Abstract
Objective: To evaluate the effects of intrathecal administration p38β antisense oligonucleotide on the development of bone cancer pain rats. Methods: Forty female SD rats weighing 180~220 g were randomly divided into 4 groups (n = 10 each): Group A (control group): intra-tibial injection of 3 μl Hank’s solution; group B (model group): intra-tibial injection of 3 μl MADB-106 mammary gland carcinoma cells of rats (4.8 × 103/μl); group C (p38β-SODN 20 μg); group D (p38β-ASODN 20 μg). The model procedures in group C and D were same to those in the group B. From the 14th day after operation, p38β-SODN 20 μg and p38β-ASODN 20 μg were respectively intrathecally administrated in group C and D once daily for 6 days whereas normal saline was for group A and B. Mechanical withdrawal threshold and radiant heat threshold of rat hind paws were measured before operation and every other day until 22 d of post-operation. The lumbar 4-6 spinal cord was removed on the 22nd day. The expression of spinal p38β protein was determined by Western blot. Results: No significant differences in mechanical withdrawal threshold and radiant heat threshold were found at all time points in control group. During the first 6 days after operation there were obvious differences in radiant heat stimulus between control group between the other groups (P < 0.05); During 14-22 days after operation, mechanical pain threshold and radiant heat threshold between p38β-SODN group and Model group were significantly changed compared with that in control group (P < 0.05). However, the differences were not remarkable between control group and p38β-ASODN group (P > 0.05). The expression of p38β protein in lumbar spinal cord was significantly higher between p38β-SODN group and Model group than that in control group (P < 0.05). There was no significant difference in p38β protein expression between p38β-ASODN group and control group (P > 0.05). Conclusions: Hyperalgesia induced by bone cancer can be inhibited by intrathecal administration of p38β antisense oligonucleotide, which is achieved by reducing expression of p38β protein.
Keywords: Bone cancer pain, antisense oligonucleotides, p38beta, hyperalgesia, spinal dorsal horn
Introduction
Bone pain associated with cancer is a serious clinical health problem which is difficult to treat, and its mechanisms are not well understood [1,2]. Circumstantial evidences suggest that progress has been made in understanding the changes of mitogen-activated protein kinase (MAPK) pathway in the induction and maintenance of chronic pain states [3-5]. In particular, p38 MAPK is typically activated by cellular stress and proinflammatory cytokines [6-9], and plays a critical role in inflammatory responses and spinal sensitization [10-12]. Four isoforms of p38 MAPK have been identified, each the product of distinct genes: p38α, 38β, p38γ and p38δ [5,13,14]. Among the p38 family members, p38α and p38β are two of the major isoforms in the mature nervous system. p38α is the most abundant isoform in the DRG and spinal cord [15]. We demonstrated that p38β was mainly expressed in spinal microglia after bone cancer pain was developed [16]. Inhibition of p38 pathways has been shown to effectively attenuate inflammatory and neuropathic pain in different animal models [15,17-19]. Development of knockdown for p38 pathways to target neurons and glial cells may lead to new therapeutic strategy for pain management.
The antisense strategy is a better gene therapy technique and could be a useful tool for the study of endogenous gene regulation [20,21]. Pathway analyses demonstrated that the phosphorylation of p38 and its downstream target CREB was inhibited by the antisense oligodeoxynucleotide (AS-ODN) [22] and knockdown of p38β with antisense oligonucleotides prevented acute pain sensitization [10]. However, few studies have focused on the role of p38β-ASODN in bone cancer pain.
We previously reported that p38β in the spinal cord participates in pain hypersensitivity using the bone cancer pain model [16]. In this study, we set out to investigate whether intrathecal p38β-ASODN attenuates experimental bone cancer pain and suppresses glial activation in the bone cancer pain model. The results indicate that the inhibitory effect of intrathecal p38β-ASODN on bone cancer pain is largely mediated by the downregulation of p38β.
Materials and methods
Animals
Adult female Sprague-Dawley rats weighing 200-250 g were housed at a constant ambient temperature of 24°C ± 1°C, humidity of 40%~70% under a 12 h light/dark cycle and given food and water ad libitum. The rats were individually housed in plastic cages with wood-chip bedding for at least 1 day before surgery. All experimental procedures were approved by the Institutional Animal Care and Use Committee, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology and were conducted in accordance with the NIH guide for the care and use of laboratory animals and the Ethical Issue of the IASP [23].
