Abstract
Objective: Long non-coding RNAs (lncRNAs) play important roles in diverse biological processes, such as transcriptional regulation, cell growth and tumorigenesis. However, little was known about whether lncRNA HIF 1 alpha-antisense RNA 1 (HIF1a-AS1) in regulating the proliferation and apoptosis of VSMCs in vitro and the expression of HIF1a-AS1 in serum of TAA patients. Methods: The cell viability was detected by the CCK8 assay. The cell apoptosis was assessed by annexin V-PI double-labeling staining. Expression of genes and proteins were analyzed by real-time PCR and western blotting respectively. Cells were transfected with siRNAs as a gene silencing methods. Results: In serum of TAA patients, the expression of HIF1a-AS1 was significantly increased (superior to 6 folds) compared to the normal control. Moreover, PA induced cell apoptosis in VSMCs in a time- and dose-dependent manner, and the proportion of the apoptotic cells had gained as compared to untreatment group. PA also induced upregulation expression of HIF1a-AS1. We also found that transfection of cells with HIF1a-AS1 siRNA decreased the expression of caspase3 and caspase8 and increased the expression of Bcl2, and protected PA-induced cell apoptosis in VSMCs. Conclusions: HIF1a-AS1 was overexpressed in the thoracoabdominal aorta aneurysm and the interaction between HIF1a-AS1 and apoptotic proteins plays a key role in the proliferation and apoptosis of VSMCs in vitro, which may contribute to the pathogenesis of thoracoabdominal aorta aneurysm.
Keywords: Thoracoabdominal aorta aneurysm, HIF 1alpha-antisense RNA 1, vascular smooth muscle cells, long non-coding RNA
Introduction
Aneurysm of the thoracoabdominal aorta (TAA) is relatively uncommon in the spectrum of aneurysmal disease, accounting for only 3% of diagnosed aneurysms in the United States [1]. Currently, the incidence and operations of thoracoabdominal aortic aneurysms have significantly increased. The indications for repair are considered to be a diameter of 6 cm or more and 5.5 cm for patient groups with increased risk of rupture [2]. Complex open surgical repair is associated with significant mortality and complication rates. The endovascular approach has evolved to be a good and predominant alternative to open repair of these aneurysms for older and high-risk patients as well as for aneurysms with optimal morphological suitability [3]. Nevertheless, the advances in effective therapy for TAA have been limited because the pathological mechanisms causing tumor are not known. Therefore, revealing the molecular mechanism for the TAA is indispensable for developing effective treatment.
The aortic media is mainly composed of vascular smooth muscle cells (VSMCs), which are the main source of extracellular matrix proteins such as collagen and elastin. VSMCs associated with the extracellular matrix largely determine the biomechanical properties of the aortic wall [4]. Increased apoptosis of VSMCs observed in the aortic wall of patients with TAAs is considered to be an important cause for TAA [5]. Functional research shows that Ang II induces calpain-1 expression in the aortic walls in vivo and ex vivo and VSMC in vitro. The Ang II mediated, age-associated increased MMP2 activity and migration in VSMC. Over-expression of calpain-1 in young VSMC results in cleavage of intact vimentin, and an increased migratory capacity mimicking that of old VSMC [6]. The above research results show that the functional dysfunction of VSMCs may be correlated with cardiovascular diseases and cancers.
Eukaryotic genomes encode numerous long non coding RNAs (LncRNAs), which is defined as endogenous cellular RNAs with length longer than 200 nucleotides, but lack open reading frames of significant length [7]. Within 4 years, the number of identified lncRNA genes increase more than 8000 [8]. Although the function of most lncRNAs is still unknown, their increasing numbers and the accumulating evidence for their involvement in many biologic processes provide compelling arguments in support of the dysregulation of lncRNAs has been correlated to cancer development, invasion and metastasis in the malignant cell [8-10] (Table 1). To date, the underlying mechanisms for lcnRNAs regulation VSMCs proliferation and apoptosis are quite limited.
Table 1.
