Abstract
Accurate lymph nodal staging of lung cancer is critical for determining the treatment options. With the help of 18F-fluorodeoxyglucose positron emission tomography/computer tomography (18F-FDG-PET/CT), the clinician can rule out/in the regional lymph nodes positive for metastasis in the patients with lung cancer in a majority of cases. However, a small proportion of cases with false positivity of metastasis have been reported. Transbronchial needle aspirations and mediastinoscopic biopsies are still necessary to determine whether enlarged hypermetabolic mediastinal lymph nodes are positive for lung cancer metastasis. Here we report three intricate cases showing hypermetabolic activity in the mediastinal lymph nodes in the patients with pathologically diagnosed lung cancer on PET/CT. The first patient had squamous cell carcinoma in the left upper lobe of the lung with surrounding necrotizing granulomas and concurrent with silicosis and granulomatous inflammation in the lymph nodes; the second presented with symptoms of viral pneumonia, which was pathologically diagnosed as a lung adenocarcinoma, stage IA, concurrent with sarcoidosis involving the lymph nodes; the last case was diagnosed as squamous cell carcinoma in the right upper lobe of the lung, but lymph nodes showed reactive hyperplasia. These cases suggest that some cases are so complex that avid 18F-FDG uptake in the mediastinal lymph nodes in the patients with pathologically diagnosed lung cancer should be carefully analyzed based on individual patients’ clinical background.
Keywords: FDG, PET-CT, hypermetabolic, mediastinal lymph nodes, lung cancer
Introduction
Lung cancer is the leading cause of cancer death around the world [1]. Patients with non-small cell lung cancer (NSCLC), which accounts for 85-90% of all lung cancer cases, often present with advanced disease at initial diagnosis. For 2001-2007, the overall 5-year survival of this aggressive disease was only 16% in the United States [2]. For clinicians, accurate lymph nodal staging of lung cancer is a keystone for determining the treatment options. Even with the help of new technology such as 18F-fluorodeoxyglucose positron emission tomography/computer tomography (PET/CT), and transbronchial needle aspiration (TBNA) and mediastinoscopic biopsies, it still remains difficult for clinicians to make clear whether the enlarged mediastinal lymph nodes are involved by lung cancer metastasis, especially being concurrent with other diseases such as sarcoidosis, sillicosis and pulmonary tuberculosis. Here we describe three intricate cases from the First Affiliated Hospital of Soochow University, which all showed hypermetabolic activity in the mediastinal lymph nodes on PET/CT. The first presented with a clinically unresectable mass and the bronchial biopsy showed a squamous cell carcinoma. After the chemotherapy, the lobectomy was performed with lymph node staging and the final diagnosis was stage IA squamous cell carcinoma of the lung, surrounded by necrotizing granulomas and sillicosis and the lymph nodes were granulomatous inflammation and they were negative for metastasis. The second presented with symptoms of viral pneumonia, which was pathologically diagnosed as a lung adenocarcinoma, stage IA, concurrent with sarcoidosis. The last case received video-assisted thoracic surgery (VATS) biopsy. Only the sample from the mass in the right upper lobe of the lung near hilum was confirmed as squamous cell carcinoma, but none of six FDG-avid mediastinal lymph nodes showed evidence of metastasis. These cases suggest that some cases are complex and require careful differential diagnosis to develop the best-individualized treatment plan.