Cell preparation
MADB-106 mammary gland carcinoma cells were donated by Page GG and LY Liu and prepared as described previously [16,24]. Cells were diluted in Hank’s medium to the required concentration for injection (3 × 103 cells/10 μl) and kept on ice.
38β antisense oligodeoxynucleotides
Antisense and missense oligonucleotides to the rat p38β subunit were designed according to the cloned 5’ end fragment of the rat p38β gene [10]. The 38β sense ODN sequence was 5’-TCCACGCGAGGAGGACATAC-3’ and the antisense ODN sequence was 5’-GTATGTCCTCCTCGCGTGGA-3’. 2’-O-Methoxyethylribose (MOE)-modified phosphodiester/phosphorthioate (PO/PS) chimeric oligonucleotides and MOE-modified phosphorthioate (PS) oligonucleotides crossreactive with rat 38β were synthesized, purified, and provided by Shanghai Bio-engineering Technology Co. [22,25,26]. The wings of these chimeric ASO consist of 2’-MOE modified PO linkages whereas the gap consists of 2’H/PS nucleotides demonstrating RNase Hdependent antisense activity [22]. Synthetic oligonucleotides were dissolved in artificial cerebrospinal fluid (ACSF) to a final concentration of 20 µg per 10 µl before intrathecal administration. The ACSF contained (µM): Na+, 151.1; K+, 2.6; Mg2+, 0.9; Ca2+, 1.3; Cl-, 122.7; HCO3, 21.0; HPO4, 2.5 and was bubbled with 95% O2/5% CO2 before use to adjust the final pH to 7.2.
Animal groups and surgical procedures
Rats were randomly divided into five groups (n = 10). Group Naive received neither intra-tibial operation nor intrathecal catheter implantation. Group Control received intra-tibial injection of 3 μL Hank’s solution; Group Model, group p38β-SODN and group p38β-ASODN received intra-tibial injection of 3 μL MADB-106 mammary gland carcinoma cells of rats (4.8 × 109 cells μL). From the 14th day after injection, p38β-SODN and p38β-ASODN (20 μg per 10 μl) were administered intrathecally into group p38β-SODN and group p38β-ASODN on each of six consecutive days, respectively, while ACSF (10 μl) was injected into group Control and group Model by intrathecal catheter. In group Control and group Model, animals were allowed to survive for 22 days after surgery (n = 5 for immunohistochemistry and n = 5 for Western blotting).
All procedures were performed under pentobarbital anesthesia (50 mg/kg, i.p.). After 1 week after the implantation of the intrathecal catheter, rats were anesthetized and 3 μL solution was injected into the tibial bone of the left hind paw using 0.3 ml insulin syringe with 29.5 gauge needle, as described previously [16,27].
The implantation technique of the intrathecal catheter was modified and performed [28,29]. Briefly, after rats were adequately anesthetized, a PE10 catheter (o.d. = 0.61 mm) was inserted into the subarachnoid space between the L5 and L6 vertebrae, with its tip at the lumbar enlargement. The proper location of the catheter was tested by assessing sensory and motor blockade after intrathecal injection of 6 μl lidocaine (20 mg/ml). Following the PE10 catheter, ACSF or p38β-SODN and p38β-ASODN was injected into the subarachnoid space through catheter, respectively.
Behavioral assessment of mechanical and thermal stimulation
Rats were left to acclimatize to the area for 30 min before testing. All rats were tested for mechanical and heat hypersensitivity of the plantar surface of the hindpaw on 1 day before surgery and 2, 4, 6, 8, 10, 12, 14, 16, 18, 20 or 22 days after surgery.
Paw withdrawal threshold in response to a mechanical stimulus was determined using an automated testing device (dynamic plantar aesthesiometer; Ugo Basile, Stoelting, USA) as described previously [30-33]. When the animal withdrew its hindpaw, the mechanical stimulus was automatically stopped, and the force at which the animal withdrew its paw was recorded to the nearest 0.1 g. The thermal nociceptive threshold was detected by a Hargreaves apparatus (Chinese Academy of Medical Sciences Institute of Biomedical Engineering, Beijing, China) according to a previous method [34-36]. The heat stimulation was repeated three times at an interval of 5-10 min for each paw and the mean was calculated.
