Abstract
The distinguishing of intrapulmonary metastases from multiple primaries is of great clinical importance. Although comprehensive histological assessment (CHA) was recommended for addressing this problem, the limitations of CHA have been addressed. We hypothesized that a nonmucinous lepidic component with mild nuclear atypia (NLCMA) may be one of the important sign suggesting primary lesions. In this study, we measured the value of NLCMA in distinguishing multiple primaries from intrapulmonary metastases. We retrospectively analyzed a cohort of 54 patients with 116 lesions (70 comparisons). Intrapulmonary metastases and multiple primaries were differentiated on the basis of CHA (Method I) and CHA combined with the assessment of NLCMA (Method II), respectively. Then, the results of two methods were compared with survival analysis. 33 cases were defined as multiple primaries and 21 cases as metastases by Method I, while 41 cases as multiple primaries and 13 cases as metastases by Method II. On univariate analysis, there was a better DFS in patients with a tumor ≤ 3 cm (P=0.012), female gender (P=0.011), highest N0 (P=0.002), absent micropapillary (P=0.013), multiple primaries (P=0.008 by method I, P < 0.001 by method II). A multivariate analysis adjusting for gender, tumor size, micropapillary and multiple primaries/metastases (by methodI and method II, respectively) indicated that multiple primaries (by method II) was an independent predictors for DFS. The presence of NLCMA may indicate that a lesion should be defined as primary in multifocal adenocarcinoma.
Keywords: Lepidic, multifocal lung adenocarcinoma, comprehensive histological assessment
Introduction
The incidence of multifocal primary lung cancer has been reported to range from 3.7% to 8.0% [1-4] and has increased due to advances in clinical diagnostic techniques. Adenocarcinoma is the most common histological subtype of multifocal lung cancer [1,5-7]. The pathologic identification intrapulmonary metastases and multiple primaries play an important role, which is of great clinical importance as this influences staging, prognosis and therapeutic strategy.
Atypical adenomatous hyperplasia (AAH) and adenocarcinoma in situ (AIS) are considered to be the preinvasive lesions of peripheral lung adenocarcinoma [8]. The neoplastic cells of AIS are usually nonmucinous, and nuclear atypia is inconspicuous. A lepidic component (neoplastic cells growing along preexisting alveolar structure) is the most distinctive characteristic of AIS, and the presence of a precancerous lesion is a strong evidence for diagnosing primary carcinoma. However, it is also reported that lepidic component can be observed in the metastatic tumors, the cancer cells of lepidic component are usually mucinous [9-11] and severe nuclear atypia [12,13]. Aokage et al. [13] reported that the atypia of tumor cells at the peripheral lepidic area was fairly mild in primary lung cancer, which was not observed in the metastatic tumors. So, we hypothesized that a nonmucinous lepidic component with mild nuclear atypia (NLCMA) may be one of the important sign suggesting primary lesions.
Comprehensive histological assessment (CHA) [14] was recommended by the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) for differentiating multiple primary adenocarcinomas of the lung from metastases [8]. But, the limitations of CHA have been addressed. In this study, a cohort of patients with multifocal lung adenocarcinoma was retrospectively reviewed. We wanted to measure the value of the assessment of NLCMA in distinguishing multiple primaries from intrapulmonary metastases by combining with CHA.
Materials and methods
Patients and methods
The study was performed retrospectively on patients who underwent resection between February 2003 and August 2012 at Cancer Hospital Chinese Academy of Medical Sciences. Fifty-four patients with multifocal lung adenocarcinoma were chosen for this research. All patients had complete follow-up records. The clinical data for the study included gender, age, smoking, tumor size, type of resection, adjuvant therapy and TNM stage. The histological subtype and TNM stage of the specimens were determined according to the IASLC/ATS/ERS classification and the 7th edition of the TNM classification of the American Joint Committee on Cancer (AJCC) [15]. Disease-free survival (DFS) was defined as the time from resection of the last tumor to first recurrence or lung-cancer-related death. The study design was approved by an institutional ethics review board.
Histological assessment
All surgically resected specimens were routinely fixed by 10% formalin and embedded in paraffin, and a 4-μm-thick section was prepared and stained with hematoxylin and eosin (HE). All the slides were reviewed by DM Lin and W Sun. In this study, morphology evaluation was performed according to the new IASLC/ATS/ERS classification [8]; each histological component present was recorded in 5% increments. The predominant pattern was defined as the pattern with the largest percentage. The amount of nonmucious lepidic component present and assessment of the presence and absence of stromal, lymphovascular and pleural invasion were recorded in the diagnosis of AIS, minimally invasive adenocarcinoma (MIA). The tumors were also assessed for variants such as invasive mucinous adenocarcinoma and colloid.
Nuclear atypia in nonmucinous lepidic area was classified as mild atypia, and severe atypia. The mild atypia: the tumor cells were uniform or slightly irregularity nuclei in size and shape, arranged loosely and monolayerly, and showed clara cell and/or type II cell differentiation (Figure 1A, 1B). The severe atypia was enlarged nuclei of varied sizes and irregular, with prominent nucleoli. The tumor cells were arranged multilayerly (Figure 1C, 1D), or budding to the lumen (Figure 2).
Figure 1.
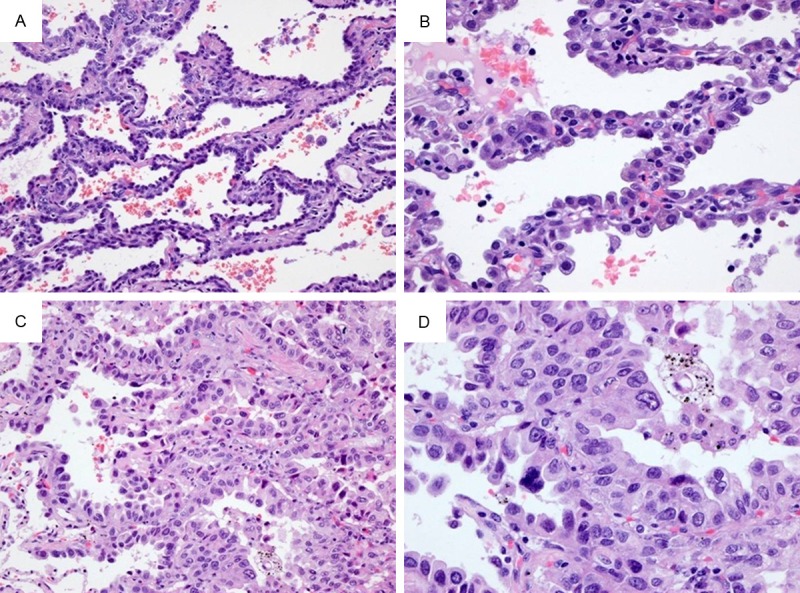
Nuclear features of lung adenocarcinoma. Mild atypia: the tumor cells spread along the alveolar wall (lepidic growth) (A, 200×), and arranged loosely and monolayerly. Tumor cells were uniform or slightly irregularity nuclei in size and shape, and showed clara cell and/or type II cell differentiation (B, 400×). Severe atypia: the tumor cells proliferated in lepidic pattern (C, 200×), and arranged multilayerly. Tumor cells were enlarged nuclei of varied sizes and irregular, with prominent nucleoli (D, 400×).