Case 1
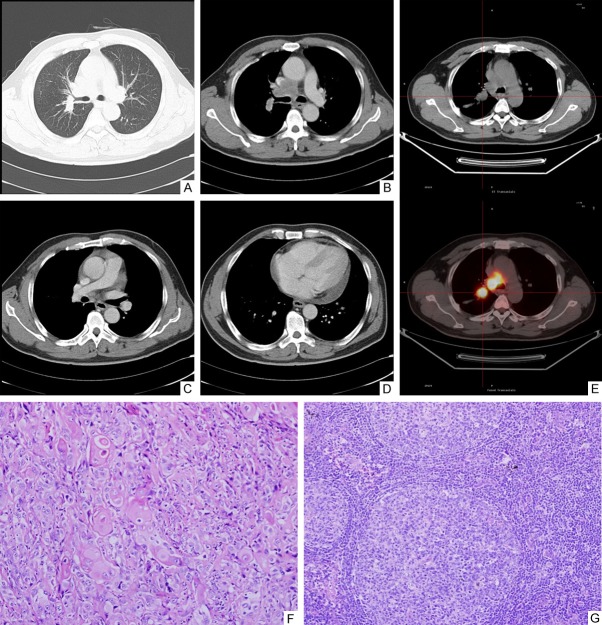
A 69-year-old male patient was admitted to the hospital in January, 2009, complaining of cough with intermittent bloody sputum for 2 months. He had a 20-pack-year smoking history, but quit 30 years ago. Nearly fifty years ago, he was diagnosed with silicosis at a local occupational hospital based on chest x-ray and three years history of being a mountain tunnel miner. He also suffered from pulmonary tuberculosis many years ago. In the recent ten years, he occasionally had seizures. The patient denied any purulent sputum, weight loss or fever. Physical examination on admission did not reveal any abnormal findings such as clubbed fingers, moist rales or palpable supraclavicular lymph nodes. His blood pressure was 126/78 mmHg, temperature 36.4°C, heart rate 82 beats/min and respiratory rate 18 breaths/min. Laboratory results showed hemoglobin 142 g/L and white blood cell count 8.69×109/L, with a differential of 62% neutrophils. Protein derivative of tuberculin was negative. Serum ferritin level was elevated to 628.2 g/L (normal, 30-400 g/L). Carbohydrate antigen 125 (CA125) was mildly elevated to 38.9 KIU/L (normal, 0.00-35.00 KIU/L) and the other serum level of tumor mark including CEA, CYFRA21-1, NSE and CA72-4 were normal. Pulmonary function tests revealed a forced expiratory volume in one second (FEV1) was 1.49 L and maximal ventilatory volume (MVV) was 56.7% of predicted values. Breath-holding test was 29.4 seconds. The parameters of arterial blood gas in room air were pH 7.44, PCO2 39 mmHg, and PO2 85 mmHg. Chest-CT performed on admission showed two masses, one (21.6 × 33.0 mm) in the right upper lobe (Figure 1A, 1B) and the other (60.8 × 48.2 mm) in the left upper lobe (Figure 1C, 1D), and multiple enlarged mediastinal lymph nodes (Figure 1D). Moreover, the size of the left mass was increased but the size of the right mass was unchanged, compared with those in the previous CT performed in the local hospital two years ago. 18F-FDG-PET/CT performed in our hospital revealed high metabolic activity in both lung masses with the maximum of standardized uptake value (SUVmax), left mass 9.7 and right mass 6.7 (Figure 1E, 1F), and enlarged mediastinal lymph nodes with SUVmax: 4.6 (Figure 1G). Magnetic resonance imaging (MRI) of the brain revealed no any abnormalities. Bronchoscopic examination showed a mass in the left upper lobe bronchial lumen, and the biopsy was performed. The pathological diagnosis is an invasive squamous cell carcinoma. Bronchial brush specimen in the right upper lobe and TBNA from the mediastinal lymph nodes showed no evidence of cancer or tuberculosis. He received two cycles of oxaliplatin-docetaxel chemotherapy pretreatment with dexamethasone and the disease was stable. Then, the patient received the left upper lobe lobectomy and lymph node biopsies from left 5, 6, 7, and 10 stations. To our surprise, moderately differentiated squamous cell carcinoma (Figure 1H), 1.4 cm in greatest dimension, was located within the left upper lobe bronchus with invasion into the submucosa whereas the lesion surrounding the left upper lobe bronchus showed areas of necrotizing granulomas (Figure 1I) and conglomerate silicosis (Figure 1J). The biopsied mediastinal lymph nodes showed granulomatous inflammation without tumor metastasis (Figure 1K). This patient was finally diagnosed as stage IA (T1aN0M0) lung squamous cell carcinoma, being concurrent with silicosis and necrotizing granulomas. Although the acid-fast bacillus (AFB) and Grocott’s methenamine silver (GMS) stains were negative, the recurrent active tuberculosis (TB) was considered based on the clinical history of TB. The patient received systemic anti-tuberculosis treatment, but without any further chemotherapy or radiation therapy. The patient was healthy until the recurrence of the tumor in the left lower lobe of the lung 56 months later.
Figure 1.