Mechanical withdrawal thresholds and radiant heat threshold of rat hind paws were measured every other day from one day before operation until 22 days later. The lumbar 4-5 spinal cord was removed on the 22nd day. The expression of p38β protein in the spinal cord was determined using Western blotting.
Tissue preparation and immunohistochemistry
Rats were deeply anesthetized on 22 d after surgery. After the perfusion by 4% paraformaldehyde in 0.1 M phosphate buffer, lumbar (L4-6) spinal segments were removed and post-fixed in perfusate for 6 h and transferred, first to 10% sucrose for 4 hs and then to 30% sucrose until they sank for cryoprotection. The tissue was cut into smaller segments and frozen on dry ice for 1 h, then stored at -80°C until use. Tissue was sectioned serially at a thickness of 25 μm.
Sections were mounted on glass slides and labeled with rabbit anti-OX-42 (microglia, 1:200) and mouse anti-glial fibrillary acidic protein (GFAP) (astrocyte, 1:500). After a 5 min washing in PBS, sections were incubated for 2 h at R.T. with the secondary antibody, biotinylated goat anti-rabbit IgG (Vector Laboratories Inc., Burlingame, USA) for 1 h at 4°C. Immunoreactive signals were further amplified by ABC solution (1:100; Vector Laboratories Inc.). Control sections were processed similarly, except that primary antibodies were omitted.
Western blottting
On 22 days after the intra-tibial injection, the lumbar spinal cord (L4-6) was removed. Total proteins from lumbar spinal segment were prepared by addition of 1 ml of ice cold solubilization buffer containing protease inhibitors. The tissue was homogenized. After being placed on ice for 30 min, the homogenate was centrifuged at 12,000 × g for 30 minutes at 4°C. The supernatant was assayed for protein content using the BCA assay method and stored at -20°C. Total protein (60 μg) was electrophoresed on a 6% sodium dodecyl sulfate polyacrylamide gel, as suggested by the manufacturer. After electrophoresis, the proteins were transferred and blocked with 5% nonfat dry milk. The primary antibody (rabbit anti-p38β, 1:500) was added and incubated for 2 h at room temperature in fresh blocking buffer. The membrane was washed for 30 minutes in washing buffer at room temperature, before the secondary antibody (1:500 dilution of alkaline phosphatase (AKP) coupled goat anti-rabbit immunoglobulin G (KPL, USA) was added for 1 h at room temperature in blocking buffer. The membrane was washed in washing buffer for another 30 min and the antibodies were then revealed using western blot reagent plus. For analysis, the blots were scanned and quantified with software and the results were expressed as the ratio of p38β immunoreactivity to β-actin immunoreactivity [37].
Data analysis
Investigators were blinded to all treatments in all tests. All values were expressed as the mean ± standard error of the mean (S.E.M.) and subjected to statistic evaluation using one-way analysis of variance (one-way ANOVA) followed by post hoc comparison (Student-Newman-Keuls test) to confirm significant differences between the groups. P < 0.05 is set as the level of statistical significance.
Results
Physiological functions
All rats kept on good health after surgery as assessed by general activity, normal weight gain, and grooming. Rats treated with p38β-ASODN consumed similar amount of food and fluid compared with p38β-SODN or ACSF over the entire observation period (P > 0.05). No significant difference was observed in terms of body weight, rectal temperature, and respiratory rate among four groups (P > 0.05).
p38β-ASODN reverses mechanical and thermal hyperalgesia
Model group developed a marked hypersensitivity to innocuous due to mechanical and thermal stimulation of the lateral surface of the left hind paw (sural nerve skin area) on 14 d after surgery (Figure 1). During the first 6 days after operation there were obvious differences in radiant heat stimulus between control group between the other groups (P < 0.05, Figure 2); During 14-22 days after operation, mechanical pain threshold and radiant heat threshold between p38β-SODN group and Model group were significantly changed compared with that in control group (P < 0.05). However, the differences to mechanical and thermal stimulation were not remarkable between control group and p38β-ASODN group (P > 0.05). Comparison among the four groups, the mechanical withdrawal threshold in p38β-ASODN group was significantly higher than that in Model group or p38β-SODN group on 22 days after surgery (P < 0.01, Figures 1 and 2).
Figure 1.