Disease-related LncRNA in human
| Symbol | Cancers | Chromosome location | Start | End | Strand | Species | Alias | NCBI No. | |
|---|---|---|---|---|---|---|---|---|---|
| 7SK | Cancer | chr6 | 52860418 | 52860749 | + | Human | RN7SK; 7SK | NR_001445.2 | 22377309 |
| BCAR4 | Breast cancer | chr16 | 11913687 | 11922689 | - | Human | BCAR4 | NR_024049.1 | 21506106 |
| BCYRN1 | Breast cancer | chr2 | 47562454 | 47562653 | + | Human | BCYRN1; BC200; BC200a; LINC00004; NCRNA00004 | NR_001568.1 | 15240511 |
| BOK-AS1 | Testicular cancer | chr2 | 242483799 | 242498558 | - | Human | BOK-AS1; BOKAS; NAToB; BOK-AS; NCRNA00151 | NR_033346.1 | 19287972 |
| C1QTNF9B-AS1 | Prostate cancer | chr13 | 24463028 | 24466242 | + | Human | C1QTNF9B-AS1; PCOTH | BC073902 | 15930275 |
| CASC2 | Endometrial cancer | chr10 | 119806332 | 119969665 | + | Human | CASC2; C10orf5 | NR_026939.1 | 15024726 |
| CBR3-AS1 | Prostate cancer | chr21 | 37504065 | 37528606 | - | Human | CBR3-AS1; PlncRNA-1 | NR_038892.1 | 22264502 |
| CCAT1 | Colorectal cancer | chr8 | 128219629 | 128231724 | - | Human | CCAT1 | XR_108886.3 | 23416875 |
| CDKN2B-AS1 | Breast cancer | chr9 | 21994790 | 22121096 | + | Human | CDKN2B-AS1; ANRIL; p15AS; CDKN2BAS; CDKN2B-AS; NCRNA00089; RP11-145E5.21 | NR_003529.20 | 17440112 |
| DNM3OS | Ovarian cancer | chr1 | 172107724 | 172113975 | - | Human | DNM3OS; DNM3-AS1; MIR199A2HG | NR_038397.1 | 20400975 |
| DSCAM-AS1 | Breast cancer | chr21 | 41755010 | 41757285 | + | Human | DSCAM-AS1; M41 | NR_038896.1 | 12177779 |
| EPB41L4A-AS1 | Cancer | chr5 | 111496223 | 111498198 | + | Human | EPB41L4A-AS1; TIGA1; C5orf26; NCRNA00219 | NR_015370.2 | 16973895 |
| GAS5 | Breast cancer | chr1 | 173833039 | 173837125 | - | Human | GAS5; SNHG2; NCRNA00035 | NR_002578.7 | 20673990 |
| H19 | Bladder cancer | chr11 | 2016406 | 2019065 | - | Human | H19; ASM; BWS; WT2; ASM1; PRO2605; D11S813E; LINC00008; NCRNA00012 | NR_002196.5 | 11193051 |
| HIF1A-AS1 | Kidney cancer | chr14 | 62147759 | 62162536 | - | Human | HIF1A-AS1; 5’aHIF-1A | 21897117 | |
| HOTAIR | Breast cancer | chr12 | 54356096 | 54362515 | - | Human | HOTAIR; HOXAS; HOXC-AS4; HOXC11-AS1; NCRNA00086 | NR_047517.15 | 19182780 |
| IGF2-AS | Prostate cancer | chr11 | 2161758 | 2169896 | + | Human | IGF2-AS; PEG8; IGF2AS; IGF2-AS1 | NR_028044.1 | 19767753 |
| KCNQ1OT1 | Colorectal cancer | chr11 | 2661768 | 2721228 | - | Human | “KCNQ1OT1; LIT1; KvDMR1; KCNQ10T1; KCNQ1-AS2; KvLQT1-AS; NCRNA00016 | 16965397 | |
| lincRNAp21 | Lung cancer | N/A | N/A | N/A | N/A | Human | N/A | N/A | 22535282 |
| LSINCT5 | Breast cancer | N/A | N/A | N/A | N/A | Human | N/A | N/A | 21532345 |
| MALAT1 | Cancer | chr11 | 65265233 | 65273940 | + | Human | MALAT1; HCN; NEAT2; MALAT-1; PRO2853; LINC00047; NCRNA00051 | NR_002819.6 | 20711585 |
| MEG3 | Bladder cancer | chr14 | 101292445 | 101327363 | + | Human | MEG3; GTL2; FP504; prebp1; PRO0518; PRO2160; LINC00023; NCRNA00028 | NR_002766.7 | 14602737 |
| MIR31HG | Breast cancer | chr9 | 21454267 | 21559697 | - | Human | MIR31HG | NR_027054.1 | 22289355 |
| PCA3 | Prostate cancer | chr9 | 79379354 | 79402465 | + | Human | PCA3; DD3; NCRNA00030 | NR_015342.12 | 18602209 |
| PCAT1 | Prostate cancer | chr8 | 128025399 | 128033259 | + | Human | PCAT1; PCAT-1 | NR_045262.1 | 21804560 |
| PCGEM1 | Prostate cancer | chr2 | 193614571 | 193641625 | + | Human | PCGEM1; LINC00071; NCRNA00072 | NR_002769.2 | 16515751 |
| PCNCR1 | Prostate cancer | N/A | N/A | N/A | N/A | Human | N/A | N/A | 22535282 |
| PVT1 | Breast cancer | chr8 | 128806779 | 129113499 | + | Human | PVT1; LINC00079; NCRNA00083 | NR_003367.6 | 17908964 |
| RRP1B | Cancer | chr21 | 45079432 | 45115960 | + | Human | RRP1B; Nnp1; RRP1; NNP1L; KIAA0179 | NM_015056.2 | 19710015 |
| SRA1 | Breast cancer | chr5 | 139929653 | 139937678 | - | Human | SRA1; SRA; SRAP; STRAA1; pp7687 | NM_001035235.6 | 20079837 |
| TDRG1 | Testicular cancer | chr6 | 40346163 | 40347631 | + | Human | TDRG1; LINC00532 | NR_024015.1 | 21243750 |
| UCA1 | Bladder cancer | chr19 | 15939757 | 15946230 | + | Human | UCA1; CUDR; LINC00178; NCRNA00178 | NR_015379.3 | 20117985 |
| WRAP53 | Cancer | chr17 | 7589389 | 7606820 | + | Human | WRAP53; DKCB3; TCAB1; WDR79 | NM_001143990.1 | 21441950 |
| XIST | Breast cancer | chrX | 73040495 | 73072588 | - | Human | XIST; SXI1; swd66; DXS1089; DXS399E; LINC00001; NCRNA00002 | NR_001564.3 | 17545591 |
| Yiya | Cancer | chr1 | 214098092 | 214099997 | + | Human | LINC00538; Yiya | NR_046189.1 | 22258142 |
| ZNFX1-AS1 | Breast cancer | chr20 | 47894715 | 47905797 | + | Human | ZNFX1-AS1; HSUP1; HSUP2; ZFAS1; C20orf199; NCRNA00275 | NR_003604.2 | 21460236 |
In this study, we performed a hierarchical cluster analysis of the differentially expressed Lnc-RNA in the serum of TAA patients to identify the lncRNA HIF 1 alpha-antisense RNA 1 (HIF1A-AS1) that associated with Lnc-RNA expression. We also investigated the role of HIF1A-AS1 in vitro in regulating the proliferation and apoptosis of aortic VSMCs.
