Abstract
Objective: This study aims to explore the function of Integrin-β/FAK in the mechanical signal transduction and the connection with downstream ERK signal pathways. Methods: Human osteosarcoma MG63 cell lines were used in this study. The effects of mechanical strain on the Integrin-β1 expression, FAK and ERK signal pathway in Human osteosarcoma MG63 cells were detected using RT-PCR and Western-blotting methods. The localization of FAK in Human osteosarcoma MG63 cells were determined using immunofluorescent method. The interaction between Integrin-β1 and FAK were detected by using co-immunoprecipitation method. Results: The expression of Integrin-β1 shows a notable bimodel distribution, mechanical strain stimulation can promote Integrin-β1 expression and the phosphorylation of FAK and ERK, mechanical strain activated FAK and ERK mediated by Integrin-β1. Conclusion: Integrin-β1 may play an important role in osteoblast proliferation differentiation process, it might feel external strain stimulation through ECM composition and makes FAK phosphated through the interaction with FAK, thus causing a series of activation of signal molecules. Finally it reduces MAPK (ERK) activation and cellular responses to finish mechanical signal transduction.
Keywords: Integrin-β, human osteosarcoma MG63 cells, mechanical stimulation, focal adhesion kinase (FAK), ERK signal pathway
Introduction
Interactions between extracellular matrix and cells affect basic life activities of cells. Integrin, a kind of important cell-surface molecules which are the main receptor of extracellular matrix and prevalent in the surface of various cells, play an important role in mechanical signal transduction [1-3]. Many in vitro and in vivo studies have discovered that human osteoblasts can expressed many kinds of integrin molecules such as α1, α2, α3, α5, αV, β1, β3, β5 and so on, among them β1 is in rich [4-6]. Many studies showed that integrin-β1 mediated cells combining synthesis of ECM including type I collagen, fibronectin and so on. It shows that integrin-β1 might play an important role in osteoblast signal transduction [7-10]. It also involves in the formation of tissues and organs. Focal adhesion kinase (FAK), the non-receptor tyrosine kinase in cytoplasm, is activated with the aggregation of macula adherens. FAK is the key point of integrin signal transduction. Previous study showed that Integrin Signaling Pathway plays an important role in the mechanical signal transduction. In this study, we explored the function of Integrin-β/FAK in the mechanical signal transduction and the connection with downstream ERK signal pathways.
Materials and methods
Cell culture
Human osteosarcoma MG63 cell lines were produced by American type culture collection (ATCC number: CRL1427). They were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS, 50 μg/ml ascorbic acid, 100 U/ml penicillin and 100 μg/ml gentamicin. The culture medium was changed once every three days throughout the experiment. The cells were digested by a 25% trypsin-EDTA treatment, and then vaccinated to cell plate tension in a 104/cm2 density.
RNA extraction and RT-PCR
Total RNA was extracted using Trizol RNA extraction kit. cDNA was synthesized by reverse transcription: extract total RNA 0.2 µg, synthesize cDNA by using reverse transcription kits in operate specification. Experimental conditions were as follows: total RNA 0.2 µg mixed with olig(dT) 18 primer (0.5 μg/μl) 1 μl and DEPC to 12 μl, centrifuged for 35 s, then chilled on ice. Adds 4 μl of 5 × buffer, RNAI (20 U/μl) 1 μl and 10 mM dNTP mix 21 μl to the mixture, then centrifuged for 35 s and incubated for 5 min at 37°C. Finally, adds MMuLV reverse transcription (200 U/μl) to terminal volume 20 μl. Incubates the mixture for 60 min at 42°C, 10 min at 70°C, and then chills on ice. Using Roche Molecular LightCycler, the amplification reaction system was: 25 µl of PCR reaction mixture containing 10 μl SYBR Green PCR Master Mix, upstream primer (25 μmol/L) 1 μl, downstream primer (25 μmol/L) 1 μl, dNTPs (10 mmol/L) 1 μl, cDNA 2 μl, adding ddH2O to 25 μl. The reaction conditions were as follows: force-denatured the temple cDNA for 4 min at 94°C, then denatured at 94°C for 15 s, primer annealing at 60°C for 10 s, extension at 72°C for 10 s, 40 cycles in total, finally extend at 72°C for 7 min. First we double diluted and amplified the cDNA to improve that there was a linear relationship between cDNA temple amount and Ct value. And we also try to improve the amplification efficiency of target gene is the same with the house-keeping gene. The real-time monitoring method is used on fluorescence collection every cycle at annealing temperature. Using resolution melting analysis 95°C 0 s 20°C/s, 65°C 15 s 20°C/s, 95°C/s, 0.1°C/s. The premier sequences of Integrin-β1 are as follows: Forward: 5’-GGGAAACTTGGTGGCATTG-3’; Reverse: 5’-GCTCCTTGTAAACAGGCTGAAA-3’. After reaction finished, we calculated it according to the Ct value of target gene and β-actin amplification curve.
