Abstract
Aim: To investigate the changes and contributions of superior mesenteric venous perfusion (SMVP) and splenic venous perfusion (SpVP) to portal venous CT perfusion in canine model of hepatic diffuse disease. Materials and methods: By selective catheterization in superior mesenteric and splenic arteries respectively after CT perfusion scanning, SMVP and SpVP became available. Sixteen dogs were adopted and induced by carbon tetrachloride after data under normal conditions were collected. After 3, 6, 9 and 12 months from carbon tetrachloride intervention, liver biopsies by puncture or operation were performed after CT perfusion scanning. SMVP and SpVP under different pathologic conditions were compared and analyzed. Results: Three stages of hepatic diffuse lesions were defined according to pathologic changes, namely hepatitis, hepatic fibrosis, and cirrhosis. The number of dogs which survived from each stage was: 16 from normal, 12 from hepatitis, 10 from hepatic fibrosis and 4 from cirrhosis. During this progressive period, SpVP ml/(min·100 ml) declined slightly, but there were no significant differences between different stages (P > 0.05). SMVP ml/(min·100 ml) in stage of normal (64.1 ± 8.1) and hepatic fibrosis (44.4 ± 4.5), normal and cirrhosis (42.6 ± 5.4), hepatitis (61.3 ± 6.4) and hepatic fibrosis, hepatitis and cirrhosis was significantly different, but there was no significant difference of SMVP between normal and hepatitis (P = 0.326) or hepatic fibrosis and cirrhosis (P = 0.668). Conclusions: With our evidence of interventional CT perfusion, it is mesenteric, not splenic, perfusion that might coincide with hepatic portal venous perfusion during the progressive period of hepatic diffuse disease.
Keywords: Portal venous perfusion, hepatic diffuse disease, computed tomography
Introduction
Under circumstances of portal hypertension (PHT), systemic and visceral hemodynamic changes mainly include increase of portal blood flow and pressure, visceral congestion (increased blood flow in superior mesenteric artery) and decrease of pressure in superior mesenteric artery [1]. It is of importance clinically to investigate such hemodynamic changes before and after hepatic cirrhosis with some invasive and non-invasive methods. CT perfusion (CTP) has shown its clinical feasibility by providing hepatic perfusion data noninvasively [2]. It has been proved after cirrhosis that hepatic blood flow and volume are decreased, hepatic arterial perfusion is increased, and portal venous perfusion is decreased [3]. Portal venous perfusion is the added perfusion from superior mesenteric vein plus splenic vein. By CT perfusion after selective catheterization in superior mesenteric artery (SMA) and splenic artery (SpA) respectively [4], this study was to investigate the changes and contributions of superior mesenteric venous perfusion (SMVP) and splenic venous perfusion (SpVP) to portal venous CT perfusion in canine models of hepatic diffuse disease.
Materials and methods
Animals
Sixteen healthy adult dogs (23.5-26 kg) were adopted in this study with no consideration of gender. One week was given for adaptation to the environment in independent ventilating rooms. After CT perfusion data under normal conditions were collected, all dogs were fed with high fat diet and intervened twice one week by injecting carbon tetrachloride (CCl4; Sigma Chemical Co., St. Louis, MO, USA) intra-abdominally. After 3, 6, 9 and 12 months from carbon tetrachloride intervention, liver biopsies by puncture or operation were performed after CT perfusion scanning (within 24 hours). Liver samples were collected for further pathological assessment by hematoxylin-eosin staining and electron microscopy. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Shengjing Hospital of China Medical University.
CT imaging
After 24-hour fast, the dog received narcosis and was fixed on a special board supinely. Bi-inguinal regions exposed largely enough, femoral arteries were punctured and selective catheterization into SMA and SpA was performed by Seldinger technique with 5F Coude’s catheter. After a quick angiography for confirmation, the dog was transported to the CT room.
All perfusion examinations were completed on Phillips Brilliance 64 spiral CT scanner. Plain scan (slice thickness = 3 mm) was performed to select a slice in which the superior mesenteric vein (SMV) or splenic vein (SpV), and the hepatic right lobe were clearly visualized. With this slice at the centre, eight-section sequential dynamic contrast enhancement CT scans were done according to the protocol of 0.5 s scan time, 1 s cycle time, 30 s total scan time; 120 kv, 150 mAs; 5-mm slice; 512 × 512 matrix, 220 mm FOV. A total amount of 10 ml Omnipaque (350 mg iodine/ml; GE Healthcare, Princeton, NJ, USA) was injected simultaneously with CT scanning with flow rate of 2 ml/s by an automated high pressure syringe. Scanning interval for SMVP and SpVP was more than 30 minutes.