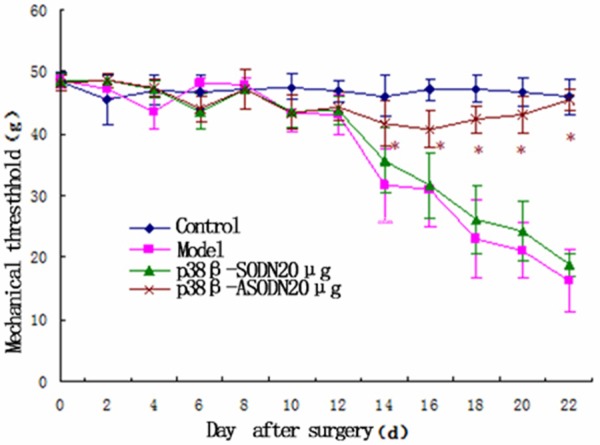
Effects of p38β-ASODN treatment on mechanical hyperalgesia. In first 12 days after intra-tibial operation, PWT in Model group was not significantly different from that in Control group (P > 0.05), whereas on 14-22 day after operation, differences were remarkable between the two groups (*P < 0.01). On 14-22 day after operation, PWT in p38β-SODN group was not significantly different from that in Control group (P > 0.05), whereas significant differences in PWT were found between p38β-ASODN group and p38β-SODN group (*P < 0.05), and there were obvious differences in PWT between p38β-ASODN group and Model group (*P < 0.05). Data are expressed as mean ± SEM.
Figure 2.
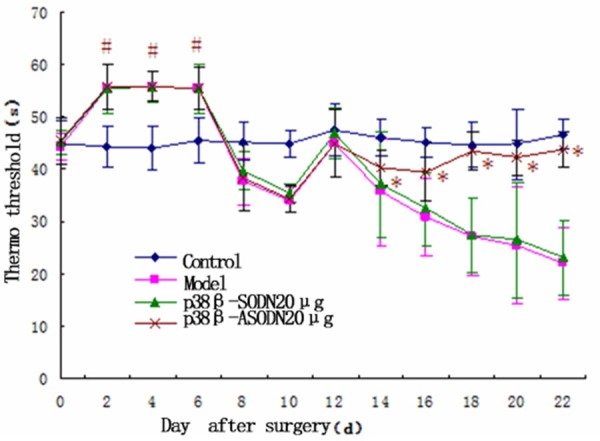
Effects of p38β-ASODN treatment on thermal hyperalgesia. No significant differences in radiant heat threshold were found at all-time points in control group. During the first 6 days after operation there were obvious differences in radiant heat stimulus between control group between the other groups (*P < 0.05); During 14-22 days after operation, radiant heat threshold between p38β-SODN group and Model group were significantly changed compared with that in the p38β-ASODN group (*P < 0.05). Data are expressed as mean ± SEM.
p38β-ASODN decreased bone cancer pain-induced p38β expression
Effect of p38β-ASODN on spinal p38β protein expression was evaluated by western blottting (n = 5 each group, Figure 3). No significant differences in p38β protein expression were found at 22 d after operation between Control group and p38β-ASODN group, between Model group and p38β-SODN group, respectively. At day 22 after operation, p38β protein in Model group evidently augmented when compared with that in Control group (*P < 0.01), whereas p38β protein in p38β-ASODN group significantly decreased when compared with that in Model group (*P < 0.01). p38β protein in p38β-SODN group was not significantly different from that in Control group at 22 d after operation (P > 0.05).
Figure 3.
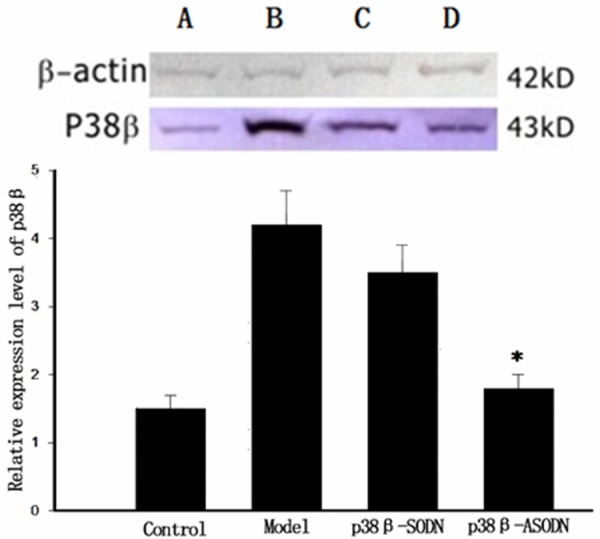
p38β protein expression of the lumbar spinal cord on day 22 after operation. No significant differences in p38β protein expression were found between p38β-ASODN group and Control group, between Model group and p38β-SODN group, respectively. p38β protein in Model group evidently augmented when compared with that in Control group (*P < 0.01), whereas p38β protein in p38β-ASODN group significantly decreased when compared with that in Model group (*P < 0.01). p38β protein in p38β-ASODN group was not significantly different from that in Control group at day 22 after operation (P > 0.05).