Imaging studies and pathologic findings in case 1. Chest-CT (A and C, lung window; B and D, mediastinal window) shows small nodules (A, C), two masses with calcifications, one (21.6 × 33.0 mm) in the right upper lobe (A, B) and the other (60.8 × 48.2 mm) in the left upper lobe (C, D) and enlarged mediastinal lymph nodes with calcifications (D). PET-CT shows accumulation of FDG in both masses (SUVmax: left mass 9.7; right mass 6.7) (E, F) and enlarged mediastinal lymph nodes (SUVmax: 4.6) (G). Histological section from the left upper lobe bronchus shows squamous cell carcinoma with focal invasion into the submucosa (original magnification 100 ×) (H). Section obtained from the lesion surrounding the bronchus in the left upper lobe shows necrotizing granulomas (original magnification 100 ×) (I). Section from the lesion surrounding the left upper lobe bronchus shows conglomerate silicosis (original magnification × 100) (J). Section of the mediastinal lymph node at the left 10 station shows granulomatous inflammation (original magnification, 100 ×) (K).
Case 2
A 51-year-old female patient was admitted to the hospital in January, 2010 because of ground glass opacity in the left lung discovered on a routine chest X-ray. Chest-CT confirmed a 25.0 × 19.2 mm area with patchy opacity in the left upper lobe (Figure 2A) and multiple enlarged mediastinal lymph nodes (Figure 2B). She also had fever, dry cough and mild nasal discharge during that time. Her temperature was 37.8°C. But no respiratory rales were detected. Laboratory results showed hemoglobin 145 g/L and white blood cell 4.72 × 109/L, with a differential of 64% neutrophils. Tumor markers including CEA were normal. At that time, China experienced outbreaks of respiratory illness caused by 2009 pandemic influenza A (H1N1) virus, real-time reverse transcriptase polymerase-chain-reaction (RT-PCR) was performed on her nasopharyngeal brush specimen, and the results indicated that she was infected by H1N1 virus. She received anti-infection treatment including moxifloxacin hydrochloride and oseltamivir for two weeks. After treatment, she did not have fever but a 2 week follow-up chest-CT showed the lesion in the left upper lobe that was not absorbed at all. Sarcoidosis, tuberculosis and cancer of the lung were considered in the differential diagnoses. She received further examination. Angiotensin-converting enzyme blood levels and 24-hour urine calcium were normal. PET/CT scan showed high metabolic activity in the left upper lobe lesion (SUVmax: 2.5) (Figure 2C) and mediastinal lymph nodes (SUVmax: 3.9) (Figure 2D). Transbronchial lung biopsy from the left upper lobe lesion and transbronchial needle aspirations from mediastinal lymph nodes were performed and all the samples were negative for malignancy. As the pulmonary function tests were normal, this patient received video-assistant thoracoscopic surgery (VATS) wedge biopsy for a definite diagnosis. In addition, mediastinoscopic biopsies were done. The frozen sections showed a lung adenocarcinoma in the left upper lobe and non-necrotizing granuloma in lymph nodes at station 5 and 10 of the left side. The left upper lobe lobectomy was performed following the frozen diagnosis. Postoperative histopathological examination showed a moderately differentiated acinar adenocarcinoma, moderately differentiated (Figure 2E). The mediastinal nodes showed non-necrotizing granulomas, compatible with sarcoidosis (Figure 2F). Special stains including AFB and GMS stains were negative for microorganisms. Finally, she was diagnosed as stage IA (T1aN0M0) lung adenocarcinoma, being concurrent with sarcoidosis and the upper respiratory tract infection by H1N1 virus. After the operational procedure, she received four cycles of cisplatin-docetaxel chemotherapy pretreatment with dexamethasone because the mediastinal lymph nodes were not completely staged. Now she is healthy and the enlargement of mediastinal lymph nodes has decreased.
Figure 2.