Protein extraction and western blotting
Total proteins were extracted and analyzed with SDS-PAGE electrophoresis. Then it was electrotransferred to the PVDF membrane. The membrane containing the proteins was used for immunoblotting with required antibodies. The first antibodies were anti-Integrin-β1, anti-FAK, anti-p-FAK, anti-ERK, anti-p-ERK and anti-β-actin antibodies. The second antibodies were anti-rabbit and anti-mouse antibody with Horseradish Peroxidase. The protein bands were scanned and quantified as a ratio to β-actin.
Cellular immunofluorescence
Human osteosarcoma MG63 cells were divided into 6 orifice plate with aseptic glasses. When the cell fusion reached 50%~60%, they were washed with PBS for three times, disposed of 4% paraformal-dehyde for 30~40 minutes, washed by PBS for 3 times, then air dried. They were treated with 0.25% Triton X-100 at 37°C for 20 min, cleaned of film, washed by PBS for 3 times, air dried. Then added blocking serum, kept moisture and closed for 30 min at 37°C. Added rabbit anti-FAK (1:200 diluting) after serum deprivation, kept moisture at 4°C overnight. Kept for 30 min at 37°C, washed by PBS for 3times (10 min every time), added second antibody with FITC (1:200 diluting) for 90 min at 37°C. Washed by PBS for 3 times (10 min every time). Added DAPI (1:800 diluting) and marked nucleus for 8 min at 37°C, then washed by PBS for 3 times (10 min every time). The expression of FAK and DAPI nuclear localization were examined under fluorescence microscope.
Co-immunoprecipitation
1. Lysis of each intervention group, taken 500 μg protein from each group into 1.5 ml EP tube, added cell lysis buffer to 0.5 ml. 2. Added anti-FAK 10 μl into each tube, away for 2 h at 4°C. 3. Added Protein A/G PLUS-Agaross 30 μl into each tube sway at 4°C overnight. 4. The cells were centrifuged at the speed of 5000 r/min for 5 min at 4°C, then supernatant were abandoned. 5. Added 1 ml cell lysis buffer into immune precipitate, then washed and shook for 20 min at 4°C (Horizontal shaking bed). 6. Centrifuged at the speed of 5000 r/min for 5 min at 4°C, the supernatant were abandoned, then added cell lysis buffer to wash, repeated for 3 times. 7. Collected the immuno-precipitate, added 40 μl 1 × SDS buffer in each tube to resuspend the immune complex, then boiled it for 3~5 min at 100°C and centrifuged at the speed of 5000 r/min for 5 min. The supernatant was collected to another tube. 10. Taken 20 μl for Western blotting.
Cell intervention experiment
The change of Integrin-β1 expression during the proliferation and differentiation of human osteosarcoma MG63 cells: vaccinated the human osteosarcoma MG63 cells in the cell culture bottle with DMEM (supplemented with 10% FBS, 50 μg/ml ascorbic acid, 100 U/ml penicillin, 100 μg/ml gentamicin). Started to add 10 mM β-sodium glycerophosphate in the culture medium the third day after vaccination, the culture medium was changed every 3days. The total cellular protein and total cellular RNA were extracted for 1, 3, 6, 12, 18 and 24 days after vaccination. The content of total cellular protein was examined by BCA Protein Assay Kit (see the first part). The reverse transcription and fluorescence quantitative PCR reaction were used to evaluate the extracted total cellular RNA.
Effect of mechanical strain on Integrin-β1 expression in Human osteosarcoma MG63 cells: vaccinated the Human osteosarcoma MG63 cells on the plate with DMEM (supplemented with 10% FBS, 50 μg/ml ascorbic acid, 100 U/ml penicillin, 100 μg/ml gentamicin). The cells were intervened by mechanical stimulation the third day after vaccination. The cells were cultured under serum free DMEM with 1% BSA to starvation for 24 h before intervention, then under mechanical stimulation (2000 μstain, 0.2 Hz) for 1-12 h.
Effect of mechanical strain on FAK and ERK signal pathway in Human osteosarcoma MG63 cells: vaccinated the Human osteosarcoma MG63 cells on the plate with DMEM (supplemented with 10% FBS, 50 μg/ml ascorbic acid, 100 U/ml penicillin, 100 μg/ml gentamicin). The cells were intervened by mechanical stimulation the third day after vaccination. The cells were cultured under serum free DMEM with 1% BSA to starvation for 24 h before intervention, then under mechanical stimulation (2000 μ stain, 0.2 Hz) for 0, 10, 20, 30, 60, 120min. The phosphorylation protein was extracted after intervention.