Materials and methods
Serum samples and cell culture
Human serum samples were obtained with written informed consent from The Fourth Hospital of Hebei Medical University. The study was approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University. 50 serum samples of TAA patients and 50 cases of normal control group were collected between 02/2010 and 12/2014.
The vascular smooth muscle cells (VSMCs) were maintained in RPMI-1640 (Invitrogen, USA) supplemented with 10% FBS (Invitrogen, USA) at 37°C in a humidified incubator (Thermo, USA), 5% CO2, 95% air atmosphere. The medium was replenished every day. Confluent cells were treated with various concentrations of palmitic acid (Sigma, USA).
Cell viability detection by CCK8
VSMCs (5.0×103/well) were plated and treated in 96-well plates (three wells per group) with various concentrations of palmitic acid (0, 0.2, 0.4 or 0.8 mM) for 24 h, 48 h or 72 h respectively. 10 μL of CCK8 (Beyotime, China) was added to the cells, and the OD value of the cells was measured at 450 nm using an ELISA reader (BioTek, USA) according to the manufacturer’s instructions.
Quantification of apoptosis by flow cytometry
Apoptosis was assessed using annexin V, a protein that binds to phosphatidylserine (PS) residues which are exposed on the cell surface of apoptotic cells. VSMCs (5.0×105/well, 1 ml) were plated and treated in 6-well plates (three wells per group). After treatment, cells were washed twice with PBS (pH=7.4), and re-suspended in staining buffer containing 10 μl PI and 5 μl annexin V-FITC. Double-labeling was performed at room temperature for 15 min in the dark before the flow cytometric analysis. Cells were immediately analyzed using FACScan and the Cellquest program (Becton Dickinson). Quantitative assessment of apoptotic cells was also assessed by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) method, which examines DNA-strand breaks during apoptosis by using BD ApoAlertTM DNA Fragmentation Assay Kit. The cells were trypsinized, fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton-X-100 in 0.1% sodiumcitrate. After being washed, the cells were incubated with the reaction mixture for 60 min at 37°C. The stained cells were then analyzed with flow cytometer.
Quantitative real-time PCR
VSMCs (5.0×105/well) were plated and treated in 6-well plates (three wells per group) after 24 h with treatment for 48 h. The VSMCs RNA extraction was performed according to the TRIzol manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Synthesis of cDNAs was performed by reverse transcription reactions with 2 μg of total RNA using moloney murine leukemia virus reverse transcriptase (Promega, Switzerland) with oligo dT (15) primers (Fermentas) as described by the manufacturer. The first strand cDNAs served as the template for the regular polymerase chain reaction (PCR) performed using a DNA Engine (ABI 9700). The cycling conditions were 2-min polymerase activation at 95°C followed by 40 cycles at 95°C for 15 s and 55°C for 60 s. PCR with the following primers: as shown in Table 2. U6 as an internal control was used to normalize the data to determine the relative expression of the target genes. The reaction conditions were set according to the kit instructions. After completion of the reaction, the amplification curve and melting curve were analyzed. Gene expression values are represented using the 2-ΔΔct method.
Table 2.