Figure 2.
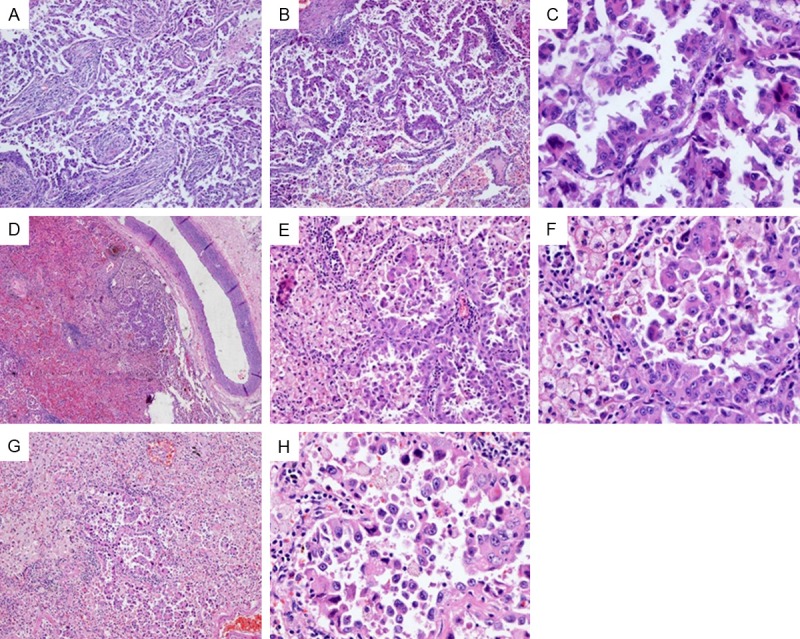
The tumor A of case 29 (A-C) was composed of micro-papillary and lepidic component. Tumor cells in lepidic area were severe atypia, and budding to the lumen. Small intrapulmonary metastatic tumors (D-H) was found around tumor A, and showed similar morphology. The tumors were composed of micro-papillary and lepidic component. Tumor cells in lepidic area showed severe atypia.
In this study, CHA was defined as Method I [14]. Briefly, Method I included evaluation of not only the percentages of histological subtypes, but also additional histological features such as cytological features, and patterns of stroma. CHA combined with NLCMA was defined as Method II (Figure 3). First of all, NLCMA was evaluated to comparing adenocarcinoma. If two lesions in one comparison present NLCMA, it was diagnosed as primary. Then, the rest of comparisons were compared by using of CHA. The distinction of intrapulmonary metastases from multiple primaries was identified by the two methods (Method I and Method II). Then, the results of two methods were compared with survival analysis.
Figure 3.
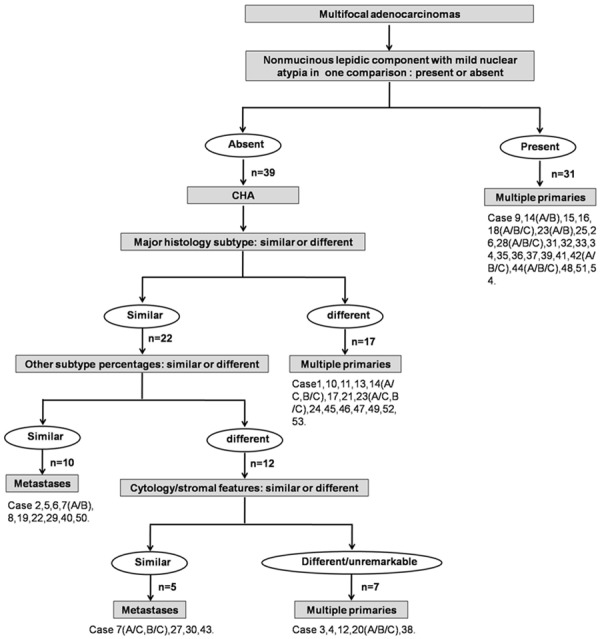
The histologic assessment of Method II in the discrimination of multiple primary lung cancers from intrapulmonary metastases.
Statistical analyses
The statistical comparisons were performed using Fisher’s exact test. DFS was assessed using the Kaplan-Meier method. The log-rank test was used to compare survival curves. The Cox proportional hazards model was employed for multivariate analysis. Results were considered significant at the 0.05 level. All statistical analyses were performed using the SPSS software program (Chicago, IL), version 18.0.
Results
Patient’s characteristics and histological assessment
Of the 54 patients, 46 had double lesions and 8 had triple lesions. Cancers were synchronous in 45 patients and metachronous in 9 patients. The median interval between metachronous tumors was 59.0 months (range 14.0-72.0 months). The median age was 62 years (range 44-78), and 33 (61.1%) patients were female. A total of 18 patients (33.3%) were current or ex-smokers (Table 1).
Table 1.
The clinical features of the patients with multi-focal lung adenocarcinoma
| Characteristics | No. (%) or median (range) |
|---|---|
| Age (years) | 62 (44-78) |
| Gender | |
| Male | 21 (38.9) |
| Female | 33 (61.1) |
| Smoking status | |
| Smoker | 18 (33.3) |
| Non-smoker | 36 (66.7) |
| Largest tumor size (cm) | 2.0 (0.3-5.0) |
| Location | |
| Same lobe | 18 (33.3) |
| Ipsilateral different lobe | 23 (42.6) |
| Bilateral | 13 (24.1) |
| Tumor number | |
| Two | 47 (87.0) |
| Three | 7 (13.0) |
| Highest pN descriptor | |
| N0 | 36 (66.7) |
| N1/N2 | 18 (33.3) |
The percentages of histological subtypes, cytological features and stromal characteristics are shown in Table 2. The histopathologic assessment showed that 3.4% (n=4) of the tumors were AIS; 3.4% (n=4) were MIA; 37.1% (n=43) were acinar predominant (AP); 12.1% (n=14) were papillary predominant (PP); 25.9% (n=30) were nonmucinous lepidic predominant (LP); 12.9% (n=15) were solid predominant (SP); and 1.7% (n=2) were micropapillary predominant (MPP). In cases 13, 22, and 49, 4 lesions were classified as variants of invasive adenocarcinoma. Lepidic component was present in 67 (57.8%) lesions, and MP component was present in 16 (13.8%) lesions (regardless of percentage), respectively. 65 lesions were nonmucinous; 2 lesion (case 13, tumor B and case 49, tumor A) was mucinous, and diagnosis as invasive mucinous adenocarcinoma. NLCMA appeared in 64 of 66 lesions, only 2 lesions (case 1, tumor B and case 29, tumor A) presented nonmucinous lepidic component with severe nuclear atypia. In case 29, several small metastatic nodules (range 0.1-0.4 cm) were separately around the dominant mass. The small metastatic tumors were mainly composed of the lepidic pattern with severe atypia and micropaplliary component (Figure 2). AAH was incidentally observed in peripheral lung far away from the tumor in 8 cases (case 9, 15, 23, 31, 32, 41, 42, and 49). Vascular invasion was found in 4 cases (case 27, 29, 32, and 44).
