Abstract
The aim of this study was to investigate the effect of PI3K/AKT signaling pathway in the activity of recombinant human angiotensin converting enzyme 2 (rhACE2) promoted the activity of endothelial nitric oxide synthase (eNOS). The human umbilical vein endothelial cells (HUVEC) were cultured in vitro. Then treated with Ang II (1×10-6 mol/L) for 24 h. The rhACE2 (100 μmol/L) was added and incubated for 5, 10, 15, 30, 60 min respectively which was based on Ang II intervention. The effect of rhACE2 on phosphorylation eNOS level was also observed in the presence of LY294002 (10 μmol/L) (PI3K/AKT inhibitors). Griess reagent method was applied to measure NO contents in cell culture supernatant, RT-PCR to detect the expression of eNOSmRNA in HUVEC, and Western blot to detect the expression of eNOS and phosphorylated eNOS. In Ang II intervention group, NO contents were significantly lower than control group (P < 0.05). Through rhACE2 treatment, the NO contents in cell culture medium and the expression level of phosphorylated eNOS were significantly higher than in Ang II intervention group (P < 0.05), but eNOSmRNA and non-phosphorylated eNOS protein expression level showed no significant difference (P > 0.05). After HUVEC was intervened by PI3K/AKT pathway inhibitor LY294002, the expression level of phosphorylated eNOS was significantly lower than that in the rhACE2 30 min treatment group (P < 0.05). rhACE2 may reduce the activity of Ang II inhibited endothelial cell eNOS, which can be blocked by PI3K/AKT pathway inhibitor LY294002, suggesting PI3K/AKT signaling pathway plays an important role in rhACE2’s promotion of the activity of endothelial cell eNOS.
Keywords: Recombinant human ACE2, nitric oxide synthase, PI3K/AKT signaling pathway
Introduction
Recent studies show that ACE2 is closely related to cardiovascular diseases. It generates cardiovascular protective peptide Ang1-7 by degrading Ang II to antagonize Ang, from raising blood pressure, and promote atherosclerosis and cardiac hypertrophy, fibrosis and other cardiovascular toxic effects [1-6], through its receptor Mas [7,8]. Endothelial dysfunction is an important start factor, which causes the elevation of blood pressure, atherosclerosis, cardiac dysfunction, etc. Endothelial NO synthase (eNOS) is the rate-limiting enzyme regulating NO synthesis in endothelial cells. Studies have shown that rhACE2 can enhance eNOS activity [9], increase endothelial NO synthesis, but the mechanism is unclear.
According to basic researches, PI3K/AKT signaling pathway is composed of serine/threonine protein kinase in the PI3K family and its downstream, and plays an important role in inhibiting apoptosis and promoting cell proliferation. Gene mutation, PTEN deletion or increased expression of growth factor receptors can activate the PI3K signaling pathway. The activated PI3K signaling pathway phosphorylates the AKT, activated by the endothelial nitric oxide synthase (eN0S) of the activated AKT phosphorylation, producing NO. eNOS activity is related to the phosphorylation of eNOS protein serine 1179, and PI3K/AKT signaling pathway mediates this process [10-12]. Sampaio et al [13]. Found that in CHO which expresses Mas and in human aortic endothelial cells, Ang [1-7] stimulates eNOS phosphorylation, causing the generation of NO, through PI3-kinase’s dependence on AKT phosphorylation. Therefore, the new idea is whether ACE2 increases eNOS protein and further produces NO though PI3K/AKT pathway.
This study mainly observed the effect of PI3K/AKT signaling pathway in the rhACE2 enhanced eNOS activity in order to identify the mechanism of rhACE2’s improvement of endothelial function.
Materials and methods
Culture of HUVEC
RPMI-1640 medium (containing 10% fetal bovine serum, 100 U/mL penicillin and 100 U/mL streptomycin) (Sigma) was kept in incubator at 37°C with 5% CO2. 0.25% trypsin was used for digestion and passage.