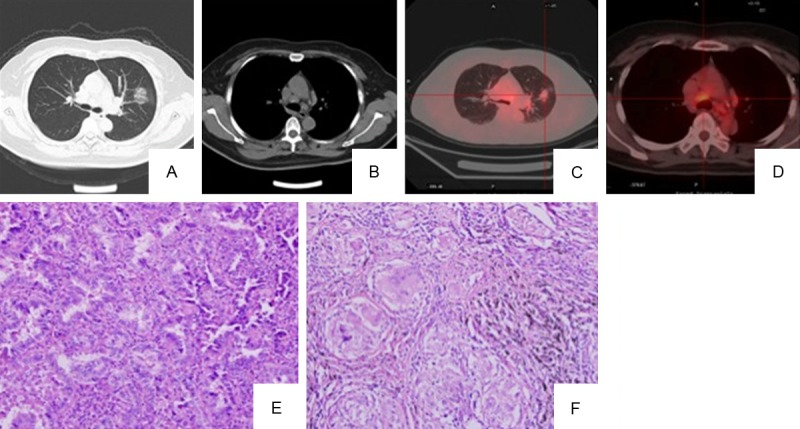
Imaging studies and pathologic findings in case 2. Chest-CT shows 25.0 × 19.2 mm ground glass opacity in the left upper lobe (A, lung window) and multiple enlarged mediastinal lymph nodes (B, mediastinal window). PET-CT shows high metabolic activity in the left upper lobe lesion (C, SUVmax: 2.5) and mediastinal lymph nodes (D, SUVmax: 3.9). Histological sections reveal moderately differentiated acinar adenocarcinoma in left upper lobe of the lung (E, original magnification 200 ×) and non-necrotizing granulomas in mediastinal nodes (F, original magnification, 200 ×).
Case 3
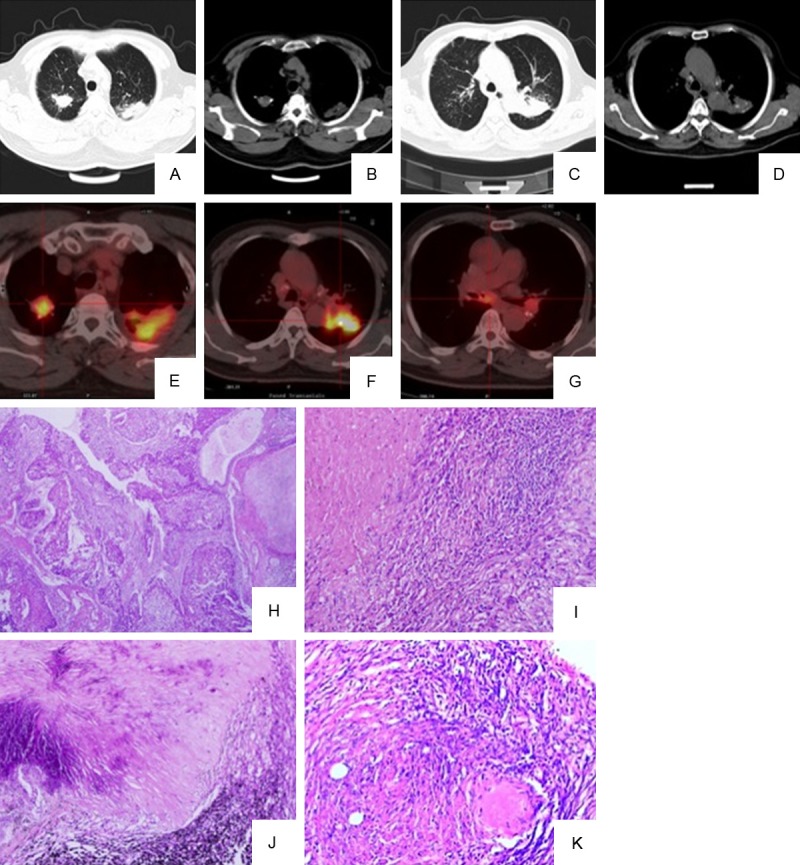
A 53-year-old male patient was admitted in the hospital in August, 2010, complaining of cough with a little white mucus sputum. He had a 20-pack-year smoking history, but quit 10 years ago. In October, 2007, he received cryoablation for treatment of the left facial skin squamous cell carcinoma. In September, 2008, he received the left neck lymph node dissection because of squamous cell carcinoma metastasis. The patient denied fever, hemoptysis or weight loss. Physical examination on admission showed surgical scars on the left maxillary and neck skin, respectively, but no other abnormal findings such as palpable supraclavicular lymph node or moist rales in the lung. Tumor markers and blood biochemistry examination were normal. Purified protein derivative of tuberculin test, rheumatoid factor and anti-nuclear antibody were negative. Chest-CT scan on August 19, 2010 showed a 23.3 × 21.0 mm mass in the right upper lobe near the hilum, pericardial effusion and multiple enlarged mediastinal lymph nodes (Figure 3A-D). There were no abnormalities identified in the abdomen by ultrasound examination. Two separate bronchoscopic examinations revealed mucosal granularity in the right bronchus near the carina, and narrow entrance of posterior segment of the right upper lobe bronchus. The samples from these mucosal lesions showed squamous metaplasia; no carcinoma was found in the samples obtained from subcarinal and right paratracheal lymph nodes by TBNA. Biopsies from the mediastinal lymph nodes were performed. The enlarged right station 4, lower paratracheal lymph node was negative for malignancy. The patient was discharged for recovery. He was readmitted after a month. PET/CT was performed on October 21, 2010 showed hypermetabolic activity in the right upper lobe mass (SUVmax: 21.7) and mediastinal lymph nodes (SUVmax: 30.0) (Figure 3E). VATS biopsy from the mass in the right upper lobe and mediastinoscopic biopsies from the lymph nodes at the right station 4 and 7 were performed. Finally, he was diagnosed as a moderately differentiated squamous cell carcinoma in the right upper lobe (Figure 3F) but hypermetabolic mediastinal lymph nodes were negative for metastasis and they all showed reactive hyperplasia (Figure 3G). Metastatic disease was clinically considered because the patient had pericardial effusion and extremely hypermetabolic mediastinal lymph nodes. The patient received four cycles of cisplatin-gemcitabine chemotherapy and local radiation therapy. He died of massive airway hemoptysis caused by local progression nine months after diagnose.