Data analysis
All images were transmitted to the Philips Brilliance 64 workstation (Extented Brilliance Workspace, EBW) for data analysis using the accompanied perfusion software. Two regions of interest (ROI) were drawn in the SMV or SpV, and right lobe of the liver respectively to get satisfactory time-density curves, SMVP and SpVP. The ROI of liver parenchyma was contoured to be as large as possible and large blood vessels or liver margins should meanwhile not be involved.
Statistical analysis
Data were shown by expression of mean ± SD. The Student’s paired t test (P < 0.05 as statistically significant) was used to compare the perfusion changes under normal and different pathologic conditions.
Results
Pathological alterations
The process of hepatic diffuse lesions was classified into three stages according to pathologic alterations, namely hepatitis, hepatic fibrosis, and cirrhosis. The number of dogs which survived from each stage was: 16 from normal, 12 from hepatitis, 10 from hepatic fibrosis and 4 from cirrhosis. By pathological investigations, there were hepatocyte swelling and steatosis in stage of hepatitis, aggravating sinusoid capillarization and collagen deposition in Disse’s spaces in stage of fibrosis and finally pseudolobule appearance in stage of cirrhosis.
CT images
CT perfusion changes were shown in Table 1 and Figures 1, 2. During this progressive period, SpVP [unit: ml/(min·100 ml)] declined slightly (from 22.3 ± 3.9 at normal to 20.1 ± 2.6 at cirrhosis), but there were no significant differences between different stages (P > 0.05). The results of statistical analysis revealed that SMVP [unit: ml/(min·100 ml)] in stage of normal (64.1 ± 8.1) and hepatic fibrosis (44.4 ± 4.5), normal and cirrhosis (42.6 ± 5.4), hepatitis (61.3 ± 6.4) and hepatic fibrosis, hepatitis and cirrhosis was significantly different, but there was no significant difference of SMVP between normal and hepatitis (P = 0.326) or hepatic fibrosis and cirrhosis (P = 0.668).
Table 1.
SMVP and SpVP at different stages of hepatic diffuse disease (mean ± SD) [unit: ml/(min·100 ml)]
| Normal | Hepatitis | Fibrosis | Cirrhosis | |
|---|---|---|---|---|
| SMVP | 64.1±8.1 | 61.3±6.4 | 44.4±4.5 | 42.6±5.4 |
| SpVP | 22.3±3.9 | 25.4±8.7 | 20.4±5.6 | 20.1±2.6 |
Figure 1.
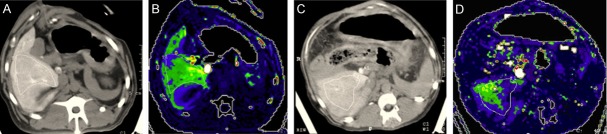
SMVP at hepatic normal stage (A, B. SMVP = 63.9 ml/100 g) and hepatic cirrhosis stage (C, D. SMVP = 19.7 ml/100 g). Unsmooth liver border and ascites were shown on (C) and decreased perfusion was shown on (D) compared to (B).
Figure 2.
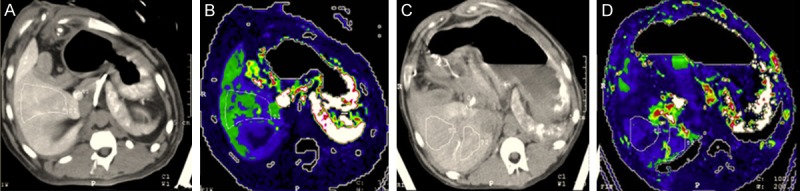
SpVP at hepatic normal stage (A, B. SpVP = 24.6 ml/100 g) and hepatic cirrhosis stage (C, D. SpVP = 22.3 ml/100 g). Nodular surface of the live and ascites were shown on (C) and decreased perfusion was shown on (D) compared to (B).
Discussion
Portal hypertension (PHT) is a syndrome of blood flow blockage or increase in portal venous system caused by various problems, among which hepatic cirrhosis is a common one [5]. Mechanisms of PHT hemodynamic changes have deeply been investigated with varied techniques on different hepatic cirrhosis models [6-8].