Table 2.
The histological analysis of the multifocal lung adenocarcinoma
| Subtype | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Case | Presentation (Interval) | Tumor | Location | Largest size (cm) | N stage | Acinar (%) | Solid (%) | Papillary (%) | Micropapillary (%) | Nonmucinous Lepidic component (%) | Cytology or Stroma or Variants of invasive adenocarcinoma | Type of adenocarcinomas |
| 1 | Synchronous | A | LUL | 3.1 | N1 | 25 | 0 | 60 | 10 | 5 | PP | |
| B | LLL | 2.5 | N1 | 10 | 0 | 0 | 0 | 90 | MIA | |||
| 2 | Synchronous | A | LLL | 2.6 | N2 | 100 | 0 | 0 | 0 | 0 | AP | |
| B | LLL | 1.8 | N2 | 100 | 0 | 0 | 0 | 0 | AP | |||
| 3 | Synchronous | A | RLL | 4.0 | N0 | 0 | 0 | 90 | 10 | 0 | Inflammatory stroma | PP |
| B | RML | 4.0 | N0 | 0 | 0 | 80 | 20 | 0 | PP | |||
| 4 | Synchronous | A | RLL | 3.5 | N2 | 0 | 100 | 0 | 0 | 0 | SP | |
| B | RLL | 2.5 | N2 | 80 | 0 | 20 | 0 | 0 | AP | |||
| 5 | Synchronous | A | RUL | 2.0 | N2 | 0 | 100 | 0 | 0 | 0 | SP | |
| B | RUL | 3.2 | N2 | 0 | 100 | 0 | 0 | 0 | SP | |||
| 6 | Metachronous (59.0 mo) | A | LUL | 4.0 | N0 | 100 | 0 | 0 | 0 | 0 | Lymphoid hyperplasia | AP |
| B | LLL | 3.3 | N0 | 100 | 0 | 0 | 0 | 0 | Lymphoid hyperplasia | AP | ||
| 7 | Synchronous | A | LLL | 2.0 | N0 | 20 | 80 | 0 | 0 | 0 | Lymphoid hyperplasia | SP |
| B | LLL | 1.5 | N0 | 10 | 90 | 0 | 0 | 0 | Lymphoid hyperplasia | SP | ||
| C | LLL | 0.3 | N0 | 0 | 100 | 0 | 0 | 0 | Lymphoid hyperplasia | SP | ||
| 8 | Synchronous | A | LUL | 5.0 | N0 | 90 | 0 | 0 | 10 | 0 | AP | |
| B | LLL | 3.5 | N0 | 80 | 0 | 0 | 20 | 10 | AP | |||
| 9 | Synchronous | A | LLL | 4.5 | N1 | 40 | 0 | 0 | 0 | 60 | LP | |
| B | RLL | 2.0 | N0 | 30 | 0 | 0 | 0 | 70 | LP | |||
| 10 | Metachronous (69.0 mo) | A | RUL | 3.0 | N0 | 60 | 0 | 0 | 0 | 40 | AP | |
| B | RLL | 1.5 | N0 | 30 | 0 | 50 | 20 | 0 | PP | |||
| 11 | Metachronous (14.0 mo) | A | RML | 2.0 | N2 | 60 | 0 | 0 | 40 | 0 | AP | |
| B | LLL | 1.5 | N0 | 20 | 0 | 0 | 0 | 80 | LP | |||
| 12 | Synchronous | A | LUL | 4.5 | N0 | 30 | 70 | 0 | 0 | 0 | AP | |
| B | LUL | 5.0 | N0 | 0 | 100 | 0 | 0 | 0 | SP | |||
| 13 | Synchronous | A | LUL | 1.0 | N0 | 70 | 0 | 0 | 0 | 30 | AP | |
| B* | LUL | 0.8 | N0 | N/A | N/A | N/A | N/A | N/A | Invasive mucinous adenocarcinoma | |||
| 14 | Synchronous | A | LUL | 1.5 | N0 | 80 | 0 | 0 | 0 | 20 | AP | |
| B | LUL | 1.5 | N0 | 60 | 0 | 0 | 0 | 40 | AP | |||
| C | LUL | 0.7 | N0 | 40 | 0 | 60 | 0 | 0 | PP | |||
| 15 | Synchronous | A | RLL | 2.0 | N0 | 30 | 0 | 0 | 0 | 70 | LP | |
| B | RUL | 1.0 | N0 | 0 | 0 | 0 | 0 | 100 | AIS | |||
| 16 | Synchronous | A | LLL | 1.5 | N0 | 30 | 0 | 0 | 0 | 70 | LP | |
| B | LLL | 0.8 | N0 | 40 | 0 | 0 | 0 | 60 | Clear cells | LP | ||
| 17 | Synchronous | A | LLL | 3.0 | N0 | 25 | 0 | 0 | 0 | 75 | LP | |
| B | LUL | 1.5 | N0 | 100 | 0 | 0 | 0 | 0 | Mucinous | AP | ||
| 18 | Synchronous | A | RLL | 3.6 | N2 | 80 | 10 | 0 | 0 | 10 | AP | |
| B | RUL | 1.8 | N2 | 10 | 0 | 0 | 0 | 90 | MIA | |||
| C | RUL | 1.8 | N2 | 10 | 0 | 60 | 0 | 30 | PP | |||
| 19 | Synchronous | A | RUL | 2.0 | N1 | 20 | 80 | 0 | 0 | 0 | SP | |
| B | RLL | 1.1 | N1 | 10 | 90 | 0 | 0 | 0 | SP | |||
| 20 | Synchronous | A | RLL | 3.5 | N0 | 50 | 0 | 30 | 20 | 0 | AP | |
| B | RLL | 3.5 | N0 | 80 | 0 | 10 | 10 | 0 | Clear cells | AP | ||
| C | RUL | 0.5 | N0 | 100 | 0 | 0 | 0 | 0 | AP | |||
| 21 | Synchronous | A | LUL | 1.0 | N2 | 0 | 0 | 0 | 0 | 100 | AIS | |
| B | LLL | 2.5 | N2 | 0 | 0 | 60 | 40 | 0 | PP | |||
| 22 | Synchronous | A* | RUL | 1.6 | N2 | N/A | N/A | N/A | N/A | N/A | Colloid | |
| B* | RLL | 3.0 | N2 | N/A | N/A | N/A | N/A | N/A | Colloid | |||
| 23 | Synchronous | A | LLL | 1.5 | N0 | 40 | 0 | 0 | 0 | 60 | LP | |
| B | RUL | 1.7 | N0 | 20 | 0 | 0 | 0 | 80 | LP | |||
| C | LLL | 1.8 | N0 | 70 | 0 | 30 | 0 | 0 | AP | |||
| 24 | Synchronous | A | RUL | 2.0 | N0 | 100 | 0 | 0 | 0 | 0 | AP | |
| B | RLL | 2.5 | N0 | 30 | 0 | 0 | 0 | 70 | LP | |||
| 25 | Synchronous | A | RUL | 1.9 | N0 | 0 | 0 | 70 | 0 | 30 | PP | |
| B | LLL | 1.0 | N0 | 30 | 0 | 0 | 0 | 70 | LP | |||
| 26 | Synchronous | A | RUL | 0.6 | N0 | 20 | 0 | 0 | 0 | 80 | MIA | |