Figure 3.
Imaging studies and pathologic findings in case 3. Chest-CT shows a mass in the right upper lobe of the lung near the hilum (A, lung window; B, mediastinal window), mutiple enlarged mediastinal lymph nodes (B and C, mediastinal window) and pericardial effusion (D, mediastinal window). PET-CT shows accumulation of FDG in the right upper mass (SUVmax: 21.7) and mediastinal lymph nodes (SUVmax: 30.0) (E). Histological sections show invasive moderately differentiated squamous cell carcinoma in the right upper lobe near the hilum (original magnification, 200 ×) (F) and reactive hyperplasia in FDG-avid mediastinal lymph nodes (G) (original magnification, 200 ×).
Discussion
Staging of NSCLC is important for determining the options of treatment and prognosis. PET-CT has emerged as a more accurate non-invasive staging modality in detecting mediastinal lymph node metastases from NSCLC than chest-CT [3], but some benign pulmonary diseases, such as granulomatous disease, silicosis, calcification and fibrosis could be accompanied by false 18F-FDG avid uptake in benign lymph nodes [4,5]. These lymph nodes might be mistaken as being positive for metastasis when such benign pulmonary diseases and lung cancer are co-existent in the same patient. Therefore, if there is positive PET/CT finding in the mediastinal lymph nodes they should be biopsied for pathologic examination, as recommended by the National Comprehensive Cancer Network (NCCN) guidelines. Conventional TBNA or direct real-time endobronchial ultrasound (EBUS)-guided TBNA and mediastinoscopic biopsy make it possible for clinicians to confirm the pathologic status of mediastinal lymph nodes. However, due to one or several underlying diseases, it still remains challenging for clinicians to confirm mediastinal lymph node involvement in the metastasis of lung cancer in intricate cases, even with the help of these technologies.
Silicosis is a common occupational disease caused by inhaling tiny bits of crystalline silicon dioxide or silica [6]. The characteristic radiologic features in the simple silicosis are multiple small round opacities and often symmetrically distributed with upper-zone predominance [7]. Chronic silicosis usually develops after 10 years exposure. Enlargement of hilar and mediastinal lymph nodes is common, and these lymph nodes can become calcified, sometimes in an egg-shell pattern [6,7]. Several disorders, including mycobacterial infection, airway obstruction and lung cancer are associated with silica exposure [8-13]. Occasionally, TB and lung cancer may be complicated by silicosis in the same patient. And moreover, some patients with silicosis progress to massive fibrosis which could mimic complications of TB or lung cancer, as the size of the lesion increases over time. In our patient (case 1), the mass in the left upper lobe was increased in size, and was considered active TB in the resected lung specimen TB occurred before neoadjuvant chemotherapy, so it was not likely complicated by chemotherapy. This patient also showed the enlargement of hilar and mediastinal lymph nodes and the mass in the right upper lobe with FDG-avidity, but the sizes didn’t increase over time. These findings supported that the enlargement of lymph nodes and the mass in the right upper lobe were caused by silicosis or TB, not metastasis of lung cancer. The cytological samples from the right upper lobe and mediastinal lymph nodes showed no evidence of cancer. All these evidences make the decision of surgical resection the tumor. This case suggests that every silicosis patient with suspected lung cancer should be excluded for the coexistence of tuberculosis, and status of the enlarged mediastinal lymph node should be evaluated.
