Western blot and ELISA. Results: α-NF dose-dependently decreased TNF-α and IL-6 and increased IL-10 expression induced by IDM (Insulin, dexamethasone, isobutylmethylxanthine) in 3T3-L1 pre-adipocytes. Conditioned medium from α-NF treated 3T3-L1 differentiated cells inhibited inflammatory response in mouse macrophage cell line RAW264.7 in contrast to IDM control medium. NFĸB activation elicited by IDM was suppressed by α-NF in a dose-response manner. Consequently, decreased TNF-α and increased IL-10 secretion, downstream targets of NFĸB signaling pathway, were observed with α-NF in macrophages. Finally, Conditioned medium from α-NF pre-treated, LPS-activated macrophages ameliorated the suppression of 3T3-L1 adipogenesis by LPS activated macrophages. Conclusion: Our results suggest that α-NF regulates inflammation response in both adipocytes and macrophages and adipocyte-macrophage interaction which contributes to pre-adipocyte differentiation.
Keywords: Adipocyte-macrohage interaction, α-NF, condition medium, inflammation
Introduction
Obesity is a state of low-grade chronic inflammation characterized by abnormal cytokine production, increased synthesis of acute phase reactants and activation of pro-inflammatory signaling pathways [1-3]. The imbalance of pro- and anti-inflammatory status has been linked to an increased risk of developing insulin resistance, type 2 diabetes and cardiovascular diseases [4-6]. Moreover, macrophage infiltration is a vital event in the initiation of pathologic obesity and macrophage secreted factors impair human adipogenesis [7]. Available evidence from obese mice and humans revealed that macrophages, adipocytes and pre-adipocytes produce a variety of adipokines and cytokines including TNF-α and IL-6 [8], contribute to the elevation of circulating inflammatory markers in obesity. Local interaction under different conditions [9,10] suggesting the cross-talk between adipocytes and macrophages is a potential mechanism that aggravates inflammatory changes in obese adipose tissue.
Flavonoids are a large family of plant secondary metabolites that are typical dietary component although they are not considered as nutritive elements. They have a wide arrange of biological activities including anti-oxidative, anti-inflammatory, and anti-cancer [11-13]. Alpha-naphthoflavone (α-NF) is a synthetic flavonoid and used as an antagonist for the aromatic hydrocarbon receptor (AhR) [14]. Our previous study demonstrated that α-NF inhibit IDM induced 3T3-L1 adipogenesis [15]. Due to the important role of adipocyte-macrophage interaction in pre-adipocyte differentiation and obese development, we hypothesize that the suppression of α-NF on 3T3-L1 differentiation might involve in the modulation of inflammation in adipocyte-macrophage interaction. Therefore, the present study is to investigate the role of α-NF in the inflammatory response during 3T3-L1 adipogenesis, the interaction between adipocytes and macrophages, and the possible underlying mechanism.
Materials and methods
Cell culture and preparation of conditioned medium
Mouse 3T3-L1 pre-adipocyte was from the Chinese Academy of Science (Shanghai, China) and Mouse macrophage cell line RAW264.7 was a kind gift from Prof. Ouyang Jingping (Wuhan University). Mature adipocytes were differentiated from 3T3-L1 pre-adipoctyes as described in our previous study [15]. Adipocyte-conditioned medium was prepared by differentiating 3T3-L1 cells in 6-well plates for 10 days with different does of α-NF treatment and control medium was from IDM differentiated cells alone. Macrophage-conditioned medium was collected from the 24 h LPS activated RAW264.7 macrophage with or without α-NF pre-treatment. Control medium was RPM1-10% FBS kept at 37°C for 24 h in the absence of macrophages. All conditioned media were pooled from at least three individuals and store at -80°C until used.
Measurement of secreted cytokines by ELISA
TNF-α, IL-10 levels in cells culture medium were measured with ELISA kits according to the manufacturer’s instructions (R&D systems).
qRT-PCR
Cells harvested in 1 ml of Trizol reagent (Invitrogen), RAN extraction and qRT-PCR were performed as previously described [15]. Generally, 1 μg of total RNA was used for cDNA synthesis and qPCR was performed in 96-well plates with the SYBR Green kit (ABI) in a Step-one Plus real-time PCR detection system. Gene expression was quantified by the comparative cycle threshold method. Details of primer sets are available upon request.