| B | RUL | 1.0 | N0 | 0 | 0 | 80 | 0 | 20 | PP | |||
| 27 | Synchronous | A | RLL | 2.0 | N1 | 80 | 0 | 10 | 10 | 0 | Lymphoid hyperplasia | AP |
| B | RUL | 4.0 | N1 | 100 | 0 | 0 | 0 | 0 | Lymphoid hyperplasia | AP | ||
| 28 | Metachronous (29.0 mo) | A | RLL | 3.0 | N0 | 20 | 0 | 0 | 0 | 80 | LP | |
| B | RML | 3.0 | N0 | 10 | 0 | 0 | 10 | 80 | LP | |||
| C | LLL | 1.3 | N0 | 0 | 0 | 60 | 0 | 40 | PP | |||
| 29 | Synchronous | A | LLL | 3.5 | N2 | 0 | 0 | 0 | 80 | 20 | MPP | |
| B | LUL | 1.0 | N2 | 0 | 0 | 0 | 80 | 20 | MPP | |||
| 30 | Metachronous (31.0 mo) | A | LLL | 2.8 | N0 | 70 | 0 | 0 | 0 | 30 | Mucinous | AP |
| B | LUL | 2.0 | N2 | 100 | 0 | 0 | 0 | 0 | Mucinous | AP | ||
| 31 | Synchronous | A | LUL | 1.5 | N0 | 0 | 0 | 0 | 0 | 100 | AIS | |
| B | LUL | 1.5 | N0 | 10 | 0 | 0 | 0 | 90 | MIA | |||
| 32 | Synchronous | A | RML | 1.2 | N0 | 70 | 0 | 20 | 0 | 10 | AP | |
| B | RUL | 0.8 | N0 | 80 | 0 | 0 | 0 | 20 | AP | |||
| 33 | Synchronous | A | RML | 1.5 | N0 | 40 | 0 | 0 | 0 | 60 | LP | |
| B | RUL | 3.0 | N0 | 80 | 0 | 0 | 0 | 20 | AP | |||
| 34 | Synchronous | A | LUL | 2.2 | N0 | 60 | 0 | 30 | 0 | 10 | Clear cells | AP |
| B | RUL | 1.4 | N0 | 20 | 0 | 0 | 0 | 80 | LP | |||
| 35 | Synchronous | A | RUL | 2.0 | N0 | 0 | 0 | 0 | 0 | 100 | AIS | |
| B | RUL | 2.0 | N0 | 20 | 0 | 0 | 0 | 80 | LP | |||
| 36 | Synchronous | A | LUL | 1.5 | N0 | 20 | 0 | 0 | 0 | 80 | LP | |
| B | LUL | 2.0 | N0 | 20 | 0 | 0 | 0 | 80 | LP | |||
| 37 | Synchronous | A | RLL | 2.5 | N0 | 70 | 0 | 0 | 0 | 30 | AP | |
| B | LLL | 0.9 | N0 | 40 | 0 | 0 | 0 | 60 | LP | |||
| 38 | Synchronous | A | RUL | 3.9 | N1 | 60 | 40 | 0 | 0 | 0 | AP | |
| B | RUL | 2.2 | N1 | 80 | 0 | 0 | 0 | 20 | AP | |||
| 39 | Synchronous | A | RLL | 2.0 | N0 | 30 | 0 | 0 | 0 | 70 | LP | |
| B | RUL | 3.0 | N0 | 40 | 0 | 0 | 0 | 60 | LP | |||
| 40 | Synchronous | A | RML | 2.7 | N0 | 100 | 0 | 0 | 0 | 0 | AP | |
| B | RUL | 0.6 | N0 | 100 | 0 | 0 | 0 | 0 | AP | |||
| 41 | Synchronous | A | RML | 2.5 | N0 | 25 | 0 | 0 | 0 | 75 | LP | |
| B | LUL | 1.3 | N0 | 0 | 0 | 80 | 0 | 20 | PP | |||
| 42 | Synchronous | A | RLL | 1.5 | N0 | 70 | 0 | 0 | 0 | 30 | AP | |
| B | LUL | 1.5 | N0 | 80 | 0 | 0 | 0 | 20 | AP | |||
| C | LUL | 2.0 | N0 | 40 | 0 | 0 | 0 | 60 | LP | |||
| 43 | Metachronous (35.0 mo) | A | RUL | 3.0 | N0 | 60 | 0 | 30 | 0 | 10 | Mucinous | AP |
| B | LLL | 4.0 | N2 | 70 | 0 | 20 | 10 | 0 | Mucinous | AP | ||
| 44 | Synchronous | A | RUL | 2.8 | N2 | 30 | 10 | 0 | 0 | 60 | LP | |
| B | RML | 2.2 | N2 | 30 | 0 | 0 | 0 | 70 | LP | |||
| C | RLL | 1.1 | N2 | 20 | 0 | 0 | 0 | 80 | LP | |||
| 45 | Metachronous (63.0 mo) | A | LUL | 3.0 | N1 | 70 | 0 | 10 | 0 | 20 | LP | |
| B | LLL | 2.0 | N1 | 10 | 90 | 0 | 0 | 0 | SP | |||
| 46 | Synchronous | A | RUL | 2.5 | N0 | 0 | 100 | 0 | 0 | 0 | Necrosis | SP |
| B | RML | 0.4 | N0 | 100 | 0 | 0 | 0 | 0 | AP | |||
| 47 | Synchronous | A | RUL | 1.3 | N1 | 80 | 20 | 0 | 0 | 0 | AP | |
| B | RUL | 3.1 | N1 | 20 | 80 | 0 | 0 | 0 | Signet ring cells | SP | ||
| 48 | Synchronous | A | LUL | 1.7 | N0 | 30 | 0 | 60 | 0 | 10 | PP | |
| B | LUL | 0.7 | N0 | 30 | 0 | 20 | 0 | 50 | LP | |||
| 49 | Metachronous (63.0 mo) | A | LLL | 4.3 | N0 | N/A | N/A | N/A | N/A | N/A | Invasive mucinous adenocarcinoma | |
| B | RLL | 1.5 | N0 | 0 | 0 | 70 | 30 | 0 | PP | |||
| 50 | Metachronous (72.0 mo) | A | LLL | 1.2 | N0 | 0 | 100 | 0 | 0 | 0 | SP | |
| B | LLL | 1.0 | N0 | 0 | 100 | 0 | 0 | 0 | SP | |||
| 51 | Synchronous | A | RUL | 2.0 | N0 | 70 | 0 | 0 | 0 | 30 | AP | |
| B | RUL | 1.2 | N0 | 0 | 0 | 70 | 0 | 30 | PP | |||
| 52 | Synchronous | A | LLL | 1.3 | N0 | 30 | 0 | 0 | 0 | 70 | Clear cells | LP |
| B | RLL | 1.1 | N0 | 80 | 0 | 0 | 0 | 20 | AP | |||
| 53 | Synchronous | A | LUL | 2.1 | N0 | 20 | 80 | 0 | 0 | 0 | SP | |
| B | LUL | 0.6 | N0 | 100 | 0 | 0 | 0 | 0 | AP | |||
| 54 | Synchronous | A | LLL | 1.1 | N0 | 30 | 0 | 0 | 0 | 70 | LP | |
| B | RLL | 4.5 | N0 | 60 | 0 | 20 | 0 | 20 | AP | |||
N/A, not applicable; LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; LP, lepidic predominant; AP, acinar predominant; PP, papillary predominant; SP, solid predominant; MPP, micropapillary predominant.