Sarcoidosis is a multisystem disorder characterized by non-necrotizing granulomas and pulmonary and mediastinal involvement is extremely common. Pulmonary sarcoidosis may manifest variably from mediastinal and bilateral hilar lymphadenopathy to parenchymal opacities or fibrosis on chest imaging at difference stages [14]. Some subtypes of lung adenocarcinoma, such as adenocarcinoma in situ, also can manifest as solitary or multifocal nonsolid or bubble-like opacities on chest-CT [15,16], which could be mistaken as sarcoidosis if there is co-existence of these two diseases in the same patient. Sarcoid reactions in the regional lymph nodes also have been reported in lung cancer [17], but sarcoid reactions in lymph node couldn’t stop cancer metastasis [17,18]. The lymph nodes involved by sarcoidosis can be hypermetabolic and some authors even reported that osteolytic or osteosclerotic lesions in vertebrae and femurs caused by sarcoidosis showed increased FDG uptake on fused PET/CT [19,20]. These mixed clinical manifestations make clinicians difficultly interpret the status of enlarged and hypermetabolic mediastinal lymph nodes. Our patient (case 2) first presented as viral pneumonia, but this didn’t explain the multiple enlarged hilar and mediastinal lymph nodes. After two weeks’ treatment with oseltamivir, she recovered from fever and cough, but the patchy opacity in the left upper lobe remained unchanged. The findings raised the suspicion of sarcoidosis, lung cancer or lymphoma. As the diagnosis could not be confirmed by bronchoscopy, the patient received VATS wedge biopsy. Fortunately, there was no evidence of tumor metastasis in lymph nodes, and the patient received diagnostic wedge resection and lymph node staging. This case suggests that if a new patchy opacity or ground glass opacity appears in the lung of a sarcoidosis patient, especially a focal lesion; we cannot assume that it has progressed into sarcoidosis stage 2. It also could represent early stage lung cancer.
The third patient (case 3), was even more difficult to diagnose. The primary clinical diagnosis of this patient was facial skin squamous cell carcinoma metastasis to mediastinal lymph nodes. He had a history of facial skin squamous cell carcinoma and multiple enlarged mediastinal lymph nodes. To our surprise, only the sample from the mass in right upper lobe was confirmed as squamous cell carcinoma. All the samples obtained from mediastinal lymph nodes revealed reactive hyperplasia, although they all showed high 18F-FDG uptake on PET-CT images. These findings were considered false negative [21] because avid 18F-FDG uptake with SUVmax 30 is extremely unusual in the reactive lymph nodes.
In conclusion, we present three intricate NSCLC cases which all showed hypermetabolic lymphadenopathy with false-positive or false-negative FDG-PET findings. These findings indicated FDG-PET findings should be carefully evaluated and this process will help us obtain an accurate lung cancer staging and choose an optimal treatment for the patients.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Wahl RL, Quint LE, Greenough RL, Meyer CR, White RI, Orringer MB. Staging of mediastinal non-small cell lung cancer with FDG PET, CT, and fusion images: preliminary prospective evaluation. Radiology. 1994;191:371–377. doi: 10.1148/radiology.191.2.8153308. [DOI] [PubMed] [Google Scholar]
- 4.Shim SS, Lee KS, Kim BT, Chung MJ, Lee EJ, Han J, Choi JY, Kwon OJ, Shim YM, Kim S. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–1019. doi: 10.1148/radiol.2363041310. [DOI] [PubMed] [Google Scholar]