Intrathecal p38β-ASODN suppressed glial activation in the bone cancer pain model
A low-power image showed that the activation of GFAP and OX-42 was observed among four groups at 22 d after operation (n = 5 each group, Figure 4). And activated astrocytes demonstrated profoundly cell proliferation and hypertrophy in Model group, whereas the resting astrocytes displayed small round nuclei and slender processes in Control group. The result found that p38β-ASODN treatment markedly inhibited the activation of GFAP and OX-42 at 22 d after operation. In terms of immunohistochemical scores (Figure 4), there was no significant difference for GFAP and OX-42 immunostaining in spinal dorsal horns between Model group and p38β-SODN group, where there was significant difference for GFAP and OX-42 immunostaining between Model group and p38β-ASODN group.
Figure 4.
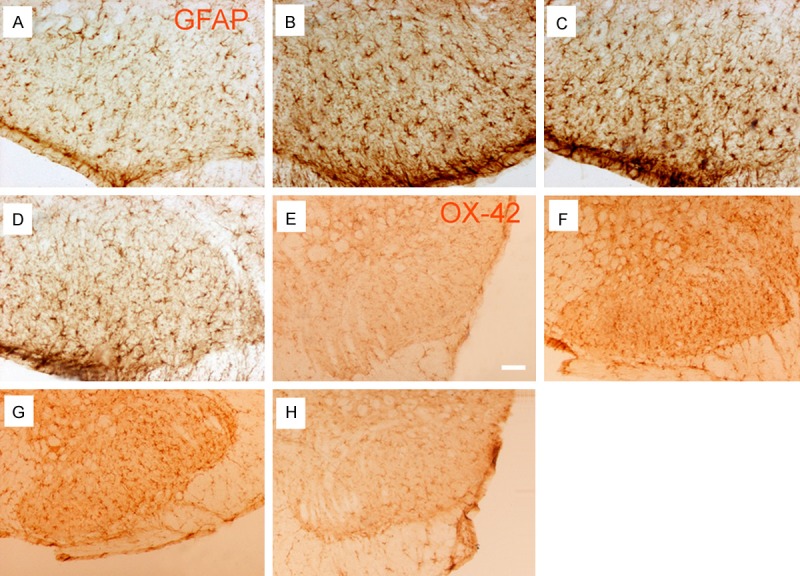
GFAP and OX-42 immunostaining of the lumbar (L4-6) spinal cord on 14 d after intra-tibial injection (n = 4 each group). The representative images were from Control group (A, E), Model group (B, F), p38β-SODN group (C, G), and p38β-ASODN group (D, H). Immunohistochemistry results showed that the up-regulation of GFAP and OX-42 between model group and p38β-SODN group was distributed in dorsal horn of spinal cord to the tibia with cancer at 14 d after operation, and p38β-ASODN treatment (D, H) markedly inhibited the up-regulation of GFAP and OX-42. Scale bars are 100 µm.
Discussion
Our data demonstrated that (1) rat with down-regulation of spinal p38β are viable with no obvious health problems; (2) intrathecal injection of p38β-ASODN decreased mechanical and thermal hyperalgesia in bone cancer pain animals; (3) bone cancer pain significantly increased the expression of p38β protein in the spinal cord; (4) expression of p38β protein in the spinal cord was significantly down-regulated by administration of p38β-ASODN; (5) intrathecal injection of p38β-ASODN significantly reduced the activation of spinal glia. Therefore, these results suggest that p38β-ASODN may alleviate mechanical allodynia and thermal hyperalgesia through the p38β-related pathway. Beardmore et al reported that mice with knockouts of spinal p38β were viable with no obvious health problems, suggesting that this mild phenotype can be compensation between different p38 isoforms [38]. Thus, down-regulation of p38β inhibits spinal nociceptive processing and represents a potential target for bone cancer pain therapy.