PCR primers used in this study
| Symbol | Forward | Reverse |
|---|---|---|
| 7SK | AAACAAGCTCTCAAGGTC | CCTCATTTGGATGTGTCT |
| BCAR4 | GGACTCATTGTTGTTCTAC | ACCTATGGCTATCATTGTT |
| BCYRN1 | CTGGGCAATATAGCGAGAC | TGCTTTGAGGGAAGTTACG |
| BOK-AS1 | CTTGGCAGTTCTGATTGTG | TTGTCCGCTGTGGATAAG |
| C1QTNF9B-AS1 | AGACACCTGAACATTCCT | CTGAGCAAGTTTCCTTCTT |
| CASC2 | CTATTCCGAGTAAGAAGTG | TCTGTGTTGATGTTGATT |
| CBR3-AS1 | CTTCTGGTTACAATGATTCTC | CACTTACTGCCTACATTAGA |
| CDKN2B-AS1 | TCATCATCATCATCATCATC | TGCTTCTGTCTCTTCATA |
| DNM3OS | ATAGAGCAAGTCTGGATT | GGATGAGGCAATAACATT |
| DSCAM-AS1 | ACTAGCACAGATGGCATTC | CAGGAAGCATCGTGAACA |
| EPB41L4A-AS1 | TAAGACAGTGAGGATGTGAAT | ATTATGGTGACAGCAGTGA |
| GAS5 | CACAGGCATTAGACAGAA | AGGAGCAGAACCATTAAG |
| H19 | CTCCACGACTCTGTTTCC | TCTCCACAACTCCAACCA |
| HIF1A-AS1 | AATGTGTTCCTTGCTCTT | GTATGTCTCAGTTATCTTCCT |
| HOTAIR | AATAGACATAGGAGAACACTT | AATCTTAATAGCAGGAGGAA |
| IGF2-AS | CGCCACTGTGTTACCATT | TTGCCCATCCCAGATAGAA |
| KCNQ1OT1 | GCATATCTGTCTTCCGTAT | CCTCTTCCTTCGTTCAAT |
| LSINCT5 | TAGACAACTTACTTAACCTCAT | TCCTTATCCACCTTATCCA |
| MALAT1 | CCGCTGCTATTAGAATGC | CTTCAACAATCACTACTCCAA |
| MEG3 | TGGCATAGAGGAGGTGAT | AGACAAGTAAGACAAGCAAGA |
| MIR31HG | ACTTCCACGATAGCAATG | GAATGAATCCTCTGTCCTC |
| PCA3 | AATCATACTGGTCACTTATCT | TTAACAACTGGTCCTGAG |
| PCAT1 | TAGAGCCTTGAAGATGAG | TCGTGTAGTTGTAAGATGA |
| PCGEM1 | TAGTTAAGCAGATTCATAGA | GATGTCATAGTCCTCTTC |
| PVT1 | CTTGAGAACTGTCCTTACG | CAGATGAACCAGGTGAAC |
| RRP1B | CAGTATATCTCAACTCAGT | TTCTTCTTCCTTCTTCTC |
| SRA1 | TTACAGAGATTAGAACCACATT | GGCAAGTCAGAGTTACAAT |
| TDRG1 | GATTCGTCTGGTTCCTTA | TTCCTCTTGACTGATTCTAA |
| UCA1 | TTCCTTATTATCTCTTCTG | TCCATCATACGAATAGTA |
| WRAP53 | CAATAGTGCTGATAACAT | CAGTAATCATAGATGGTAT |
| XIST | GAACCACCTACACTTGAG | TGCTATGCGTTATCTGAG |
| Yiya | TATCCTATTCTTAGCAACTG | ACATACCTGGCATATAGT |
| ZNFX1-AS1 | CCAGTTCCACAAGGTTAC | GCAGGTAGGCAGTTAGAA |
| U6 | CTCGCTTTGGCAGCACA | AACGCTTCACGAATTTGCGT |
Western blotting
The VSMCs were homogenized and extracted in NP-40 buffer, followed by 5-10 min boiling and centrifugation to obtain the supernatant. Samples containing 50 μg of protein were separated on 10% SDS-PAGE gel, transferred to PVDF Transfer Membrane (Millipore). After saturation with 5% (w/v) non-fat dry milk in TBS and 0.1% (w/v) Tween 20 (TBST), the membranes were incubated with the following antibodies, caspase3, caspase8 and Bcl-2 (Santa Cruz, USA), at dilutions ranging from 1:500 to 1:2,000 at 4°C over-night. After three washes with TBST, membranes were incubated with secondary immunoglobulins (Igs) conjugated to IRDye 800CW Infrared Dye (LI-COR), including donkey anti-goat IgG and donkey anti-mouse IgG at a dilution of 1:10,000-1:20,000. After 1 hour incubation at 37°C, membranes were washed three times with TBST. Blots were visualized by the Odyssey Infrared Imaging System (LI-COR Biotechnology). Signals were densitometrically assessed (Odyssey Application Software version 3.0) and normalized to the β-actin signals to correct for unequal loading using the monoclonal anti-β-actin antibody (Bioworld Technology, USA).
RNA interference
The small interfering (si) RNA for human PTX3 or scramble siRNA was obtained from Dharmacon (Lafayette, USA). The small interfering with the following primers: siHIF1A-AS1-1, Forward 5’-GAGUCUGUGUGGGACAAGCACUUCA-3’ and Reverse 5’-AGUAGAGGAUGUGACUCACUGUCUG-3’; siHIF1A-AS1-2, Forward 5’-GCUAACACUGGUCUGAGCAAGGU-3’ and Reverse 5’-UCCUCAAGGAGAGAGGACUAAGC-3’, siHIF1A-AS1-3, Forward 5’-GCACAGGAUUCAGUCCACUGUCUU-3’ and Reverse 5’-GACACAGGACACUGAAAGCUUGG-3’; scramble, Forward 5’-CACCAGUGGCUAUCACACGUGUGA-3’ and Reverse 5’-UCAAGAGGAGUGUAACCCACACGU-3’. The siRNA oligonucleotides (at a final concentration of 100 nM) were transfected into human umbilical vein endothelial cells using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
Statistical analysis
The data from these experiments were reported as mean ± standard errors of mean (SEM) for each group. All statistical analyses were performed by using PRISM version 4.0 (GraphPad). Inter-group differences were analyzed by one-way ANOVA, and followed by Tukey’s multiple comparison test as a post test to compare the group means if overall P < 0.05. Differences with P value of < 0.05 were considered statistically significant.