The tumors were classified as the variants of invasive adenocarcinoma, so the component was not given in this table.
In cases with 2 tumors, 1 comparison was made; in cases with 3 tumors, there were 3 comparisons. There were 116 tumors and 70 comparisons totally in the study. The maximum diameter of tumors was ranged from 0.3 cm to 5.0 cm. 33 patients were diagnosed as multiple primaries and 21 patients as metastases by Method I. While, there were 41 cases as multiple primaries and 13 cases as metastases by Method II (Figure 3). The discrepancy between the two methods comprised 8 cases that were diagnosed as multiple primaries using Method II but regarded as metastases using Method I (Table 3). The metastases cases classified by Method II associated with high incidence of lymph node metastases (8 of 13, 61.5%) (P=0.020).
Table 3.
Comparison of Method I and Method II in this study
| Case | Method I | Method II |
|---|---|---|
| 1 | Multiple primaries | Multiple primaries |
| 2 | Metastases | Metastases |
| 3 | Multiple primaries | Multiple primaries |
| 4 | Multiple primaries | Multiple primaries |
| 5 | Metastases | Metastases |
| 6 | Metastases | Metastases |
| 7 (A vs. B) | Metastases | Metastases |
| 7 (A vs. C) | Metastases | Metastases |
| 7 (B vs. C) | Metastases | Metastases |
| 8 | Metastases | Metastases |
| 9* | Metastases | Multiple primaries |
| 10 | Multiple primaries | Multiple primaries |
| 11 | Multiple primaries | Multiple primaries |
| 12 | Multiple primaries | Multiple primaries |
| 13 | Multiple primaries | Multiple primaries |
| 14 (A vs. B)* | Metastases | Multiple primaries |
| 14 (A vs. C) | Multiple primaries | Multiple primaries |
| 14 (B vs. C) | Multiple primaries | Multiple primaries |
| 15 | Multiple primaries | Multiple primaries |
| 16* | Metastases | Multiple primaries |
| 17 | Multiple primaries | Multiple primaries |
| 18 (A vs. B) | Multiple primaries | Multiple primaries |
| 18 (A vs. C) | Multiple primaries | Multiple primaries |
| 18 (B vs. C) | Multiple primaries | Multiple primaries |
| 19 | Metastases | Metastases |
| 20 (A vs. B) | Multiple primaries | Multiple primaries |
| 20 (A vs. C) | Multiple primaries | Multiple primaries |
| 20 (B vs. C) | Multiple primaries | Multiple primaries |
| 21 | Multiple primaries | Multiple primaries |
| 22 | Metastases | Metastases |
| 23 (A vs. B)* | Metastases | Multiple primaries |
| 23 (A vs. C) | Multiple primaries | Multiple primaries |
| 23 (B vs. C) | Multiple primaries | Multiple primaries |
| 24 | Multiple primaries | Multiple primaries |
| 25 | Multiple primaries | Multiple primaries |
| 26 | Multiple primaries | Multiple primaries |
| 27 | Metastases | Metastases |
| 28 (A vs. B) | Multiple primaries | Multiple primaries |
| 28 (A vs. C) | Multiple primaries | Multiple primaries |
| 28 (B vs. C) | Multiple primaries | Multiple primaries |
| 29 | Metastases | Metastases |
| 30 | Metastases | Metastases |
| 31 | Multiple primaries | Multiple primaries |
| 32 | Multiple primaries | Multiple primaries |
| 33 | Multiple primaries | Multiple primaries |
| 34 | Multiple primaries | Multiple primaries |
| 35 | Multiple primaries | Multiple primaries |
| 36* | Metastases | Multiple primaries |
| 37 | Multiple primaries | Multiple primaries |
| 38 | Multiple primaries | Multiple primaries |
| 39* | Metastases | Multiple primaries |
| 40 | Metastases | Metastases |
| 41 | Multiple primaries | Multiple primaries |
| 42 (A vs. B)* | Metastases | Multiple primaries |
| 42 (A vs. C) | Multiple primaries | Multiple primaries |
| 42 (B vs. C) | Multiple primaries | Multiple primaries |
| 43 | Metastases | Metastases |
| 44 (A vs. B) | Multiple primaries | Multiple primaries |
| 44 (A vs. C) | Multiple primaries | Multiple primaries |
| 44 (B vs. C)* | Metastases | Multiple primaries |
| 45 | Multiple primaries | Multiple primaries |
| 46 | Multiple primaries | Multiple primaries |
| 47 | Multiple primaries | Multiple primaries |
| 48 | Multiple primaries | Multiple primaries |
| 49 | Multiple primaries | Multiple primaries |
| 50 | Metastases | Metastases |
| 51 | Multiple primaries | Multiple primaries |
| 52 | Multiple primaries | Multiple primaries |
| 53 | Multiple primaries | Multiple primaries |
| 54 | Multiple primaries | Multiple primaries |
Indicates discrepancies between the two methods.
Treatment
More than half (38 of 54, 70.4%) of the patients underwent a single operation, whereas 16 patients (29.6%) underwent two or more operations. Pneumonectomy and lobectomy (including bilobectomy and multiple lobectomy) were performed for 4 and 82 tumors, respectively. Segmentectomy and wedge resection were performed for 4 and 16 tumors, respectively. 28 patients (51.9%) underwent a standard surgical resection such as a lobectomy or pneumonectomy, and 26 patients (48.1%) received at least one limited resection. More than half (38 of 54, 70.4%) of the patients underwent a single operation, whereas 16 patients (29.6%) underwent two or more operations. All patients had not received neoadjuvant therapy. 23 patients (42.6%) underwent adjuvant treatment, which consisted of chemotherapy, radiotherapy or both.
Survival
The median follow-up time was 30.0 months (range 4.0-127.0 months). Of the 54 patients, 20 had recurrence of disease or died during the study period. The patients which all the tumors present a component of NLCMA (case 9, 15, 16, 18, 25, 26, 28, 31, 32, 33, 34, 35, 36, 37, 39, 41,42, 44, 48, 51, and 54) had good clinical outcome. Only 4 patients (case 9, 18, 48, and 54) had recurrence or died. Overall, median DFS from the last resection was 93.0 months (95% confidence interval, 33.3-152.7 months). On univariable analysis, there was a better DFS in patients with a tumor ≤ 3 cm (P=0.012), female gender (P=0.011), highest N0 (P=0.002), absent micropapillary (P=0.013), multiple primaries (P=0.008 by method I, P < 0.001 by method II) (Figure 4; Table 4). A multivariate analysis adjusting for gender, N stage, tumor size, micropapillary and multiple primaries/metastases (by method I and method II, respectively) indicated that only multiple primaries (by method II) remained significantly associated with DFS (Table 5).