In agreement with our previous findings [16] and earlier reports by Dickenson and colleagues [39], the animals injected with MADB-106 mammary gland carcinoma cells into the tibia of rats showed progressive development of evoked mechanical and thermal hyperalgesia on the ipsilateral hindpaw. The contralateral hindpaw responses were the same as the sham group responses on both hindpaws and remained stable throughout the postoperative period, indicating that none of the behavioral tests cause tissue damage or hypersensitivity. These results were identical with previous published findings [40-42].
Fitzsimmons et al reported that peripheral inflammation-evoked hyperalgesia is prevented by downregulation of p38β by using intrathecal antisense oligonucleotides, suggesting that activation of spinal p38β may involve in acute facilitatory processing [43]. Svensson et al showed that down-regulation of spinal p38β prevented nocifensive flinching evoked by intraplantar formalin injection and hyperalgesia induced by intrathecal injection of substance P [10]. We previously demonstrated that the p38 isoforms were distinctly expressed in dorsal horn of spinal cord: p38β in microglia and p38alpha in neurons [16]. Results of the current study demonstrated that mechanical and thermal hyperalgesia induced by bone cancer were inhibited by intrathecal administration of p38β antisense oligonucleotide, which is achieved by reducing expression of p38β protein and suppressing glial activation.
In conclusion, our data demonstrate the specific role of spinal p38β in mediating bone cancer pain. Thus, we conclude that therapeutic intervention targeting p38β may provide a novel approach to suppress spinal glial activation associated with bone cancer pain.
Acknowledgements
The authors gratefully thank Dr. Gayle G. Page for generously providing the MADB106 cells.
Disclosure of conflict of interest
None.
References
- 1.Donovan-Rodriguez T, Dickenson AH, Urch CE. Gabapentin normalizes spinal neuronal responses that correlate with behavior in a rat model of cancer-induced bone pain. Anesthesiology. 2005;102:132–140. doi: 10.1097/00000542-200501000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci. 1999;19:10886–10897. doi: 10.1523/JNEUROSCI.19-24-10886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katsura H, Obata K, Miyoshi K, Kondo T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Sakagami M, Noguchi K. Transforming growth factor-activated kinase 1 induced in spinal astrocytes contributes to mechanical hypersensitivity after nerve injury. Glia. 2008;56:723–733. doi: 10.1002/glia.20648. [DOI] [PubMed] [Google Scholar]
- 4.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R, Sun L, Hayashi Y, Liu X, Koyama S, Wu Z, Nakanishi H. Acute p38-mediated inhibition of NMDA-induced outward currents in hippocampal CA1 neurons by interleukin-1beta. Neurobiol Dis. 2010;38:68–77. doi: 10.1016/j.nbd.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Dewil M, dela Cruz VF, Van Den Bosch L, Robberecht W. Inhibition of p38 mitogen activated protein kinase activation and mutant SOD1 (G93A)-induced motor neuron death. Neurobiol Dis. 2007;26:332–341. doi: 10.1016/j.nbd.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Hegen M, Gaestel M, Nickerson-Nutter CL, Lin LL, Telliez JB. MAPKAP kinase 2-deficient mice are resistant to collagen-induced arthritis. J Immunol. 2006;177:1913–1917. doi: 10.4049/jimmunol.177.3.1913. [DOI] [PubMed] [Google Scholar]