- 5.Kim BT, Lee KS, Shim SS, Choi JY, Kwon OJ, Kim H, Shim YM, Kim J, Kim S. Stage T1 non-small cell lung cancer: preoperative mediastinal nodal staging with integrated FDG PET/CT--a prospective study. Radiology. 2006;241:501–509. doi: 10.1148/radiol.2412051173. [DOI] [PubMed] [Google Scholar]
- 6.Leung CC, Yu IT, Chen W. Silicosis. Lancet. 2012;379:2008–2018. doi: 10.1016/S0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- 7.Kim KI, Kim CW, Lee MK, Lee KS, Park CK, Choi SJ, Kim JG. Imaging of occupational lung disease. Radiographics. 2001;21:1371–1391. doi: 10.1148/radiographics.21.6.g01nv011371. [DOI] [PubMed] [Google Scholar]
- 8.Rees D, Murray J. Silica, silicosis and tuberculosis. Int J Tuberc Lung Dis. 2007;11:474–484. [PubMed] [Google Scholar]
- 9.teWaternaude JM, Ehrlich RI, Churchyard GJ, Pemba L, Dekker K, Vermeis M, White NW, Thompson ML, Myers JE. Tuberculosis and silica exposure in South African gold miners. Occup Environ Med. 2006;63:187–192. doi: 10.1136/oem.2004.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrlich RI, Myers JE, te Water Naude JM, Thompson ML, Churchyard GJ. Lung function loss in relation to silica dust exposure in South African gold miners. Occup Environ Med. 2011;68:96–101. doi: 10.1136/oem.2009.048827. [DOI] [PubMed] [Google Scholar]
- 11.Rushton L. Chronic obstructive pulmonary disease and occupational exposure to silica. Rev Environ Health. 2007;22:255–272. doi: 10.1515/reveh.2007.22.4.255. [DOI] [PubMed] [Google Scholar]
- 12.Brown T. Silica exposure, smoking, silicosis and lung cancer--complex interactions. Occup Med (Lond) 2009;59:89–95. doi: 10.1093/occmed/kqn171. [DOI] [PubMed] [Google Scholar]
- 13.Lacasse Y, Martin S, Gagne D, Lakhal L. Dose-response meta-analysis of silica and lung cancer. Cancer Causes Control. 2009;20:925–933. doi: 10.1007/s10552-009-9296-0. [DOI] [PubMed] [Google Scholar]
- 14.Criado E, Sanchez M, Ramirez J, Arguis P, de Caralt TM, Perea RJ, Xaubet A. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics. 2010;30:1567–1586. doi: 10.1148/rg.306105512. [DOI] [PubMed] [Google Scholar]
- 15.Austin JH, Garg K, Aberle D, Yankelevitz D, Kuriyama K, Lee HJ, Brambilla E, Travis WD. Radiologic implications of the 2011 classification of adenocarcinoma of the lung. Radiology. 2013;266:62–71. doi: 10.1148/radiol.12120240. [DOI] [PubMed] [Google Scholar]
- 16.Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology. 2009;253:606–622. doi: 10.1148/radiol.2533090179. [DOI] [PubMed] [Google Scholar]
- 17.Tomimaru Y, Higashiyama M, Okami J, Oda K, Takami K, Kodama K, Tsukamoto Y. Surgical results of lung cancer with sarcoid reaction in regional lymph nodes. Jpn J Clin Oncol. 2007;37:90–95. doi: 10.1093/jjco/hyl141. [DOI] [PubMed] [Google Scholar]
- 18.Aoki K, Yoshimura K, Hoashi S, Ushio T, Tai H, Itsubo K, Takagi K, Okano H. [Lung cancer with a sarcoid-like reaction in the primary tumor] . Nihon Kyobu Shikkan Gakkai Zasshi. 1997;35:466–470. [PubMed] [Google Scholar]
- 19.Prabhakar HB, Rabinowitz CB, Gibbons FK, O’Donnell WJ, Shepard JA, Aquino SL. Imaging features of sarcoidosis on MDCT, FDG PET, and PET/CT. AJR Am J Roentgenol. 2008;190:S1–6. doi: 10.2214/AJR.07.7001. [DOI] [PubMed] [Google Scholar]
- 20.Baldini S, Pupi A, Di Lollo S, Marchionni N, Shraim R, Bosi A. PET positivity with bone marrow biopsy revealing sarcoidosis in a patient in whom bone marrow metastases had been suspected. Br J Haematol. 2008;143:306. doi: 10.1111/j.1365-2141.2008.07288.x. [DOI] [PubMed] [Google Scholar]
- 21.Konishi J, Yamazaki K, Tsukamoto E, Tamaki N, Onodera Y, Otake T, Morikawa T, Kinoshita I, Dosaka-Akita H, Nishimura M. Mediastinal lymph node staging by FDG-PET in patients with non-small cell lung cancer: analysis of false-positive FDG-PET findings. Respiration. 2003;70:500–506. doi: 10.1159/000074207. [DOI] [PubMed] [Google Scholar]