CT perfusion imaging can reflect hemodynamic perfusion of tissue and organ. By adoption of arterial catheterization, CT perfusion imaging and computed tomography arterial portography (CTAP) were combined together to achieve SMVP and SpVP with maximum slop analytic method [9,10]. Portal venous system is functionally sub-divided into “mesenteric region” (also called greater visceral circulation) and “gastro-splenic region” (also called less visceral circulation), whose contributions to portal perfusion can be indexed out by SMVP and SpVP respectively. Uematsu evaluated hepatic perfusion by semi-quantitative MR measurement after selective catheterization into SMA, and found SMVP was significantly decreased in Child B and C patients compared with Child A patients [11]. We found at hepatic fibrosis and cirrhosis, SMVP was obviously decreased but SpVP remained almost unchanged. It may be attributed to the following two aspects: anatomically, the spleen is rich in blood sinus and the pressure difference is smaller between splenic artery and vein. A relatively higher pressure can be retained in splenic vein due to its greater compensation ability [12]. There are rich capillaries between superior mesenteric artery and vein, which may cause bulk weakening of visceral arterial pressure [13]. On the other side, the superior mesenteric vein, which collects mostly intestinal venous blood, is the principal nutrient vessel of the liver. The obvious decrease of SMVP may be correlated to liver function damage after fibrosis or cirrhosis, which also accords to Annet’s study [14]. The decrease of SMVP may deteriorate hepatic cirrhosis and portal hypertension.
There have existed two different theories, forward and backward theories, which are concerning on the complicated PHT hemodynamic changes at hepatic fibrosis and cirrhosis. Half a century ago, the backward theory [14,15] was held that increased portal resistance was caused by compression of hyperplastic fibrous connective tissue after normal hepatic lobules were destroyed, which is also called mechanical obstruction theory. According to forward theory [11,16,17], high cardiac output and arterial blood perfusion will occur at hepatic cirrhosis, thus vasodilators (glycagon, vasoactive intestinal peptide, nitrogen monoxidum, etc) get increased, rennin-angiotonin-aldosterone system is activated to facilitate a visceral hyperkinetic circulatory state, and finally portal hypertension is developed and retained. Recently it is generally held that increased portal resistance is the initiating factor of portal hypertension, and visceral hyperkinetic circulatory state will retain or aggravate this condition [8]. In this study, SMVP and SpVP were not reduced proportionately and SMVP showed no significance between normal and hepatitis or hepatic fibrosis and cirrhosis, which all accorded to forward theory. SpVP was not obviously decreased between different stages of hepatic diffuse damage. This may imply the “nutrient” blood from the “mesenteric” region could be more determinant in the progression of portal hypertension, which we call the “nutrient” theory.
Hepatotoxic cirrhosis model induced by carbon tetrachloride imitates posthepatitic diffuse damage well [18-20]. Sinusoid capillarization, collagen deposition in Disse’s spaces and pseudolobule formation can cause narrowness and increased pressure in hepatic sinus, reduced blood perfusion and portal venous flow, and finally portal perfusion is decreased [21]. From such models, we found that SMVP contributed more during cirrhotic portal hypertension and proposed the “nutrient” theory to indicate that future medical treatments of hepatic diffuse disease, like fibrosis and cirrhosis, should target more on the mesenteric procedures, which may receive higher medical effectiveness on improving portal perfusion as well as relieving portal hypertension.
Disclosure of conflict of interest
None.