Experimental groups
1) Control group: normal HUVEC (Sigma, America) was cultured at 37°C in 5% CO2; 2) Ang II intervention group: based on the control group, Ang II (Sigma) (1×10-6 mol/L) was added and incubated 24 h; 3) rhACE2 intervention group: based on the Ang II (1×10-6 mol/L) intervention, rhACE2 (100 μmol/L) (Sigma) was added and incubated for 5, 10, 15, 30, 60 min respectively; 4) PI3K/AKT signaling pathway inhibitor LY294002 group: based on the Ang II intervention, LY294002 (10 μmol/L) (Sigma) was added and incubated for 30 min, and then rhACE2 (100 μmol/L) was added and incubated for 30 min. Cells and supernatant in each group were collected.
Determination of NO
Using Griess reagent method, Nitrite content in cell culture supernatant was measured to indirectly reflect the content of NO. An equal volume of Griess reagent (1% sulfanilic acid, 0.1% N-naphthyl ethylenediamine, 2.5% phosphoric acid) was added to 50 μl culture supernatant and incubated at room temperature for 10 min, and automatic microplate reader was used to measure absorbance (A) at 540 nm. The different concentrations of standard samples were abscissa and their corresponding A values the vertical axis. The standard curve was drawn. Based on the A value of each group, NO content was calculated.
Real-time quantitative PCR
Using Trizol reagent (Santa Cruz, America), the total RNA of each group was extracted, and dissolved in water with 30 μl diethyl amide pyrophosphate (DEPC, RNA enzyme inhibitors). With UV spectrophotometer, the values of A260 nm and A280 nm were determined, the purity and concentration of RNA were calculated, and the A260 nm/A280 nm values were controlled between 1.8 and 2.0. 500 μg total RNA was taken, and according to the instructions of RevertAidTM First stand cDNA Synthesis kit, was then transcribed into cDNA. In accordance with the instructions of Maxima Probe/ROX qPCR Master Mix (2X), real-time quantitative PCR amplification was performed. PCR primers and probes were synthesized by Shanghai Biological Engineering Technology Co. eNOS: up stream primer, 5’-CGGCATCACCAGGAAGAAGA-3’; down stream primer: 5’-CATGAGCGGCGGAGAT-3’, probe: FAM-TTTAAAGAAGTGGCCAACGCCGTGAA-BHQ. The up stream primer of β-actin, 5’AGCGGTTCCGATGCCCT3’, down stream, primer: 5’AGAGGTCTTTACGGATGTCAACG3’, probe: FAM-CCTTCCTTCTTGGGTATGGAATCCTGTG-BHQ1. The reaction conditions of real-time quantitative PCR: predenaturation at 94°C 10 min, denaturation at 94°C 15 s, extension at 60°C 45 s, and circulation 40 times. CT (cycle threshold) values were read. First, the difference between determined gene eNOS CT and the internal control gene β-actin CT of each sample was calculated, and then ΔCT value difference of the samples in the normal control group was distracted from the ΔCT value difference between the samples in each experimental group, using 2-ΔΔCT.
Western blot
Cell lysate was used to crack cultured HUVEC to determine protein concentration. 50 μg total protein sample was separated by SDS-PAGE, transferred to PVDF membranes, and closed with 5% BSA for 4 h, 1:1000 diluted mouse-derived eNOS monoclonal antibody (Santa Cruz, America), eNOS phosphorylated monoclonal antibody (Santa Cruz, America) and β-actin monoclonal antibody (Santa Cruz, America) were added, at 4°C overnight. Then the membrane was washed, and 1:10000 diluted HRP-labeled goat anti-mouse secondary antibody (Santa Cruz, America) was added and incubated 4 h, and then was scanned and analyzed after ECL chemiluminescence coloration, using gel imaging system. The expression level of eNOS and phosphorylated eNOS protein was represented by the semi-quantitative ratio of A values and the corresponding β-actin A value in each group.
Statistical analysis
Using SPSS17.0 statistical software, the analysis was performed. All data were expressed as x̅ ± s, ANOVA was adopted to make inter-group comparisons, and t-test was applied to the comparison between two groups. P < 0.05 was considered statistically significant.