Western blot
Whole cell lysates were isolated as previously described [15]. A total of 50 μg protein was separated by 12% SDS-PAGE. Western blot was performed using antibody against NFκB (1:2000, CST) and β-actin (1:5000, Sigma).
Oil red O staining and quantification
Detail performance and measurement were described elsewhere [15]. The critical points in ORO are: Working solution should be prepared freshly and filtered by a 0.45 μm filter; ORO dye outside the cells should be wash out by running water before quantification.
Statistical analysis
Data were presented as the mean ± SEM and analyzed with one-way ANOVA by PRISM. The post-hoc tests were performed once ANOVA revealed significant. Statistical significance was set at P < 0.05.
Result
α-NF inhibits pro-inflammatory cytokines expression in 3T3-L1 cells upon differentiation
Our previous study showed that α-NF inhibited adipogenesis in 3T3-L1 pre-adipocytes. Considering the key role that the inflammatory cytokines play in the pre-adipocytes differentiation and obesity related insulin resistance, we measure the effects of α-NF on the inflammatory cytokines in 3T3-L1 cells during adipogenesis. Our results showed that IDM cocktail significantly increased pro-inflammatory cytokines TNF-α and IL-6, and repressed anti-inflammatory cytokine IL-10 in both mRNA expression and secretion (Figure 1A, 1B). In contrast to IDM cocktail, α-NF exhibited reverse effects on these cytokines expression and production (Figure 1A, 1B), although it did require higher dose of α-NF for IL-6 and IL-10 expression to reach the significant level.
Figure 1.
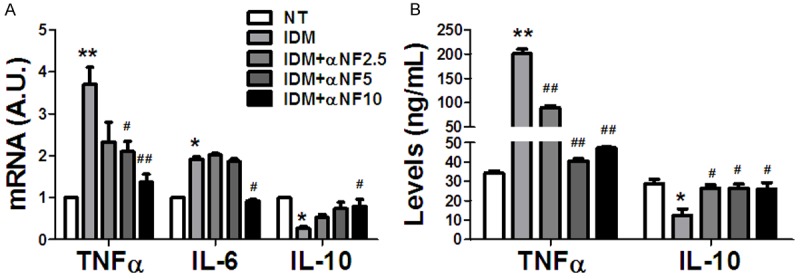
α-NF suppressed pro-inflammatory cytokines, while increased anti-inflammatory cytokines expression during 3T3-L1 adipogenesis. A: The inflammatory cytokines expression after 10 days differentiation in 3T3-L1 cells. B: The concentration of inflammatory cytokines after 10 days differentiation in 3T3L-1 cells cultured medium. *: Compared with non-treatment (NT) group, P < 0.05; **: Compared with NT group, P < 0.01; #: Compared with IDM group, P < 0.05; ##: Compared with IDM, P < 0.01.
Conditioned medium from α-NF treated 3T3-L1 differentiated cells suppresses pro-inflammatory cytokines production in RAW264 macrophages
Adipocytes-macrophages interaction plays a vital role in initiation and progression of obesity and associated chronic diseases. α-NF suppressed pre-adipocytes differentiation at dose-dependent manner [15] and it also modulated inflammatory response induced by IDM differentiated cocktail IDM (Figure 1). Therefore, we collected 3T3-L1 adipocytes conditioned medium (aCM) after 10 days differentiated with different dose of α-NF treatment to culture RAW-264.7 macrophages for 24 hours. We found that aCM up-regulated the pro-inflammatory cytokines expression in macrophage, α-NF dose-dependently repressed TNF-α and IL-6 expression compared with IDM control (Figure 2A). ELISA revealed the similar impact of α-NF on TNF-α and IL-10 production in condition medium (Figure 2B).
Figure 2.