Figure 4.
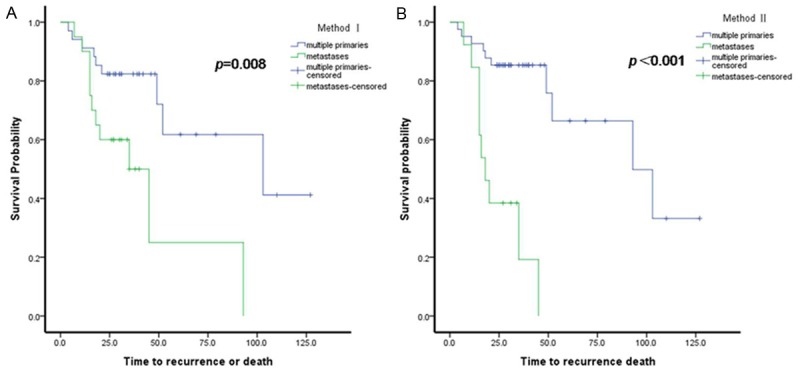
Survival analysis. Disease-free survival (DFS) in cases of multiple primaries and metastatic lung adenocarcinoma, as classified using (A) Method I and (B) Method II.
Table 4.
Univariable analysis of the disease-free survival according to clinical and pathologic characteristic
| Variable | No. | No. of event | Disease-free survival | ||
|---|---|---|---|---|---|
|
| |||||
| Median | 95% confidence interval | P-value | |||
| Total group | 54 | 20 | 93.0 | 33.3-152.7 | |
| Gender | |||||
| Male | 21 | 13 | 49.0 | 8.2-89.8 | 0.011 |
| Female | 33 | 7 | - | - | |
| Age | |||||
| > 65 | 17 | 6 | 93.0 | 0-203.7 | 0.845 |
| ≤ 65 | 37 | 14 | 52.0 | 16.0-88.0 | |
| Smoking | |||||
| Never-smoker | 36 | 12 | 103.0 | 34.4-171.6 | 0.235 |
| Smoker | 18 | 8 | 49.0 | 41.4-56.7 | |
| Largest size (cm) | |||||
| ≤ 3 cma | 36 | 9 | 103.0 | 27.8-178.2 | 0.012 |
| > 3 cm | 18 | 11 | 20.0 | 0-54.6 | |
| Location | |||||
| Same lobe | 18 | 4 | 52.0 | 46.0-58.0 | 0.050 |
| Ipsilateral different lobes | 23 | 12 | 35.0 | 12.5-57.5 | |
| Bilateral lobe | 13 | 4 | 93.0 | 0-198.4 | |
| Vascular invasion | |||||
| Yesa | 4 | 2 | 20.0 | - | 0.307 |
| No | 50 | 18 | 93.0 | 33.3-152.7 | |
| Micropapillary | |||||
| Yesa | 11 | 8 | 20.0 | 0-44.7 | 0.013 |
| No | 43 | 12 | 93.0 | 18.1-168.0 | |
| Highest N stage | |||||
| N0 | 36 | 7 | - | - | 0.002 |
| N1-2 | 18 | 13 | 20.0 | 13.8-26.2 | |
| Use of limited resection | |||||
| Yes | 26 | 11 | 93.0 | - | 0.112 |
| No | 28 | 9 | 103.0 | 37.0-169.0 | |
| Pneumonectomy | |||||
| Yes | 2 | 2 | 15.0 | - | 0.056 |
| No | 52 | 18 | 93.0 | 8.1-178.0 | |
| Adjuvant therapy | |||||
| Yes | 23 | 10 | 52.0 | 9.2-94.8 | 0.979 |
| No | 31 | 10 | 93.0 | 20.5-165.6 | |
| Method I | |||||
| multiple primaries | 34 | 9 | 103.0 | 10.4-195.6 | 0.008 |
| metastases | 20 | 11 | 35.0 | 17.0-53.1 | |
| Method II | |||||
| multiple primaries | 41 | 10 | 93.0 | 41.2-144.8 | < 0.001 |
| metastases | 13 | 10 | 18.0 | 12.1-23.9 | |
at least one tumor in one comparison.
-: the value was not given by statistical analyses.
Table 5.
Multivariable analysis of the disease-free survival
| Variables | Method I | Method II | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| HR ratio | 95% confidence interval | P-value | HR ratio | 95% confidence interval | P-value | |
| Gender (male vs female ) | 1.954 | 0.721-5.294 | 0.188 | 2.017 | 0.751-5.414 | 0.164 |
| Highest N stage (N0 vs. N1/N2) | 0.398 | 0.141-1.126 | 0.083 | 0.365 | 0.129-1.038 | 0.059 |
| Tumor size (≤ 3 cm vs > 3 cm) | 0.768 | 0.278-2.121 | 0.610 | 0.710 | 0.260-1.940 | 0.504 |
| Micropapillary (prenstation vs absence) | 1.721 | 0.643-4.609 | 0.280 | 1.037 | 0.363-2.962 | 0.946 |
| Metastases vs multiple primaries | 2.114 | 0.789-5.663 | 0.137 | 5.269 | 1.757-15.799 | 0.003 |
Discussion
Patients regarded as multiple primaries have a much better clinical outcome than intrapulmonary metastasis. Recent surgical data have suggested that multiple primary cancers do not adversely affect survival of lung cancer patients and may be amenable to surgical resection with prolonged survival [16,17]. The major aim of this study was to measure the value of the assessment of NLCMA in distinguishing between metastases and multiple primaries. The results showed that patients with multifocal lung adenocarcinoma which NLCMA involved had good clinical outcome. The clinical outcomes supported Method II as being clinically relevant, as patients with tumors regards as multiple primaries had better outcome compared with metastases (analysis by univariable and multivariate analysis).
Similarly to single lung adenocarcinoma, many factors affect the outcome of patients with multiple primaries, such as tumor size, T stage, N stage and gender [16,18-20]. Our results were consisted these results before. We found that small tumor size, female gender, highest N0 were associated with long term DFS. Several studies have shown that lymph node status being a significant prognostic factor of survival among patients who underwent resection for treatment of multifocal lung adenicarcinoma [18] Chang et al. [21] found that the occurrence of lymph node metastasis was more commonly observed in intrapulmonary metastases. In the present study, the metastases cases classified by Method II associated with high incidence of lymph node metastases.