Results
Levels of NO
NO content (3.495 ± 0.362 nmol/L) in Ang II intervention group was significantly lower than in the control group (11.513 ± 0.392) (P < 0.05). And NO content in the cell culture medium of rhACE2 subgroups were higher than in the Ang II intervention group, P < 0.05. After rhACE2 incubation of each subgroup for 5, 10, 15, 30, 60 min, NO levels were (5.823 ± 0.310 nmol/L), (7.182 ± 0.633 nmol/L), (9.532 ± 0.609 nmol/L), (10.398 ± 0.508 nmol/L), (8.502 ± 0.776 nmol/L), respectively, with the level at 30 min the highest (Figure 1A).
Figure 1.
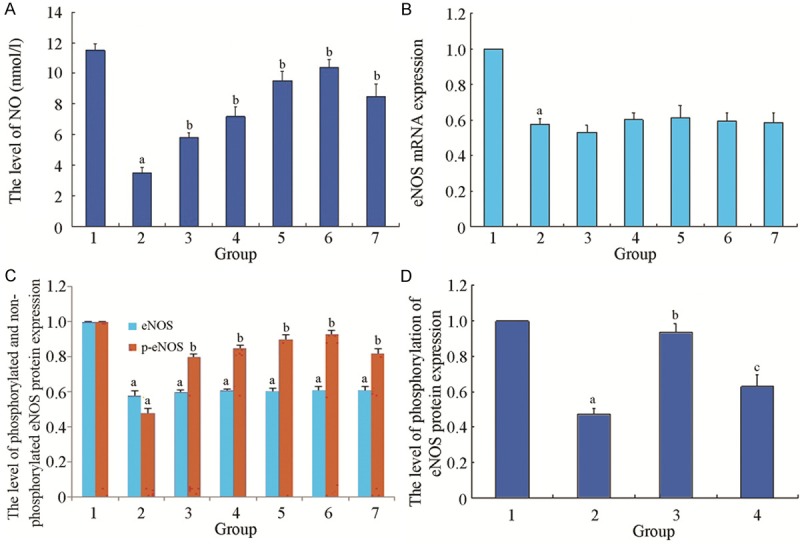
A. The effect of rhACE2 on the NO content of HUVEC cultured supernatant. 1: normal control group; 2: Ang II intervention group; 3: rhACE2 5 min group; 4: rhACE2 10 min group; 5: rhACE2 15 min group; 6: rhACE2 30 min group; 7: rhACE2 60 min group. Compared with normal control group, a P < 0.05; Compared with Ang II intervention group, b P < 0.05; n=6 in each group. B. The effect of rhACE2 on the relative expression of eNOS mRNA of each group. 1: normal control group; 2: Ang II intervention group; 3: rhACE2 5 min group; 4: rhACE2 10 min group; 5: rhACE2 15 min group; 6: rhACE2 30 min group; 7: rhACE2 60 min group. Compared with normal control group, a P < 0.05; n=6 in each group. C. The effect of rhACE2 on the relative expression of non-phosphorylated and phosphorylated eNOS protein of each group. 1: normal control group; 2: Ang II intervention group; 3: rhACE2 5 min group; 4: rhACE2 10 min group; 5: rhACE2 15 min group; 6: rhACE2 30 min group; 7: rhACE2 60 min group. Compared with normal control group, a P < 0.05; Compared with Ang II intervention group, b P < 0.05; n=6 in each group. D. The effect of inhibitor P13K/AKT on the relative expression of phosphorylated eNOS protein of each group. 1: normal control group; 2: Ang II intervention group; 3: rhACE2 15 min group; 4: LY294002 group. Compared with normal control group, a P < 0.05; Compared with Ang II intervention group, b P < 0.05; n=6 in each group; Compared with rhACE2 intervention group, c P < 0.05.
ENOS mRNA expression
In normal control group, the relative expression level of eNOS mRNA was set as 1, the relative expression of eNOS mRNA in each group was expressed as the ratio to it. In Ang II intervention group, the relative expression of eNOS mRNA was lower than in the normal control group (0.578 ± 0.031), P < 0.05. The relative expression levels of eNOS mRNA cells in each rhACE2 subgroup (incubated 5, 10, 15, 30, 60 min) were (0.532 ± 0.040), (0.602 ± 0.040), (0.613 ± 0.069), (0.593 ± 0.050), and (0.585 ± 0.055), respectively showing no significant difference, compared with Ang II intervention group, P > 0.05 (Figure 1B).