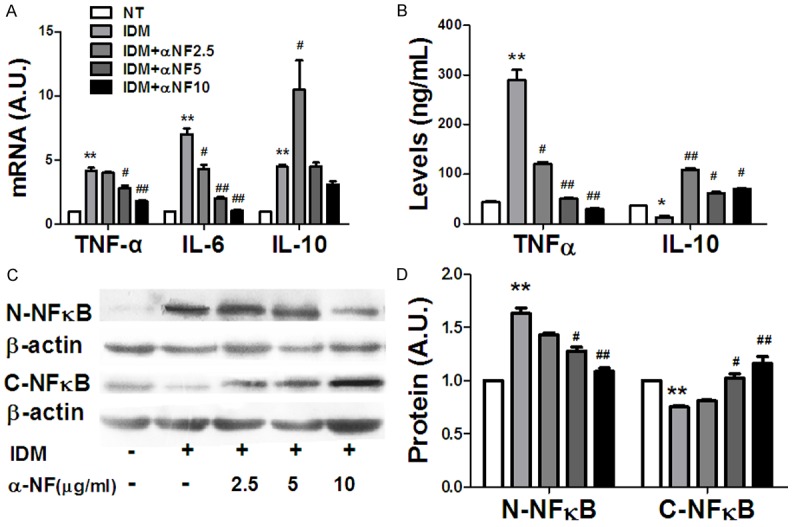
Pro- and Anti-inflammatory cytokines expression in RAW264.7 macrophages when cultured with α-NF treated adipocytes conditioned medium (aCM). A: The cytokines mRNA expression in RAW264 macrophages. B: The cytokines concentration in RAW264 cultured medium. C, D: The NF-κB activation in RAW264 macrophages. N-NFκB: Nucleus NFκB; C-NFκB: Cytoplasm NFκB. *: Compared with NT, P < 0.05; **: Compared with NT, P < 0.01; #: Compared with IDM, P < 0.05; ##: Compared with IDM, P < 0.01.
Conditioned medium from α-NF treated 3T3-L1 differentiated cells inhibits NF-κB activation in RAW264 macrophages
TNF-α, IL-6 and IL-10 are downstream targets of NF-κB. The modulating role of α-NF in TNF-α, IL-6 and IL-10 production prompts us to observe the NF-κB activation. NF-κB is a canonical pro-inflammatory signaling pathway. Once activated by the extracellular signals, NF-κB rapidly translocates from the cytoplasm to the nucleus and activates target gene expression. We found significantly elevated nucleus NF-κB and concurrently repressed cytoplasm NF-κB expression after IDM hormone cocktail treatment (Figure 2C, 2D). α-NF suppressed NF-κB activation induced by IDM in dose-dependent manner (Figure 2C, 2D).
To further investigate the role of α-NF in modulating NF-κB target genes, we used pyrrolidine dithiocarbamate (PDTC), a specific antagonist of NF-κB, to block NF-κB activation. We found that PDTC did suppress IDM induced NF-κB activation and α-NF exerted a synergistic effect (Figure 3A, 3B). For the downstream target genes expression, α-NF and PDTC exhibited synergistic suppression on TNF-α production (Figure 3D), but not on its mRNA expression (Figure 3C). Furthermore, PDTC ablated IL-10 secretion elicited by α-NF (Figure 3D), suggested a key role of NF-κB in its regulating mechanism.
Figure 3.