Results
Hierarchical cluster analysis and HIF1a-AS1 expression in vivo
HIF 1alpha-antisense RNA 1 (HIF1a-AS1) plays a key role in the proliferation and apoptosis of vascular smooth muscle cells in vitro, which may contribute to the pathogenesis of thoracic aortic aneurysms. We then investigated the possible mechanisms that Lnc-RNA regulates the thoracoabdominal aorta tumorigenesis. We performed a hierarchical cluster analysis of the differentially expressed Lnc-RNA in the serum of TAA patients that associated with Lnc-RNA expression. After the removal of redundant and unannotated sequences, 10 genes were found to be significantly up-regulated and 15 genes to be significantly down-regulated in the TAA group compared to the normal control group. The results showed that the overexpression of HIF1a-AS1 was associated with TAA, the expression of which was at the highest levels in all 33 Lnc-RNAs in vivo (Figure 1A). To further validated the interaction between the TAA and HIF1a-AS1, large sample statistics results showed that compared to the normal control the expression of HIF1a-AS1 was significantly increased (superior to 6 folds) in serum of TAA patients.
Figure 1.

Hierarchical cluster analysis of the differentially expressed long non-coding RNAs (LncRNA) and sHIF1a-AS1 expression in serum of TAA patients. The figure is drawn by MeV software (version 4.2.6). A. Differentially expressed LncRNAs chosen from lncRNA and disease database. Correlation similarity matrix and average linkage algorithms are used in the cluster analysis. Each row represents an individual LncRNA, and each column represents a sample. The dendrogram at the left side and the top displays similarity of expression among LncRNAs and samples individually. The color legend at the right represents the level of mRNA expression, with red indicating high expression levels and blue indicating low expression levels; B. The expression of HIF 1alpha-antisense RNA 1 (HIF1a-AS1) in serum of TAA patients is measured by Quantitative real-time PCR, 50 serum samples of TAA patients and 50 cases of normal control group were collected. Values are expressed as mean ± SEM, n=50 in each group.
PA-induced cell apoptosis and LncRNA HIF1a-AS1 expression in VSMCs
To evaluate the potential cell apoptosis of PA in VSMCs, we analyzed the effect of PA on cell survival in VSMCs. The CCK8 assay was used to measure cell viability. The viabilities of HUVECs treated with PA were significantly lower than those of untreatment group. Treatment of HUVECs with PA induced cell death in a time and dose-dependent manner by using CCK8 assay (Figure 2A). We next investigated whether PA induces cell death through an apoptotic mechanism. Annexin V-PI double-labeling was used for the detection of PS externalization, a hallmark of early phase of apoptosis. Consistent with the CCK8 assay, the results showed that the proportion of the apoptotic cells had gained as compared to untreatment group (Figure 2B and 2C). Moreover, the percentage of the apoptotic cells in a dose-dependent manner. LncRNA HIF1a-AS1 is highly associated with CVD, and HIF1a-AS1 is highly expressed in advanced atherosclerosis tissues. The current study suggested that HIF1a-AS1 was associated with PA-induced dysfunction of VSMCs. The RNA expression of HIF1a-AS1 was significantly higher in VSMCs with PA (0.8 mM) than those of untreatment group (Figure 2D). Therefore, our data suggest that up-regulation the expression of HIF1a-AS1 was involved in PA-induced cell death.
Figure 2.
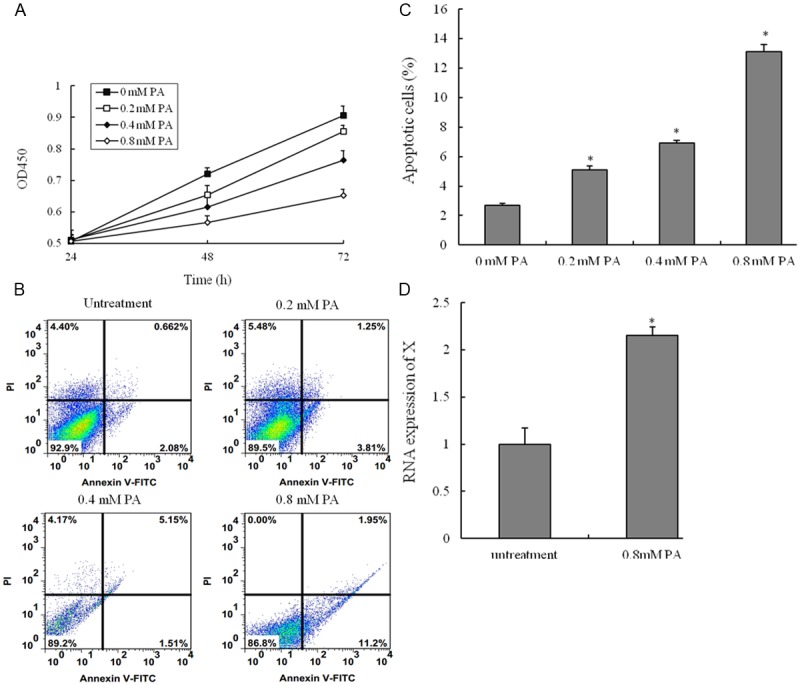
Palmitic acid-induced the apoptosis of vascular smooth muscle cells (VSMCs). VSMCs are incubated with various concentrations of palmitic acid (PA) for 24 h, 48 h or 72 h, and the cell viability was examined by CCK8 assay. (A) Cells are treated with vehicle, 0.2 mM PA, 0.4 mM PA or 0.8 mM PA for 48 h; (B) The percentage of apoptotic cells is also analyzed by flow cytometric analysis of annexin V/PI double staining and (C) the percentage of apoptotic cells (at the right of pictures). (D) Cells are treated with PA (80 mM) for 48 h, RNA expression of HIF 1alpha-antisense RNA 1 (HIF1a-AS1) in VSMCs is measured by quantitative real-time PCR. Values are expressed as mean ± SEM, n=3 in each group. *P < 0.05, versus untreatment group.