eNOS protein expression
In the normal control group, the relative expression of non-phosphorylated eNOS protein was set as 1, and non-phosphorylated eNOS protein expression of each group was expressed as the ratio to it. In Ang II intervention group, the relative expression of non-phosphorylated eNOS protein (0.575 ± 0.026) was significantly lower than in the normal control group, P < 0.05. And the relative expressions of non-phosphorylated eNOS protein of the cells (incubated 5, 10, 15, 30, 60 min) in each rhACE2 subgroup were (0.603 ± 0.019), (0.615 ± 0.015), (0.613 ± 0.019), (0.623 ± 0.021), and (0.620 ± 0.027), respectively, but with no significant difference compared with Ang II intervention group, P > 0.05 (Figure 1C).
Similarly, the relative phosphorylation of eNOS protein expression in the normal control group was set as 1. The phosphorylated eNOS protein expression in each group was expressed as the ratio to it. In Ang II intervention group, the relative the expression levels of phosphorylated eNOS protein (0.483 ± 0.031) were significantly lower than in the normal control group, P < 0.05. And in each rhACE2 subgroup, the relative expression levels of phosphorylated eNOS protein of cells were (0.822 ± 0.023), (0.873 ± 0.017), (0.903 ± 0.031), (0.930 ± 0.033), and (0.842 ± 0.027), respectively, with no significant increase, compared with Ang II intervention group, P < 0.05, among which, the relative expression level of rhACE2 30 min group was highest (Figure 1C).
Expression of phosphorylated eNOS protein
In Ang II intervention group, the relative expression levels of phosphorylated eNOS protein (0.472 ± 0.033) were significantly lower than in the control group (the relative expression level of phosphorylated eNOS protein of control group was set as 1), P < 0.05. After incubation with rhACE2 30 min, the relative expressions (0.935 ± 0.049) of phosphorylated eNOS protein were significantly higher than in Ang II intervention group, P < 0.05. But, with the addition of PI3K/AKT pathway inhibitor LY294002, the relative expressions 0.628 ± 0.069) of phosphorylated eNOS protein decreased, significantly lower than in rhACE2 intervention group, P < 0.05 (Figure 1D).
Discussion
Studies confirmed that the RAS is the key component in ACE2, which plays an important role. ACE2 can degrade Ang II, generate Ang [1-7], and also hydrolyze Ang I to generate Ang [1-9], which may further generate Ang [1-7] under the action of the ACE. Current researches show that like Ang [1-7], Ang [1-9] is also a cardiovascular protective peptide, and AT2 receptor has confirmed its specific receptor, Ang [1-9], combined with AT2, plays a role in antagonizing the cardiovascular toxic effects of AT1 mediated Ang II, such as contracting the blood vessels, injuring vascular endothelial function, and promoting atherosclerosis, myocardial hypertrophy and fibrosis [14,15]. Besides, ACE2 can also hydrolyze the peptides with vasoactive effect in the other cardiovascular systems, e.g., apelin-13, neurotension peptides, bradykinin, etc. These effects are involved in the control of blood pressure, and the regulation of heart function and vascular reactivity. ACE2 overexpression may reduce blood pressure elevation induced by Ang II [16], and recombinant human ACE2 may inhibit Ang II -induced blood pressure elevation and improve ventricular function [17-21].
In recent years, the effect of changes in endothelial cell function on blood pressure elevation and atherosclerosis is drawing more and more attention. The most important characteristics of vascular endothelial cell dysfunction are the synthesis and release of endogenous NO, and the impairment of its active function. NO, as an important cardiovascular regulating factor, is able not only to expand blood vessels, but also to inhibit the platelet adhesion and aggregation, the activation of leukocyte chemotaxis, and the proliferation and migration of vascular smooth muscle cells. It is an important protective factor for the cardiovascular system. With endothelial dysfunction, NO reduction causes vasoconstriction, platelet aggregation, smooth muscle cell proliferation and leukocyte adhesion, impairing vascular endothelial function and promoting atherosclerosis and thrombosis.