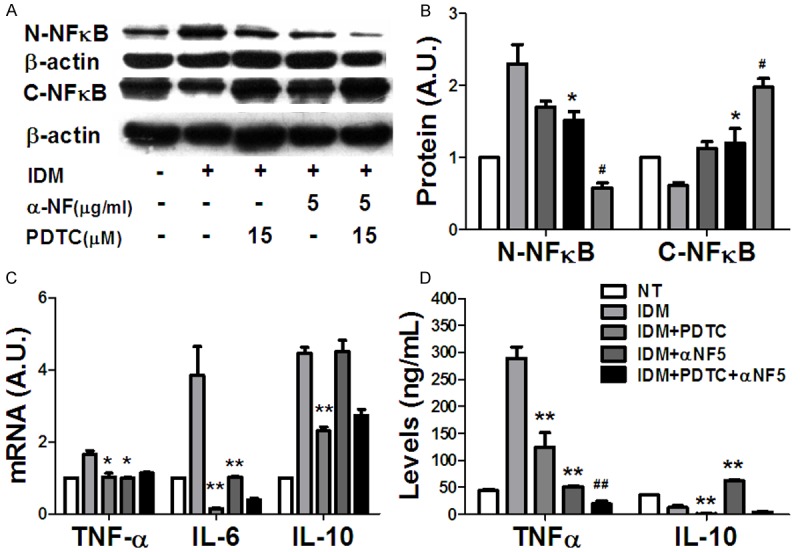
α-NF exhibited a synergistic inhibition on NF-κB activation in RAW264 macrophages with PDTC, NF-κB inhibitor. A, B: α-NF and PDTC synergistically suppressed NF-κB activation. C: α-NF exerted a synergistic inhibition on IL-6 mRNA expression with PDTC, while did not rescue IL-10 suppression. D: α-NF had a synergistic suppression on TNF-α production with PDTC, but did not reverse IL-10 secretion. *: Compared with IDM, P < 0.05; #: Compared with IDM + PDTC, P < 0.05.
α-NF pre-treated macrophages conditioned medium improves 3T3-L1 cells adipogenesis suppressed by LPS
To study the effects of α-NF in adipocyte-macrophage interaction on pre-adipocyte differentiation, we collected macrophage conditioned medium (mCM) from LPS activated RAW264 macrophages (100 ng/ml LPS, mCM) and α-NF (5 mg/ml) pre-treated activated macrophages (LPS+ α-NF, amCM) to culture 3T3-L1 cells with IDM for 8 days. Results showed that mCM from LPS activated macrophages significantly inhibited 3T3-L1 cells differentiation induced by IDM, while amCM from α-NF pre-treated activated macrophage partly improved this inhibition (Figure 4A, 4B). Real-time RT-PCR revealed that the key transcription factor PPARγ in adipogenesis was suppressed by mCM and partially rescued by α-NF pre-treatment (Figure 4C). We further observed that the pro-inflammatory cytokines (TNF-α, IL-10) expressions were up-regulated by LPS but repressed by α-NF pre-treatment in RAW264.7 macrophages (Figure 4D). ELISA revealed that α-NF production in macrophage conditioned medium was consistent with mRNA expression (Figure 4E).
Figure 4.
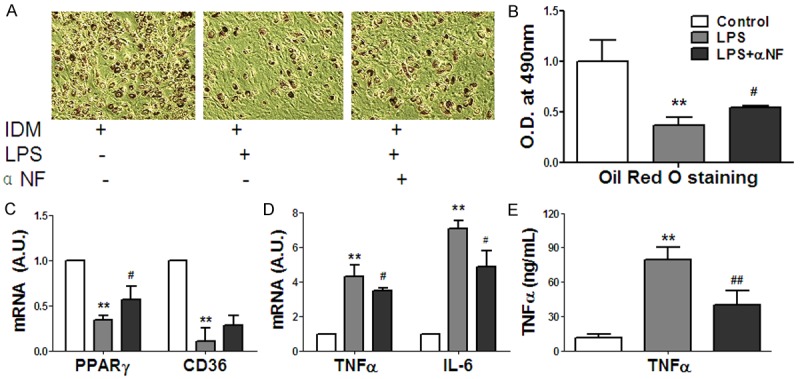
α-NF improved 3T3-L1 pre-adipocytes differentiation suppressed by LPS activated macrophage conditioned medium (mCM). A: Oil Red Staining of 3T3-L1 adipocytes with mCM for 8 days treatment. B: Quantitation of Oil Red O staining; C: Key transcription factor expression in 3T3-L1 pre-adipocyte with mCM for 48 h; D: Pro-inflammatory cytokines expression in activated macrophages RAW264. E: TNF-α production in mCM. *: Compared with control group (IDM alone), P < 0.05, **: Compared with control, P < 0.01; #: Compared with LPS group, P < 0.05.
Discussion
The present study demonstrated α-NF not only suppresses inflammatory response in 3T3-L1 adipocytes upon differentiation, also dramatically change pro-inflammatory and anti-inflammatory balance in macrophages in the presence of mature adipocytes conditioned medium. Further, α-NF suppresses IDM induced in-flammation through deactivating NFκB signaling in macrophages. Meanwhile, α-NF ameliorates the inhibition of adipogenesis by LPS activated macrophage condition medium.