In addition, we found that the presence of a micropapillary component may be a predictor of poor prognosis in multifocal lung adenocarcinoma. A micropapillary pattern has also been reported as an important factor in predicting poor prognosis in single lung adenocarcinoma [22]. Previous studies [3,23] have shown that performance of a pneumonectomy had a major adverse and independent impact on survival. In the present study, the number of patients (n=2) who underwent pneumonectomy for multiple tumors was limited. So, we did not find any statistically significant difference in DFS when comparing the group of patients who underwent pneumonectomy and the group of patients who underwent lobectomy or limited resection (P=0.056). Wedge or segmental resection might increase local recurrence, as reported by some authors [24,25]. However, use of limited resection was not associated with poor DFS in the present study.
Numerous studies showed that multifocal adenocarcinoma with lepidic component had excellent clinical outcome, and suggested that these lesions arise as independent events rather than intrapulmonary spread [26-28]. But, it was also reported that a lepidic component can appear in metastatic cancers [29]. Aokage et al [13] thought that most metastatic tumors from primary adenocarcinoma exhibited a lepidic growth in the early phase and recapitulated the morphological heterogeneity of the original tumor as the tumor grew. In case 29, small, multifocal, metastasis nodules were found around a dominant tumor. The small metastasis lesions present a lepidic and micropapillary pattern just like the dominant tumor. Most of metastasis lesions were less than 5 mm. But, the morphology of small metastasis lesion was different with AAH and AIS. We thought that a lepidic component can appear in the metastasis tumor, but the tumor cells were severe atypia, arranged multilayerly, or budding to the lumen. So, a lepidic component with severe atypia was not the evidence of primary tumor.
Some authors have reported that nearly 20% of cases of AIS show evidence of multifocality, and AAH has a close relationship with multiple primary lung adenocarcinomas [30-32]. Our result was consisted with the result. In this study, AIS and AAH were found in 4 and 8 cases respectively, which were diagnosed as multiple primaries by method II. So, we hypothesized that multicentric AIS may likewise be the pathogenesis of multiple primary lung adenocarcinomas. We thus speculated that the presence of a nonmucinous lepidic component (the tumor cells were mild atypia, and showed clara cell and/or type II cell differentiation) indicated that an adenocarcinoma could be defined as primary.
Sometimes, multifocal adenocarcinomas were encountered or presented as pneumonic consolidation. Lung adenocarcinoma presents as a diffuse, pneumonia-like, lobar consolidation, which is typical of invasive mucinous adenocarcinoma. Multiple studies indicate that tumors with mucinous lepidic (invasive mucinous adenocarcinoma) have major clinical, radiologic, pathologic and genetic differences from nonmucinous [33,34]. The presence of diffuse, pneumonia-like, lobar consolidation may be due to field cancerization of pulmonary epithelial cells. These cases seem to have a low metastatic potential [35]. Due to limited sampling, it is difficult to make a complete molecular and pathologic assessment of this type. Thus, further study of this situation should be conducted.
Several limitations of this study require consideration. First, in some cases the follow-up period was short. Long-term follow-up over several years is needed to accurately assess the prognosis of patients. Second, the sample size was limited. More patients are needed to evaluate other factors associated with clinical outcome. Third, molecular clone analysis about multifocal adenocarcinomas should be performed to support our hypothesis. The epidermal growth factor receptor (EGFR) mutation of multifocal adenocarcinoma will be investigated, and reported later.
In conclusion, the presence of NLCMA in lung multifocal adenocarcinoma might indicate a lesion as primary. The method that combines CHA with the assessment of NLCMA may potentially improve diagnosis in differentiating multiple primaries from intrapulmonary metastases. For the further study, we will evaluate more factors including more follow-up data, enlargement the number of samples and further molecular characteristics to identify more clear-cut evidence.
Acknowledgements
This work was supported by The Capital Medical Development Foundation (No. 2011-4002-01).
Disclosure of conflict of interest
None.
References
- 1.Wang X, Wang M, MacLennan GT, Abdul-Karim FW, Eble JN, Jones TD, Olobatuyi F, Eisenberg R, Cummings OW, Zhang S, Lopez-Beltran A, Montironi R, Zheng S, Lin H, Davidson DD, Cheng L. Evidence for common clonal origin of multifocal lung cancers. J Natl Cancer Inst. 2009;101:560–570. doi: 10.1093/jnci/djp054. [DOI] [PubMed] [Google Scholar]
- 2.Nakata M, Sawada S, Yamashita M, Saeki H, Kurita A, Takashima S, Tanemoto K. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann Thorac Surg. 2004;78:1194–1199. doi: 10.1016/j.athoracsur.2004.03.102. [DOI] [PubMed] [Google Scholar]
- 3.Rostad H, Strand TE, Naalsund A, Norstein J. Resected synchronous primary malignant lung tumors: a population-based study. Ann Thorac Surg. 2008;85:204–209. doi: 10.1016/j.athoracsur.2007.07.091. [DOI] [PubMed] [Google Scholar]
- 4.Trousse D, D’Journo XB, Avaro JP, Doddoli C, Giudicelli R, Fuentes PA, Thomas PA. Multifocal T4 non-small cell lung cancer: a subset with improved prognosis. Eur J Cardiothoracic Sur. 2008;33:99–103. doi: 10.1016/j.ejcts.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Kaneda H, Uemura Y, Nakano T, Taniguchi Y, Saito T, Konobu T, Saito Y. Lesions in patients with multifocal adenocarcinoma are more frequently in the right upper lobes. Interact Cardiovasc Thorac Surg. 2012;15:627–632. doi: 10.1093/icvts/ivs276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warth A, Macher-Goeppinger S, Muley T, Thomas M, Hoffmann H, Schnabel PA, Penzel R, Schirmacher P, Aulmann S. Clonality of multifocal nonsmall cell lung cancer: implications for staging and therapy. Eur Respir J. 2012;39:1437–1442. doi: 10.1183/09031936.00105911. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda K, Nomori H, Ohba Y, Shibata H, Mori T, Honda Y, Iyama K, Kobayashi T. Epidermal growth factor receptor mutations in multicentric lung adenocarcinomas and atypical adenomatous hyperplasias. J Thorac Oncol. 2008;3:467–471. doi: 10.1097/JTO.0b013e31816b4b14. [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokunaga T, Arakawa H, Kuwashima Y. A case of lepidic pulmonary metastasis from adenocarcinoma of the gallbladder mimicking acute interstitial pneumonia. Clin Radiol. 2005;60:1213–1215. doi: 10.1016/j.crad.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Burke AP. Pulmonary oligometastases: histological features and difficulties in determining site of origin. Int J Surg Pathol. 2012;20:577–588. doi: 10.1177/1066896912449039. [DOI] [PubMed] [Google Scholar]