In endothelial cells, eNOS is the rate-limiting enzyme in NO synthesis and plays an important role in regulating NO synthesis. The increase of eNOS activity will lead to more endothelial NO synthesis, and Ang II, through its AT1 receptor, inhibits the activity of endothelial cell eNOS, thereby reducing NO synthesis. ACE2 gene transfection may reverse the inhibitory effect of phosphorylation of eNOS generated by Ang II induced insulin in vascular endothelial cells, accompanied with the significant increase of NO levels, but its mechanism remains unclear. The results of this study show: In Ang II intervention group, NO content was significantly lower than in the control group, while in each rhACE2 subgroup, NO content in the cell culture medium was higher than in the Ang II intervention group, with the highest content in the 30 min group. eNOS mRNA expression detection showed that in the Ang II intervention group, eNOS mRNA relative expression level is lower than in the normal control group, and in each rhACE2 subgroup, eNOS mRNA relative expression showed no significant difference, compared with the Ang II intervention group. In Ang II intervention group, non-phosphorylated eNOS relative protein expression was significantly lower than in the control group, while in each rhACE2 subgroup, non-phosphorylated eNOS protein relative expressions (incubated 5, 10, 15, 30, 60 min) showed no significant difference, compared with the Ang II intervention group. These results suggest that after the effect of rhACE2 on each subgroup 5, 10, 15, 30, 60 min, the Enos mRNA cell level of each subgroup and non-phosphorylated eNOS protein levels do not increase. In Ang II intervention group, the relative expression levels of phosphorylated eNOS protein were significantly lower than in the control group, while in each rhACE2 subgroup, the relative expression levels of phosphorylated eNOS protein were significantly higher, compared with the Ang II intervention group, P < 0.05. The relative expression level rhACE2 in the 30 min group was the highest. Added with PI3K/AKT pathway inhibitor LY294002, the relative expression levels of phosphorylated eNOS protein were significantly lower than in the rhACE2 intervention group, P < 0.05.
The above results suggest that during the effect of rhACE2 on Ang II intervened HUVEC, although the Enos mRNA cell level and non- phosphorylated eNOS protein levels in each group do not increase, rhACE2 can significantly increase the release of endothelial NO. In this process, rhACE2 increases eNOS activity through increasing the phosphorylation of 1179 serine of eNOS protein, and this process can be blocked by PI3K/AKT pathway specific inhibitor LY294002, indicating PI3K/AKT signaling pathway was involved, which shows great significance in this process.
Acknowledgements
This study are supported by National Natural Science Foundation of China (81273878) and Science and technique Foundation of Shaanxi Province (3013JQ4002).
Disclosure of conflict of interest
None.
References
- 1.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–728. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- 2.Fraga-Silva RA, Costa-Fraga FP, Murça TM, Moraes PL, Martins Lima A, Lautner RQ, Castro CH, Soares CM, Borges CL, Nadu AP, Oliveira ML, Shenoy V, Katovich MJ, Santos RA, Raizada MK, Ferreira AJ. Angiotensin-converting enzyme 2 activation improves endothelial function. Hypertension. 2013;61:1233–1238. doi: 10.1161/HYPERTENSIONAHA.111.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oudit GY, Penninger JM. Recombinant human angiotensin–converting enzyme 2 as a new rennin-angiotensin system peptidase for heart failure therapy. Curr Heart Fail Rep. 2011;8:176–183. doi: 10.1007/s11897-011-0063-7. [DOI] [PubMed] [Google Scholar]
- 4.Moritani T, Lwai M, Kanno H, Nakaoka H, Iwanami J, Higaki T, Ishii E, Horiuchi M. ACE2 deficiency induced perivascular fibrosis and cardiac hypertrophy during postnatal development in mice. J Am Soc Hypertens. 2013;7:259–266. doi: 10.1016/j.jash.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Patel VB, Bodiga S, Basu R, Das SK, Wang W, Wang Z, Lo J, Grant MB, Zhong J, Kassiri Z, Oudit GY. Loss of angiotensin- converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: a critical role of the angiotensin II/AT1 receptor axis. Circ Res. 2012;110:1322–1335. doi: 10.1161/CIRCRESAHA.112.268029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel VB, Bodiga S, Fan D, Das SK, Wang ZC, Wang W, Basu R, Zhong JC, Kassiri Z, Qudit GY. Cardio protective effects mediated by angiotensin II type 1 receptor blockade and enhancing angiotensin 1-7 in experimental heart failure in angiotensin-converting enzyme 2-null mice. Hypertension. 2012;59:1195–1203. doi: 10.1161/HYPERTENSIONAHA.112.191650. [DOI] [PubMed] [Google Scholar]