These findings suggest that α-NF can suppress inflammatory response and regulate adipocyte differentiation and inflammation through a mechanism including NFκB. α-NF, as a structural analog of flavone, is an antagonist to the aromatic hydrocarbon receptor (AhR) [16] and a potent antiplatelet flavonoid [17]. α-NF is also reported to induce vasorelaxation in endothelium [14] and attenuate B[a]P-induced migration and invasion of vascular smooth muscle cells [18]. Adipose tissue inflammation has been regarded as an important central event in the initiation and maintenance of obesity via adipogenesis. α-NF suppressed inflammatory response during adipogenesis and also in macrophages which elicited by mature adipoctyes condition medium. In particular, α-NF decreased pro-inflammatory cytokines TNF-α, IL-10 expressions and increased anti-inflammatory cytokine IL-10 secretion. Pro-inflammatory cytokines, such as TNF-α and IL-6 are major regulators of adipose tissue metabolism. TNF-α can reduce lipid accumulation via inhibition of lipoprotein lipase and stimulation of hormone sensitive lipase. TNF-α also suppresses glucose uptake via GLUT4 and IRS-1. IL-10, as an anti-inflammatory cytokine, is essential for maintaining the integrity and homeostasis of adipose tissue by repressing pro-inflammatory responses and limit unnecessary tissue damage caused by inflammation.
Numerous studies suggest that cross-talk between adipocytes and macrophages promote pro-inflammatory cytokines production [19]. Macrophages in obese individual stimulated by pro-inflammatory cytokines lead to insulin resistance and macrophages block insulin action in adipocytes [20]. Further, macrophages in adipose tissue inhibit human pre-adipocytes differentiation via repression of transcriptional factors involved in adipogenesis [21]. Our finding showed that fully differentiated adipocytes CM stimulated pro-inflammatory responses in macrophage and α-NF suppressed this response in dose-dependent manner. Notably, α-NF elevated anti-inflammatory cytokines IL-10 release which repressed by aCM. The effects of α-NF on inflammatory responses in macrophages were parallel with NFκB deactivation. PDTC, as a selective NFκB inhibitor, abolished α-NF elicited IL-10 expression and secretion suggesting the important role of NFκB signaling in α-NF regulation.
TNF-α, mainly secreted by macrophages, is a strong suppressor of adipogenesis [22]. Conditioned medium from activated macrophages stimulated by LPS dramatically inhibited 3T3-L1 cells differentiation with concurrent suppression on PPARγ, in agreement with previous reports. Conditioned medium from macrophages, which pre-treated with α-NF, not only significantly suppressed TNF-α secretion, also increased PPARγ expression. However, 3T3-L1 differentiation repressed by mCM was only partially restored by α-NF pre-administration. Macrophages produce a huge variety of biomolecules including cytokines, chemokines and growth factors, it cannot be ruled out that other macrophage secreted factors may also be induced during the adipocytes-macrophages interaction. Furthermore, extracellular matrix remodeling plays a vital role in adipogenesis [23,24] and decreased expression of fibronectin is the characteristics of differential initiation in 3T3-L1 pre-adipocytes. Cytokines secreted by activated macrophage might participate in regulating fibronectin expression.
In summary, our findings demonstrated that α-NF regulates inflammation responses in adipocytes-macrophages interaction which contributes to pre-adipocytes differentiation via NFκB pathway.
Acknowledgements
This work was supported by Hubei Provincial Natural Science foundation (2012FFB04428) and Hubei Provincial Health foundation (X6B63). Suqing Wang was supported by National Natural Science Foundation of China (30972463).
Disclosure of conflict of interest
None.