Identification of HIF1a-AS1 in the regulation of VSMCs dysfunction
In this work, knock-out of endogenous HIF1a-AS1 with small-interfering RNA (siRNA), the expression of HIF1a-AS1 was down-regulated (Figure 3A). To evaluate the potential protective mechanisms of inhibition the function of HIF1a-AS1 in VSMCs, the CCK8 assay was used to measure cell viability. The viabilities of VSMCs inhibited with PA were protected by si-HIF1a-AS1 (Figure 3B). Consistent with the CCK8 assay, the Annexin V-PI double-labeling results showed that inhibition the function of HIF1a-AS1 with si-RNA could decrease the proportion of the apoptosis cells inducing by PA treatment (Figure 3C and 3D).
Figure 3.
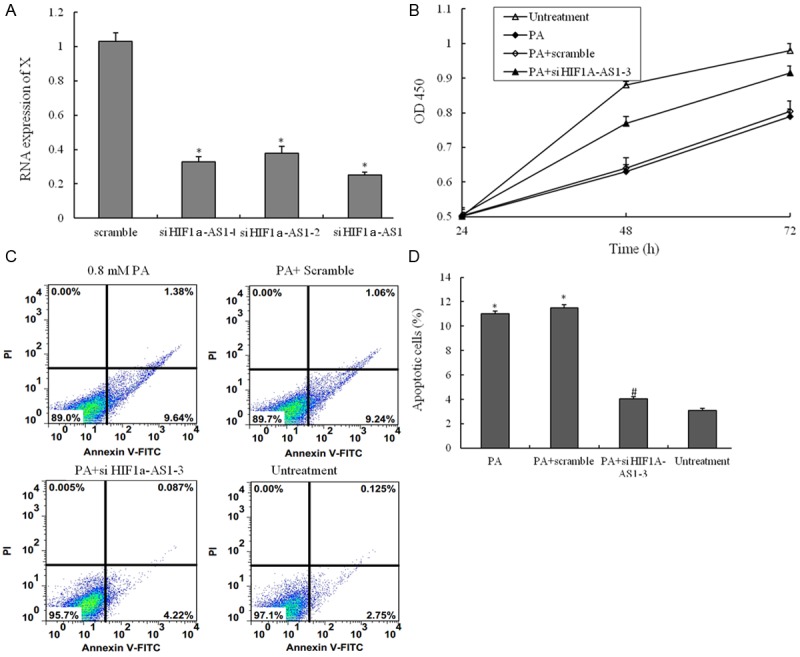
The small interfering RNA for suppressing the function of HIF1a-AS1. (A) Three different small interfering RNA were transfected into VSMCs suppressing the RNA expression of HIF1a-AS1; (B) VSMCs are treated with untreatment, 0.8 mM PA only, 0.8 mM PA plus scramble si-RNA and 0.8 mM PA plus si- HIF1a-AS1-3 for 48 h, and the cell viability was examined by CCK8 assay; (C) VSMCs are treated with untreatment, 0.8 mM PA only, 0.8 mM PA plus scramble si-RNA and 0.8 mM PA plus si- HIF1a-AS1-3 for 48 h, the percentage of apoptotic cells is also analyzed by flow cytometric analysis of annexin V/PI double staining and (D) the percentage of apoptotic cells (at the right of pictures). Values are expressed as mean ± SEM, n=3 in each group. *P < 0.05, versus untreatment group; #P < 0.05, versus PA group.
PA-mediated regulation of apoptosis-related proteins
The apoptotic response was further investigated by measuring caspase-3 and caspase-8 activity and apoptosis-related proteins with Western blot techniques. PA administration caused 4.5- and 4.4-fold increases in caspase-3 and caspase activity respectively. However, the combination PA with si-HIF1a-AS1 induced strong and specific suppression of protein expression of caspase-3 and caspase-8 Figure 4 expression, and expression of which was statistically up-regulated in the PA combination with si-HIF1a-AS1-treated group as compared to PA single treatment group. Therefore, our data suggest that up-regulation the expression of si-HIF1a-AS1 was involved in PA-induced VSMCs death.
Figure 4.
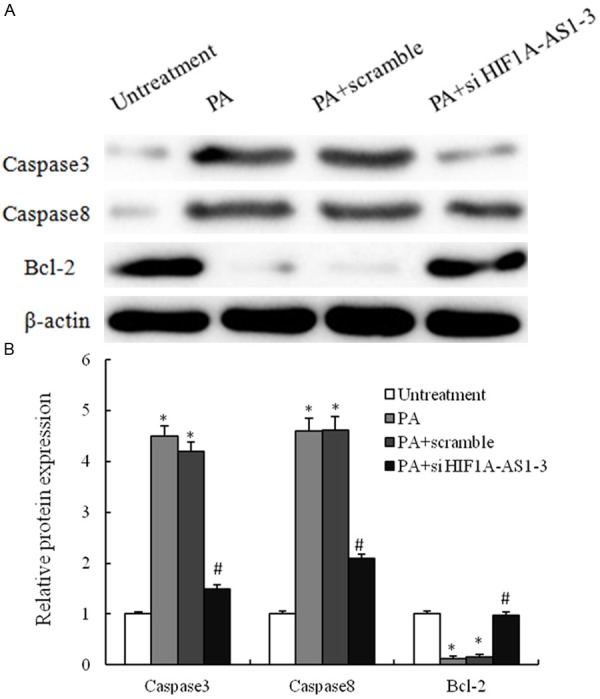
PA-mediated regulation of apoptosis-related proteins in VSMCs. A. VSMCs are treated with untreatment, 0.8 mM PA only, 0.8 mM PA plus scramble si-RNA and 0.8 mM PA plus si-HIF1a-AS1-3 for 48 h, and the expression of caspase3, caspase8 and Bcl2 are analyzed by western blotting; B. These results are confirmed by densitometric analyses. Values are expressed as mean ± SEM, n=3 in each group. *P < 0.05, versus untreatment group; #P < 0.05, versus PA group.