Effect of mechanical strain on FAK and ERK signal pathway after blocking Integrin-β1 by blocking antibody: vaccinated the Human osteosarcoma MG63 cells on the plate with DMEM (supplemented with 10% FBS, 50 μg/ml ascorbic acid, 100 U/ml penicillin, 100 μg/ml gentamicin). The cells were intervened by mechanical stimulation the third day after vaccination. The cells were cultured under serum free DMEM with 1% BSA to starvation for 24 h and added the blocking antibody of Integrin-β1 1 h before intervention. Then under mechanical stimulation for 1 h and extracted the phosphorylation protein.
Statistical analysis
Each experiment repeated independently over 3 times and shows good reproducibility. The chosen diagram was one of result of duplicate test. The data results (X̅ ± S) were analyzed by SPSS11.0 software and variance analysis was conducted for comparison between groups.
Results
Change of integrin-β1 expression in the proliferation and differentiation of human osteosarcoma MG63 cells
We detected the change of Integrin-β1 expression in the proliferation and differentiation of Human osteosarcoma MG63 cells by fluorescence quantitative PCR. We calculated the multiple of the change of Integrin-β1 expression in every time point in contrast with basal level by using ΔΔCT. The Ct values of Integrin-β and β-actin in Table 1. In the proliferation and differentiation of Human osteosarcoma MG63 cells, we found out that the expression of Integrin-β1 shows a notable bimodel distribution and expressed the highest at the sixth day and the twenty-forth day.
Table 1.
The Ct values of Integrin-β and β-actin (X̅ ± S)
| Day | Integrin-β1 | β-actin |
|---|---|---|
| 1 | 20.92 ± 0.42 | 14.38 ± 0.08 |
| 3 | 19.28 ± 0.12 | 13.92 ± 0.19 |
| 6 | 14.97 ± 0.08 | 12.19 ± 0.08 |
| 12 | 17.64 ± 0.07 | 13.75 ± 0.12 |
| 18 | 16.74 ± 0.39 | 13.67 ± 0.15 |
| 24 | 18.23 ± 0.41 | 15.59 ± 0.18 |
Effect of mechanical strain on integrin-β1 expression in human osteosarcoma MG63 cells
By comparing with the control group, we found out that mechanical strain stimulation can promote Integrin-β1 expression and the expression levels were the highest in 6-12 h (Figure 1).
Figure 1.
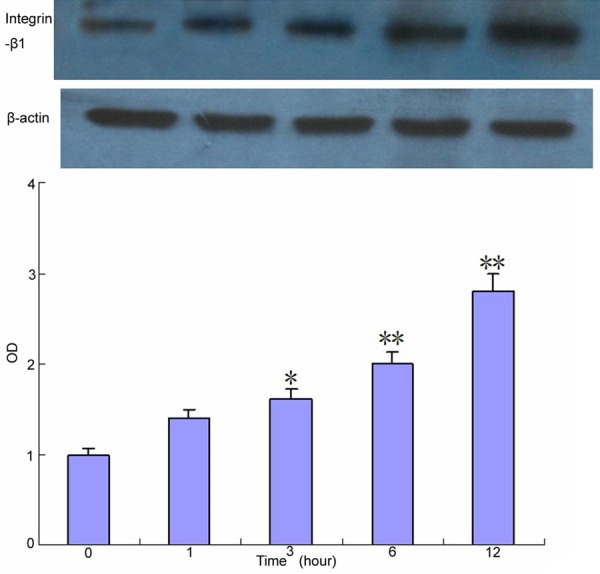
Effect of mechanical strain on Integrin-β1 expression in Human osteosarcoma MG63 cells. Compared with the control group *P < 0.05, **P < 0.01.
Immunofluorescent localization of FAK in human osteosarcoma MG63 cells
The expression of FAK in Human osteosarcoma MG63 cells was located using immunofluorescence method. The results showed that FAK was mainly located in the cytoplasm with abundant expression (Figure 2).
Figure 2.
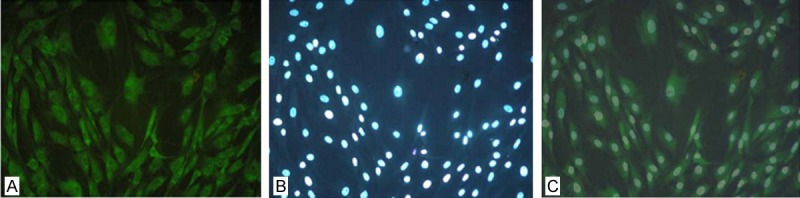
Localization of FAK in Human osteosarcoma MG63 cells. A: FITC; B: DAPI; C: Merge.