- 7.Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T. G-protein-coupled receptor MAS is a physiological antagonist of the angiotensin II type I receptor. Circulation. 2005;111:1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- 8.De Mello WC. Angiotensin (1-7) re-establishes heart cell communication previously impaired by cell swelling: Implications for myocardial ischemia. Exp Cell Res. 2014;323:359–365. doi: 10.1016/j.yexcr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhong JC, Zhu DL, Yu XY, Yu HM, Zhou M. Efects of recombinant ACE2 gene on eNOS phosphorylation Levels in human vascular endothelial cells. Space Medicine & Medical Engineering. 2009;22:172–175. [Google Scholar]
- 10.Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp BE, Pearson RB. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol. 1999;9:845–848. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- 11.Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, Snyder SH, Burnett AL. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2002;99:4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong JC, Yu XY, Lin QX, Li XH, Huang XZ, Xiao DZ, Lin SG. Enhanced angiotensin converting enzyme 2 regulates the insulin/Akt signalling pathway by blockade of macrophage migration inhibitory factor expression. Br J Pharmacol. 2008;153:66–74. doi: 10.1038/sj.bjp.0707482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185–92. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 14.Flores-Munoz M, Work LM, Douglas K, Denby L, Dominiczak AF, Graham D, Nicklin SA. Angiotensin-(1-9) attenuates cardiac fibrosis in the stroke-prone spontaneously hypertensive rat via the angiotensin Type 2 receptor. Hypertension. 2012;59:300–307. doi: 10.1161/HYPERTENSIONAHA.111.177485. [DOI] [PubMed] [Google Scholar]
- 15.Flores-Muñoz M, Smith NJ, Haggerty C, Milligan G, Nicklin SA. Angiotensin1-9 antagonises pro-hypertrophic signalling in cardiomyocytes via the angiotensin type 2 receptor. J Physiol. 2011;589:939–51. doi: 10.1113/jphysiol.2010.203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011;92:401–408. doi: 10.1093/cvr/cvr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wysocki J, Ye M, Rodriguez E, González-Pacheco FR, Barrios C, Evora K, Schuster M, Loibner H, Brosnihan KB, Ferrario CM, Penninger JM, Batlle D. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010;55:90–98. doi: 10.1161/HYPERTENSIONAHA.109.138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JA, West J, Maynard KB, Hemnes AR. ACE2 improves right ventricular function in a pressure overload model. PLoS One. 2011;6:e20828. doi: 10.1371/journal.pone.0020828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song B, Zhang ZZ, Zhong JC, Yu XY, Oudit GY, Jin HY, Lu L, Xu YL, Kassiri Z, Shen WF, Gao PJ, Zhu DL. Loss of angiotensin-converting enzyme 2 exacerbates myocardial injury via activation of the CTGF-fractalkine signaling pathway. Circ J. 2013;77:2997–3006. doi: 10.1253/circj.cj-13-0805. [DOI] [PubMed] [Google Scholar]
- 20.Murça TM, Moraes PL, Capuruço CA, antos SH, Melo MB, Santos RA, Shenoy V, Katovich MJ, Raizada MK, Ferreira AJ. Oral administration of an angiotensin-converting enzyme 2 activator ameliorates diabetes-induced cardiac dysfunction. Regul Pept. 2012;177:107–115. doi: 10.1016/j.regpep.2012.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sukumaran V, Veeraveedu PT, Gurusamy N, Yamaguchi K, Lakshmanan AP, Ma M, Suzuki K, Kodama M, Watanabe K. Cardioprotective effects of telmisartan against heart failure in rats induced by experimental autoimmune myocarditis through the modulation of angiotensin-converting enzyme-2/angiotensin 1-7/Mas receptor axis. Int J Biol Sci. 2011;7:1077–1092. doi: 10.7150/ijbs.7.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]