- 11.Gaeta M, Volta S, Scribano E, Loria G, Vallone A, Pandolfo I. Air-space pattern in lung metastasis from adenocarcinoma of the GI tract. J Comput Assist Tomogr. 1996;20:300–304. doi: 10.1097/00004728-199603000-00025. [DOI] [PubMed] [Google Scholar]
- 12.Nind NR, Attanoos RL, Gibbs AR. Unusual intraparenchymal growth patterns of malignant pleural mesothelioma. Histopathology. 2003;42:150–155. doi: 10.1046/j.1365-2559.2003.01555.x. [DOI] [PubMed] [Google Scholar]
- 13.Aokage K, Ishii G, Yoshida J, Hishida T, Nishimura M, Nagai K, Ochiai A. Histological progression of small intrapulmonary metastatic tumor from primary lung adenocarcinoma. Pathol Int. 2010;60:765–773. doi: 10.1111/j.1440-1827.2010.02596.x. [DOI] [PubMed] [Google Scholar]
- 14.Girard N, Deshpande C, Lau C, Finley D, Rusch V, Pao W, Travis WD. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am J Surg Pathol. 2009;33:1752–1764. doi: 10.1097/PAS.0b013e3181b8cf03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A. AJCC cancer staging manual. New York: Springer; 2010. [Google Scholar]
- 16.Chang YL, Wu CT, Lee YC. Surgical treatment of synchronous multiple primary lung cancers: experience of 92 patients. J Thorac Cardiovasc Surg. 2007;134:630–637. doi: 10.1016/j.jtcvs.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Aguilo R, Macia F, Porta M, Casamitjana M, Minguella J, Novoa AM. Multiple independent primary cancers do not adversely affect survival of the lung cancer patient. Eur J Cardiothorac Surg. 2008;34:1075–1080. doi: 10.1016/j.ejcts.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Voltolini L, Rapicetta C, Luzzi L, Ghiribelli C, Paladini P, Granato F, Gallazzi M, Gotti G. Surgical treatment of synchronous multiple lung cancer located in a different lobe or lung: high survival in node-negative subgroup. Eur J Cardiothorac Surg. 2010;37:1198–1204. doi: 10.1016/j.ejcts.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Jung EJ, Lee JH, Jeon K, Koh WJ, Suh GY, Chung MP, Kim H, Kwon OJ, Shim YM, Um SW. Treatment outcomes for patients with synchronous multiple primary non-small cell lung cancer. Lung Cancer. 2011;73:237–242. doi: 10.1016/j.lungcan.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Finley DJ, Yoshizawa A, Travis W, Zhou Q, Seshan VE, Bains MS, Flores RM, Rizk N, Rusch VW, Park BJ. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J Thorac Oncol. 2010;5:197–205. doi: 10.1097/JTO.0b013e3181c814c5. [DOI] [PubMed] [Google Scholar]
- 21.Chang YL, Wu CT, Lin SC, Hsiao CF, Jou YS, Lee YC. Clonality and prognostic implications of p53 and epidermal growth factor receptor somatic aberrations in multiple primary lung cancers. Clin Cancer Res. 2007;13:52–58. doi: 10.1158/1078-0432.CCR-06-1743. [DOI] [PubMed] [Google Scholar]
- 22.Cha MJ, Lee HY, Lee KS, Jeong JY, Han J, Shim YM, Hwang HS. Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg. 2014;147:921–928. e922. doi: 10.1016/j.jtcvs.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 23.Trousse D, Barlesi F, Loundou A, Tasei AM, Doddoli C, Giudicelli R, Astoul P, Fuentes P, Thomas P. Synchronous multiple primary lung cancer: an increasing clinical occurrence requiring multidisciplinary management. J Thorac Cardiovasc Surg. 2007;133:1193–1200. doi: 10.1016/j.jtcvs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Jensik RJ, Faber LP, Kittle CF, Meng RL. Survival following resection for second primary bronchogenic carcinoma. J Thorac Cardiovasc Surg. 1981;82:658–668. [PubMed] [Google Scholar]
- 25.Mathisen DJ, Jensik RJ, Faber LP, Kittle CF. Survival following resection for second and third primary lung cancers. J Thorac Cardiovasc Surg. 1984;88:502–510. [PubMed] [Google Scholar]
- 26.Chung JH, Choe G, Jheon S, Sung SW, Kim TJ, Lee KW, Lee JH, Lee CT. Epidermal growth factor receptor mutation and pathologic-radiologic correlation between multiple lung nodules with ground-glass opacity differentiates multicentric origin from intrapulmonary spread. J Thorac Oncol. 2009;4:1490–1495. doi: 10.1097/JTO.0b013e3181bc9731. [DOI] [PubMed] [Google Scholar]
- 27.Gu B, Burt BM, Merritt RE, Stephanie S, Nair V, Hoang CD, Shrager JB. A dominant adenocarcinoma with multifocal ground glass lesions does not behave as advanced disease. A Thorac Surg. 2013;96:411–418. doi: 10.1016/j.athoracsur.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 28.Vazquez M, Carter D, Brambilla E, Gazdar A, Noguchi M, Travis WD, Huang Y, Zhang L, Yip R, Yankelevitz DF, Henschke CI. Solitary and multiple resected adenocarcinomas after CT screening for lung cancer: histopathologic features and their prognostic implications. Lung Cancer. 2009;64:148–154. doi: 10.1016/j.lungcan.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yatabe Y, Borczuk AC, Powell CA. Do all lung adenocarcinomas follow a stepwise progression? Lung Cancer. 2011;74:7–11. doi: 10.1016/j.lungcan.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barsky SH, Grossman DA, Ho J, Holmes EC. The multifocality of bronchioloalveolar lung carcinoma: evidence and implications of a multiclonal origin. Mod Pathol. 1994;7:633–640. [PubMed] [Google Scholar]
- 31.Suzuki K, Takahashi K, Yoshida J, Nishimura M, Yokose T, Nishiwaki Y, Nagai K. Synchronous double primary lung carcinomas associated with multiple atypical adenomatous hyperplasia. Lung Cancer. 1998;19:131–139. doi: 10.1016/s0169-5002(97)00082-2. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Nagai K, Yoshida J, Yokose T, Kodama T, Takahashi K, Nishimura M, Kawasaki H, Yokozaki M, Nishiwaki Y. The prognosis of resected lung carcinoma associated with atypical adenomatous hyperplasia: a comparison of the prognosis of well-differentiated adenocarcinoma associated with atypical adenomatous hyperplasia and intrapulmonary metastasis. Cancer. 1997;79:1521–1526. doi: 10.1002/(sici)1097-0142(19970415)79:8<1521::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Wislez M, Antoine M, Baudrin L, Poulot V, Neuville A, Pradere M, Longchampt E, Isaac-Sibille S, Lebitasy MP, Cadranel J. Non-mucinous and mucinous subtypes of adenocarcinoma with bronchioloalveolar carcinoma features differ by biomarker expression and in the response to gefitinib. Lung Cancer. 2010;68:185–191. doi: 10.1016/j.lungcan.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 34.Hata A, Katakami N, Fujita S, Kaji R, Imai Y, Takahashi Y, Nishimura T, Tomii K, Ishihara K. Frequency of EGFR and KRAS mutations in Japanese patients with lung adenocarcinoma with features of the mucinous subtype of bronchioloalveolar carcinoma. J Thorac Oncol. 2010;5:1197–1200. doi: 10.1097/JTO.0b013e3181e2a2bc. [DOI] [PubMed] [Google Scholar]
- 35.Kerr KM. Pulmonary adenocarcinomas: classification and reporting. Histopathology. 2009;54:12–27. doi: 10.1111/j.1365-2559.2008.03176.x. [DOI] [PubMed] [Google Scholar]