- 9.Inoue T, Boyle DL, Corr M, Hammaker D, Davis RJ, Flavell RA, Firestein GS. Mitogen-activated protein kinase kinase 3 is a pivotal pathway regulating p38 activation in inflammatory arthritis. Proc Natl Acad Sci U S A. 2006;103:5484–5489. doi: 10.1073/pnas.0509188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua XY, Yaksh TL. Spinal p38beta isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J Neurochem. 2005;92:1508–1520. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- 11.Suter MR, Berta T, Gao YJ, Decosterd I, Ji RR. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol Pain. 2009;5:53. doi: 10.1186/1744-8069-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terayama R, Omura S, Fujisawa N, Yamaai T, Ichikawa H, Sugimoto T. Activation of microglia and p38 mitogen-activated protein kinase in the dorsal column nucleus contributes to tactile allodynia following peripheral nerve injury. Neuroscience. 2008;153:1245–1255. doi: 10.1016/j.neuroscience.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 14.Bohm C, Hayer S, Kilian A, Zaiss MM, Finger S, Hess A, Engelke K, Kollias G, Kronke G, Zwerina J, Schett G, David JP. The alpha-isoform of p38 MAPK specifically regulates arthritic bone loss. J Immunol. 2009;183:5938–5947. doi: 10.4049/jimmunol.0901026. [DOI] [PubMed] [Google Scholar]
- 15.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 16.Dong H, Tian YK, Xiang HB, Tian XB, Jin XG. [The cellular location and significance of p38alpha/beta isoforms in the lumbar spinal cord of the bone cancer pain rats] . Zhonghua Yi Xue Za Zhi. 2007;87:53–57. [PubMed] [Google Scholar]
- 17.Boyle DL, Jones TL, Hammaker D, Svensson CI, Rosengren S, Albani S, Sorkin L, Firestein GS. Regulation of peripheral inflammation by spinal p38 MAP kinase in rats. PLoS Med. 2006;3:e338. doi: 10.1371/journal.pmed.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mbalaviele G, Anderson G, Jones A, De Ciechi P, Settle S, Mnich S, Thiede M, Abu-Amer Y, Portanova J, Monahan J. Inhibition of p38 mitogen-activated protein kinase prevents inflammatory bone destruction. J Pharmacol Exp Ther. 2006;317:1044–1053. doi: 10.1124/jpet.105.100362. [DOI] [PubMed] [Google Scholar]
- 19.Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Pei J, Kumar D, Sakabe I, Boudreau HE, Gokhale PC, Kasid UN. Antisense oligonucleotides: target validation and development of systemically delivered therapeutic nanoparticles. Methods Mol Biol. 2007;361:163–185. doi: 10.1385/1-59745-208-4:163. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Newsome JT, Mewani R, Pei J, Gokhale PC, Kasid UN. Systemic delivery and pre-clinical evaluation of nanoparticles containing antisense oligonucleotides and siRNAs. Methods Mol Biol. 2009;480:65–83. doi: 10.1007/978-1-59745-429-2_5. [DOI] [PubMed] [Google Scholar]
- 22.Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 2007;21:3355–3368. doi: 10.1096/fj.06-6713com. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann M. Ethical considerations in relation to pain in animal experimentation. Acta Physiol Scand Suppl. 1986;554:221–233. [PubMed] [Google Scholar]
- 24.Xiang HB, Xiao JB, Zhao WX. Expression of signal transducers and activators of transcription 3 in the lumbar spinal cord induced by the tibial cancer pain in rats. J Clin Surg. 2006;14:517–518. [Google Scholar]
- 25.Baker BF, Lot SS, Condon TP, Cheng-Flournoy S, Lesnik EA, Sasmor HM, Bennett CF. 2’-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J Biol Chem. 1997;272:11994–12000. doi: 10.1074/jbc.272.18.11994. [DOI] [PubMed] [Google Scholar]
- 26.Butler M, McKay RA, Popoff IJ, Gaarde WA, Witchell D, Murray SF, Dean NM, Bhanot S, Monia BP. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51:1028–1034. doi: 10.2337/diabetes.51.4.1028. [DOI] [PubMed] [Google Scholar]
- 27.Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, Hattenberger M, Vaxelaire J, O’Reilly T, Wotherspoon G, Winter J, Green J, Urban L. A rat model of bone cancer pain. Pain. 2002;96:129–140. doi: 10.1016/s0304-3959(01)00437-7. [DOI] [PubMed] [Google Scholar]
- 28.Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods. 1999;90:81–86. doi: 10.1016/s0165-0270(99)00075-8. [DOI] [PubMed] [Google Scholar]