References
- 1.Kim MY, Baik SK, Lee SS. Hemodynamic alterations in cirrhosis and portal hypertension. Korean J Hepatol. 2010;16:347–52. doi: 10.3350/kjhep.2010.16.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma G, Bai R, Jiang H, Hao X, Ling Z, Li K. Assessment of hemodynamics in a rat model of liver cirrhosis with precancerous lesions using multislice spiral CT perfusion imaging. Biomed Res Int. 2013;2013:813174. doi: 10.1155/2013/813174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan S, Zhao WD, Zhou KR, Peng WJ, Mao J, Tang F. CT perfusion at early stage of hepatic diffuse disease. World J Gastroenterol. 2005;11:3465–7. doi: 10.3748/wjg.v11.i22.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katada Y, Shukuya T, Kawashima M, Nozaki M, Imai H, Natori T, Tamano M. A comparative study between arterial spin labeling and CT perfusion methods on hepatic portal venous flow. Jpn J Radiol. 2012;30:863–9. doi: 10.1007/s11604-012-0127-y. [DOI] [PubMed] [Google Scholar]
- 5.Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7:141–55. doi: 10.1586/egh.12.83. [DOI] [PubMed] [Google Scholar]
- 6.Lin D, Wu X, Ji X, Zhang Q, Lin Y, Chen W, Jin W, Deng L, Chen Y, Chen B, Li J. A novel canine model of portal vein stenosis plus thioacetamide administration-induced cirrhotic portal hypertension with hypersplenism. Cell Biochem Biophys. 2012;62:245–55. doi: 10.1007/s12013-011-9272-7. [DOI] [PubMed] [Google Scholar]
- 7.Avritscher R, Wright KC, Javadi S, Uthamanthil R, Gupta S, Gagea M, Bassett RL, Murthy R, Wallace MJ, Madoff DC. Development of a large animal model of cirrhosis and portal hypertension using hepatic transarterial embolization: a study in swine. J Vasc Interv Radiol. 2011;22:1329–34. doi: 10.1016/j.jvir.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Katsuta Y, Zhang XJ, Ohsuga M, Akimoto T, Komeichi H, Shimizu S, Inami T, Miyamoto A, Satomura K, Takano T. Hemodynamic features of advanced cirrhosis due to chronic bile duct ligation. J Nippon Med Sch. 2005;72:217–25. doi: 10.1272/jnms.72.217. [DOI] [PubMed] [Google Scholar]
- 9.Kanda T, Yoshikawa T, Ohno Y, Kanata N, Koyama H, Takenaka D, Sugimura K. CT hepatic perfusion measurement: comparison of three analytic methods. Eur J Radiol. 2012;81:2075–9. doi: 10.1016/j.ejrad.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Kojima H, Tanigawa N, Komemushi A, Kariya S, Sawada S. Computed tomography perfusion of the liver: assessment of pure portal blood flow studied with CT perfusion during superior mesenteric arterial portography. Acta Radiol. 2004;45:709–15. doi: 10.1080/02841850410001385. [DOI] [PubMed] [Google Scholar]
- 11.Treiber G, Csepregi A, Malfertheiner P. The pathophysiology of portal hypertension. Dig Dis. 2005;23:6–10. doi: 10.1159/000084720. [DOI] [PubMed] [Google Scholar]
- 12.Su ZZ, Shan H, Ke WM, He BJ, Zheng RQ. Portalsystemic hemodynamic changes in chronic severe hepatitis B: An ultrasonographic study. World J Gastroenterol. 2008;14:795–9. doi: 10.3748/wjg.14.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai MH, Iwakiri Y, Cadelina G, Sessa WC, Groszmann RJ. Mesenteric vasoconstriction triggers nitric oxide overproduction in the superior mesenteric artery of portal hypertensive rats. Gastroenterology. 2003;125:1452–61. doi: 10.1016/j.gastro.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Guerra M, Garcia-Pagan JC, Bosch J. Increased hepatic resistance: a new target in the pharmacologic therapy of portal hypertension. J Clin Gastroenterol. 2005;39:S131–7. doi: 10.1097/01.mcg.0000155513.17715.f7. [DOI] [PubMed] [Google Scholar]
- 15.Bosch J, Garcia-Pagan JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32:141–56. doi: 10.1016/s0168-8278(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 16.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–31. doi: 10.1002/hep.20993. [DOI] [PubMed] [Google Scholar]
- 17.Huang HC, Chang CC, Wang SS, Lee FY, Teng TH, Lee JY, Lin HC, Chuang CL, Lee SD. The roles of angiotensin II receptors in the portosystemic collaterals of portal hypertensive and cirrhotic rats. J Vasc Res. 2012;49:160–8. doi: 10.1159/000332347. [DOI] [PubMed] [Google Scholar]
- 18.He XJ, Huang TZ, Wang PJ, Peng XC, Li WC, Wang J, Tang J, Feng N, Yu MH. Morphological and biomechanical remodeling of the hepatic portal vein in a swine model of portal hypertension. Ann Vasc Surg. 2012;26:259–67. doi: 10.1016/j.avsg.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Oberholzer HM, Bester MJ, van der Schoor C. Rats on a high-energy diet showing no weight gain present with ultrastructural changes associated with liver fibrosis. Ultrastruct Pathol. 2013;37:267–72. doi: 10.3109/01913123.2013.790527. [DOI] [PubMed] [Google Scholar]
- 20.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–36. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 21.Jang JH, Kang KJ, Kim YH, Kang YN, Lee IS. Reevaluation of experimental model of hepatic fibrosis induced by hepatotoxic drugs: an easy, applicable, and reproducible model. Transplant Proc. 2008;40:2700–3. doi: 10.1016/j.transproceed.2008.07.040. [DOI] [PubMed] [Google Scholar]