References
- 1.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14:1225–1230. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- 2.Maachi M, Pieroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, Capeau J, Bastard JP. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int J Obes Relat Metab Disord. 2004;28:993–997. doi: 10.1038/sj.ijo.0802718. [DOI] [PubMed] [Google Scholar]
- 3.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2011;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 4.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Luft VC, Schmidt MI, Pankow JS, Couper D, Ballantyne CM, Young JH, Duncan BB. Chronic inflammation role in the obesity-diabetes association: a case-cohort study. Diabetol Metab Syndr. 2013;5:31. doi: 10.1186/1758-5996-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann N Y Acad Sci. 2009;892:146–154. doi: 10.1111/j.1749-6632.1999.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 7.Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology. 2007;148:868–877. doi: 10.1210/en.2006-0687. [DOI] [PubMed] [Google Scholar]
- 8.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakarai H, Yamashita A, Nagayasu S, Iwashita M, Kumamoto S, Ohyama H, Hata M, Soga Y, Kushiyama A, Asano T, Abiko Y, Nishimura F. Adipocyte-macrophage interaction may mediate LPS-induced low-grade inflammation: potential link with metabolic complications. Innate Immun. 2012;18:164–170. doi: 10.1177/1753425910393370. [DOI] [PubMed] [Google Scholar]
- 10.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Lafuente A, Guillamon E, Villares A, Rostagno MA, Martinez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 12.Guo W, Kong E, Meydani M. Dietary polyphenols, inflammation, and cancer. Nutr cancer. 2009;61:807–810. doi: 10.1080/01635580903285098. [DOI] [PubMed] [Google Scholar]
- 13.Salvamani S, Gunasekaran B, Shaharuddin NA, Ahmad SA, Shukor MY. Antiartherosclerotic effects of plant flavonoids. Biomed Res Int. 2014;2014:480258. doi: 10.1155/2014/480258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng YW, Li CH, Lee CC, Kang JJ. Alpha-naphthoflavone induces vasorelaxation through the induction of extracellular calcium influx and NO formation in endothelium. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:377–385. doi: 10.1007/s00210-003-0820-6. [DOI] [PubMed] [Google Scholar]
- 15.He Q, Huang C, Zhao L, Feng J, Shi Q, Wang D, Wang S. alpha-Naphthoflavone inhibits 3T3-L1 pre-adipocytes differentiation via modulating p38MAPK signaling. Int J Clin Exp Pathol. 2013;6:168–178. [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon YJ, Youk ES, Lee SH, Suh J, Na YJ, Kim HM. Polychlorinated biphenyl-induced apoptosis of murine spleen cells is aryl hydrocarbon receptor independent but caspases dependent. Toxicol Appl Pharmacol. 2002;181:69–78. doi: 10.1006/taap.2002.9389. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao G, Chang CY, Shen MY, Chou DS, Tzeng SH, Chen TF, Sheu JR. alpha-Naphthoflavone, a potent antiplatelet flavonoid, is mediated through inhibition of phospholipase C activity and stimulation of cyclic GMP formation. J Agric Food Chem. 2005;53:5179–5186. doi: 10.1021/jf0500738. [DOI] [PubMed] [Google Scholar]
- 18.Meng D, Lv DD, Zhuang X, Sun H, Fan L, Shi XL, Fang J. Benzo[a] pyrene induces expression of matrix metalloproteinases and cell migration and invasion of vascular smooth muscle cells. Toxicol Lett. 2009;184:44–49. doi: 10.1016/j.toxlet.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 20.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M, Poitou C, Basdevant A, Stich V, Viguerie N, Langin D, Bedossa P, Zucker JD, Clement K. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008;9:R14. doi: 10.1186/gb-2008-9-1-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 2002;51:1319–36. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima I, Yamaguchi T, Ozutsumi K, Aso H. Adipose tissue extracellular matrix: newly organized by adipocytes during differentiation. Differentiation. 1998;63:193–200. doi: 10.1111/j.1432-0436.1998.00193.x. [DOI] [PubMed] [Google Scholar]
- 24.Mori S, Kiuchi S, Ouchi A, Hase T, Murase T. Characteristic expression of extracellular matrix in subcutaneous adipose tissue development and adipogenesis; comparison with visceral adipose tissue. Int J Biol Sci. 2014;10:825–33. doi: 10.7150/ijbs.8672. [DOI] [PMC free article] [PubMed] [Google Scholar]