- 29.An K, Xu Y, Yang H, Shu HH, Xiang HB, Tian YK. Subarachnoid transplantation of immortalized galanin-overexpressing astrocytes attenuates chronic neuropathic pain. Eur J Pain. 2010;14:595–601. doi: 10.1016/j.ejpain.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Xu B, Guan XH, Yu JX, Lv J, Zhang HX, Fu QC, Xiang HB, Bu HL, Shi D, Shu B, Qin LS, Manyande A, Tian YK. Activation of spinal phosphatidylinositol 3-kinase/protein kinase B mediates pain behavior induced by plantar incision in mice. Exp Neurol. 2014;255:71–82. doi: 10.1016/j.expneurol.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 31.Cao F, Chen SS, Yan XF, Xiao XP, Liu XJ, Yang SB, Xu AJ, Gao F, Yang H, Chen ZJ, Tian YK. Evaluation of side effects through selective ablation of the mu opioid receptor expressing descending nociceptive facilitatory neurons in the rostral ventromedial medulla with dermorphin-saporin. Neurotoxicology. 2009;30:1096–106. doi: 10.1016/j.neuro.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Villetti G, Bergamaschi M, Bassani F, Bolzoni PT, Maiorino M, Pietra C, Rondelli I, Chamiot-Clerc P, Simonato M, Barbieri M. Antinociceptive activity of the N-methyl-D-aspartate receptor antagonist N-(2-Indanyl)-glycinamide hydrochloride (CHF3381) in experimental models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;306:804–814. doi: 10.1124/jpet.103.050039. [DOI] [PubMed] [Google Scholar]
- 33.Ke CB, He WS, Li CJ, Shi D, Gao F, Tian YK. Enhanced SCN7A/Nax expression contributes to bone cancer pain by increasing excitability of neurons in dorsal root ganglion. Neuroscience. 2012;227C:80–89. doi: 10.1016/j.neuroscience.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 34.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Tian XB, An K, Yang H, Tian YK. Lumbar transplantation of immortalized enkephalin-expressing astrocytes attenuates chronic neuropathic pain. Eur J Pain. 2008;12:525–533. doi: 10.1016/j.ejpain.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Zahn PK, Brennan TJ. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology. 1999;90:863–872. doi: 10.1097/00000542-199903000-00030. [DOI] [PubMed] [Google Scholar]
- 37.Wang GM, Tian YK, Chen JP, Tian XB, Gao F, Yang H, An K, Ma GP. Evaluation of NR2B peptide as subunit vaccines against experimental neuropathic pain. Chin Med J (Engl) 2007;120:643–647. [PubMed] [Google Scholar]
- 38.Beardmore VA, Hinton HJ, Eftychi C, Apostolaki M, Armaka M, Darragh J, McIlrath J, Carr JM, Armit LJ, Clacher C, Malone L, Kollias G, Arthur JS. Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol Cell Biol. 2005;25:10454–10464. doi: 10.1128/MCB.25.23.10454-10464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urch CE, Donovan-Rodriguez T, Dickenson AH. Alterations in dorsal horn neurones in a rat model of cancer-induced bone pain. Pain. 2003;106:347–356. doi: 10.1016/j.pain.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Bu H, Shu B, Gao F, Liu C, Guan X, Ke C, Cao F, Hinton AO Jr, Xiang H, Yang H, Tian X, Tian Y. Spinal IFN-gamma-induced protein-10 (CXCL10) mediates metastatic breast cancer-induced bone pain by activation of microglia in rat models. Breast Cancer Res Treat. 2014;143:255–263. doi: 10.1007/s10549-013-2807-4. [DOI] [PubMed] [Google Scholar]
- 41.Ke C, Li C, Huang X, Cao F, Shi D, He W, Bu H, Gao F, Cai T, Hinton AO Jr, Tian Y. Protocadherin20 promotes excitatory synaptogenesis in dorsal horn and contributes to bone cancer pain. Neuropharmacology. 2013;75:181–190. doi: 10.1016/j.neuropharm.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Bu H, Liu C, Gao F, Yang H, Tian X, Xu A, Chen Z, Cao F, Tian Y. Inhibition of glial activation in rostral ventromedial medulla attenuates mechanical allodynia in a rat model of cancer-induced bone pain. J Huazhong Univ Sci Technolog Med Sci. 2012;32:291–298. doi: 10.1007/s11596-012-0051-5. [DOI] [PubMed] [Google Scholar]
- 43.Fitzsimmons BL, Zattoni M, Svensson CI, Steinauer J, Hua XY, Yaksh TL. Role of spinal p38alpha and beta MAPK in inflammatory hyperalgesia and spinal COX-2 expression. Neuroreport. 2010;21:313–317. doi: 10.1097/WNR.0b013e32833774bf. [DOI] [PMC free article] [PubMed] [Google Scholar]