Discussion
Research into the pathogenesis of thoracoabdominal aortic aneurysms (TAA) is difficult, because this disease is caused by multiple factors such as hemodynamics, metabolism, inflammation and genetic influences [11]. In addition to activation of proteolysis and inflammation, apoptosis of smooth muscle cells and oxidative stress have been suggested by several clinicopathological studies, and these factors together with many others seem to be intricately interwoven to produce aneurysms [12-14]. Another difficulty is that suitable animal models are not available for the study of aortic aneurysms. In this study, apoptosis of VSMCs were considered to approximately represent the TAA cell injury model. The exposure of PA to VSMCs has been demonstrated to cause a series of dysfunction, and trends to make as the TAA cell injury model. There were two significant findings in this report: (1) patients with TAA were increased HIF1a-AS1 in the serum; (2) we found that PA could induce VSMCs apoptosis in a dose dependent manner and increase the expression of HIF1a-AS1 in VSMCs. VSMCs apoptosis was thought to be involved in TAA [12]. Thus, apoptosis was measured in the present study to better confirm and to analyze the VSMCs injury by PA. We found that the proportion of the apoptotic cells was increased.
The non-coding RNAs that are the predominant transcripts in mammalian genome exceed the number of protein coding genes. It is commonly believed that that alteration of small noncoding RNA expression, especially microRNAs contribute to the pathogenesis of cardiovascular disease [15-18]. More recently, a new class of noncoding RNAs, long non-coding RNAs, which are endogenous RNA transcripts in the genome with no or lower protein coding potential, have been reported to be abundantly transcribed [19]. Related studies indicate that lncRNAs are involved in the alteration of chromatin structure, the control of cellular functions and the regulation of related genes [20-22]. Recent studies are beginning to reveal their importance in tumorigenesis and metastasis in the malignant cell. For example, downregulation of a long noncoding RNA-ncRuPAR contributes to tumor inhibition in colorectal cancer [23], and LncRNA TARID directs demethylation and activation of the tumor suppressor TCF21 via GADD45A [24]. Recently, it has been reported that stroke-induced lncRNAs might associate with CMPs to modulate the post-ischemic epigenetic landscape [25].
In this study, we performed a LncRNA microarray technique using human samples, which can evaluate thousands genes simultaneously, and hierarchical cluster analysis of the differentially expressed Lnc-RNA in the serum of TAA patients to identify the lncRNA HIF1A-AS1 was significantly up-regulated. Our results is consistent with other studies that reported that HIF1A-AS1 is identified through BRG1 knock-down VSMCs, and the expression of HIF1A-AS1 is found to be regulated by BRG1 in VSMCs [4]. These studies will have particular relevance in the future, as the role of lncRNA in cardiovascular disease states becomes increasingly recognized. Moreover, PA dose-dependently decreased the cell viability and increased the apoptosis of VSMCs, and down-regulated the expression of Bcl2 and up-regulated the expression of caspase3 and caspase8. Interestingly, LncRNA HIF1A-AS1 knock-down could suppress PA-induced dysfunction of VSMCs in vitro. Therefore, our data suggest that up-regulation the expression of HIF1a-AS1 was involved in PA-induced cell apoptosis.
In conclusion, our results demonstrate that HIF1A-AS1 was overexpressed in the TAA patients. LncRNA HIF1A-AS1 knock-down could suppress PA-induced apoptosis of VSMCs in vitro, which may contribute to the pathogenesis of thoracoabdominal aorta aneurysm.
Disclosure of conflict of interest
None.
References
- 1.Orr N, Minion D, Bobadilla JL. Thoracoabdominal aortic aneurysm repair: current endovascular perspectives. Vasc Health Risk Manag. 2014;10:493–505. doi: 10.2147/VHRM.S46452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zanow J, Settmacher U. [Aneurysms of the thoracic and thoracoabdominal aorta] . Chirurg. 2014;85:767–73. doi: 10.1007/s00104-014-2717-y. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe Y, Kuratani T, Shirakawa Y, Torikai K, Shimamura K, Sawa Y. Hybrid endovascular repair of a dissecting thoracoabdominal aortic aneurysm with stent graft implantation through the false lumen. J Vasc Surg. 2014;59:264–267. doi: 10.1016/j.jvs.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Zhang X, Yuan Y, Tan M, Zhang L, Xue X, Yan Y, Han L, Xu Z. BRG1 expression is increased in thoracic aortic aneurysms and regulates proliferation and apoptosis of vascular smooth muscle cells through the long non-coding RNA HIF1A-AS1 in vitro. Eur J Cardiothorac Surg. 2014 doi: 10.1093/ejcts/ezu215. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Durdu S, Deniz GC, Balci D, Zaim C, Dogan A, Can A, Akcali KC, Akar AR. Apoptotic vascular smooth muscle cell depletion via BCL2 family of proteins in human ascending aortic aneurysm and dissection. Cardiovasc Ther. 2012;30:308–316. doi: 10.1111/1755-5922.12007. [DOI] [PubMed] [Google Scholar]
- 6.Jiang L, Wang M, Zhang J, Monticone RE, Telljohann R, Spinetti G, Pintus G, Lakatta EG. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS One. 2008;3:e2231. doi: 10.1371/journal.pone.0002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiao HP, Gao WS, Huo JX, Yang ZS. Long non-coding RNA GAS5 functions as a tumor suppressor in renal cell carcinoma. Asian Pac J Cancer Prev. 2013;14:1077–1082. doi: 10.7314/apjcp.2013.14.2.1077. [DOI] [PubMed] [Google Scholar]
- 8.Martens-Uzunova ES, Bottcher R, Croce CM, Jenster G, Visakorpi T, Calin GA. Long noncoding RNA in prostate, bladder, and kidney cancer. Eur Urol. 2014;65:1140–1151. doi: 10.1016/j.eururo.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taketani T, Imai Y, Morota T, Maemura K, Morita H, Hayashi D, Yamazaki T, Nagai R, Takamoto S. Altered patterns of gene expression specific to thoracic aortic aneurysms: microarray analysis of surgically resected specimens. Int Heart J. 2005;46:265–277. doi: 10.1536/ihj.46.265. [DOI] [PubMed] [Google Scholar]
- 12.Rowe VL, Stevens SL, Reddick TT, Freeman MB, Donnell R, Carroll RC, Goldman MH. Vascular smooth muscle cell apoptosis in aneurysmal, occlusive, and normal human aortas. J Vasc Surg. 2000;31:567–576. [PubMed] [Google Scholar]
- 13.Miller FJ Jr, Sharp WJ, Fang X, Oberley LW, Oberley TD, Weintraub NL. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol. 2002;22:560–565. doi: 10.1161/01.atv.0000013778.72404.30. [DOI] [PubMed] [Google Scholar]
- 14.Yajima N, Masuda M, Miyazaki M, Nakajima N, Chien S, Shyy JY. Oxidative stress is involved in the development of experimental abdominal aortic aneurysm: a study of the transcription profile with complementary DNA microarray. J Vasc Surg. 2002;36:379–385. doi: 10.1067/mva.2002.124366. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Zhi H, Li Y, Ma G, Ye X, Yu X, Yang T, Jin H, Lu Z, Wei P. Polymorphism in miRNA-1 target site and circulating miRNA-1 phenotype are associated with the decreased risk and prognosis of coronary artery disease. Int J Clin Exp Pathol. 2014;7:5093–5102. [PMC free article] [PubMed] [Google Scholar]
- 16.Choe N, Kwon JS, Kim JR, Eom GH, Kim Y, Nam KI, Ahn Y, Kee HJ, Kook H. The microRNA miR-132 targets Lrrfip1 to block vascular smooth muscle cell proliferation and neointimal hyperplasia. Atherosclerosis. 2013;229:348–355. doi: 10.1016/j.atherosclerosis.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Cheng Y, Yang J, Qin S, Chen X, Tang X, Zhou X, Krall TJ, Zhang C. Flank sequences of miR-145/143 and their aberrant expression in vascular disease: mechanism and therapeutic application. J Am Heart Assoc. 2013;2:e000407. doi: 10.1161/JAHA.113.000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kataoka M, Wang DZ. Non-Coding RNAs Including miRNAs and lncRNAs in Cardiovascular Biology and Disease. Cells. 2014;3:883–898. doi: 10.3390/cells3030883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Fu D, Qiu Y, Xing X, Xu F, Han C, Xu X, Wei Z, Zhang Z, Ge J, Cheng W, Xie HL. Genome-wide screening and identification of long noncoding RNAs and their interaction with protein coding RNAs in bladder urothelial cell carcinoma. Cancer Lett. 2014;349:77–86. doi: 10.1016/j.canlet.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell. 2011;44:667–678. doi: 10.1016/j.molcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan B, Gu W, Yang Z, Gu Z, Yue X, Gu Q, Liu L. Downregulation of a long noncoding RNA-ncRuPAR contributes to tumor inhibition in colorectal cancer. Tumour Biol. 2014 doi: 10.1007/s13277-014-2465-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Arab K, Park YJ, Lindroth AM, Schafer A, Oakes C, Weichenhan D, Lukanova A, Lundin E, Risch A, Meister M, Dienemann H, Dyckhoff G, Herold-Mende C, Grummt I, Niehrs C, Plass C. Long Noncoding RNA TARID Directs Demethylation and Activation of the Tumor Suppressor TCF21 via GADD45A. Mol Cell. 2014;55:604–614. doi: 10.1016/j.molcel.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Dharap A, Pokrzywa C, Vemuganti R. Increased binding of stroke-induced long non-coding RNAs to the transcriptional corepressors Sin3A and coREST. ASN Neuro. 2013;5:283–289. doi: 10.1042/AN20130029. [DOI] [PMC free article] [PubMed] [Google Scholar]