Effect of mechanical strain on FAK and ERK signal pathway in human osteosarcoma MG63 cells
The effects of mechanical strain on FAK and ERK signal pathway in human osteosarcoma MG63 cells were shown in Figures 3 and 4. The results showed that, compared with the control group, the mechanical strain stimulation significantly promoted the phosphorylation of FAK and ERK in osteoblast like cells MG-63. The phosphorylation of FAK was most obvious in the 60-120 minutes after mechanical strain stimulation, the phosphorylation of ERK was most obvious at 60 minutes after mechanical strain stimulation.
Figure 3.
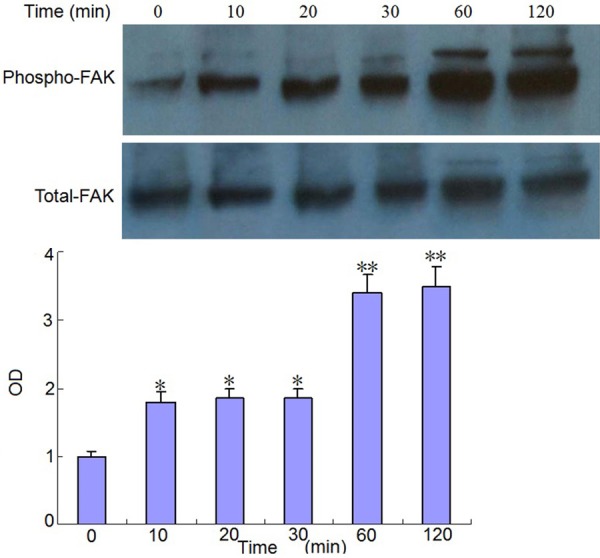
Effect of mechanical strain on FAK signal pathway in Human osteosarcoma MG63 cells. Compared with the control group *P < 0.05, **P < 0.01.
Figure 4.
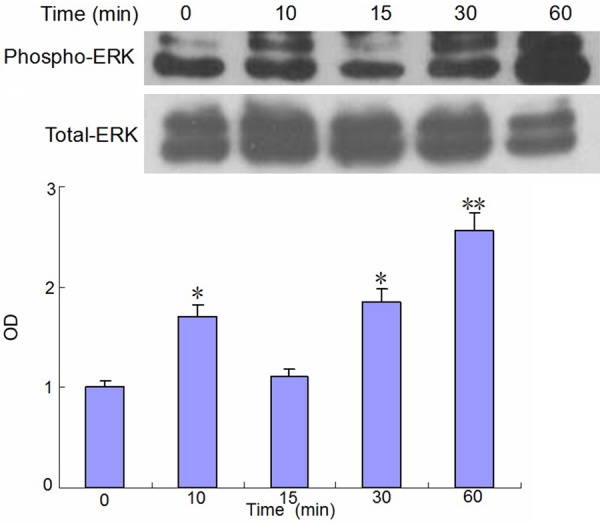
Effect of mechanical strain on ERK signal pathway in Human osteosarcoma MG63 cells. Compared with the control group *P < 0.05, **P < 0.01.
Effect of mechanical strain on FAK and ERK signal pathway after blocking integrin-β1 by blocking antibody
By using Integrin-β1 blocking antibody to block the interaction between ECM and it, we found that the activation of FAK and ERK, which was caused by mechanical strain, was inhibited obviously (Figure 5). It shows that mechanical strain activated FAK and ERK mediated by Integrin-β1.
Figure 5.
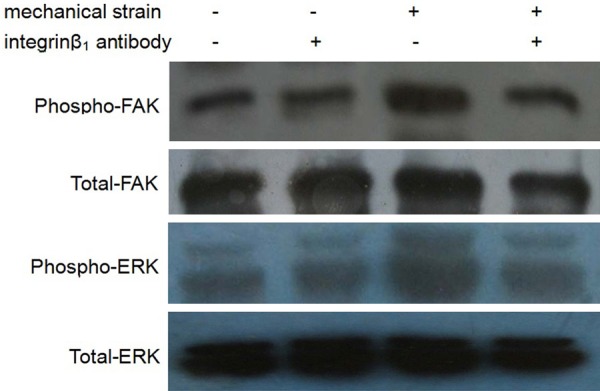
Effect of mechanical strain on FAK and ERK signal pathway after blocking Integrin-β1 by blocking antibody.
Interaction between integrin-β1 and FAK
We observed the interaction between Integrin-β1 and FAK by using co-immunoprecipitation. We found out that there have been interaction between Integrin-β1 and FAK in basic state and mechanical strain can promote the combination between them (Figure 6). It shows that mechanical strain can promote the combination between Integrin-β1 and FAK and the activation of FAK.
Figure 6.

The interaction between Integrin-β1 and FAK. IP: Co-immunoprecipitation; WB: Western blotting.
Discussion
Mechanical signal transduction is the process of cell signal transduction pathway to mechanical stimulation. From inner ear cells sound feeling to shear stress on vascular endothelial cells, the mechanical stimulation widely regulated all kinds of body physiological function. The mechanical stimulation regulated all kinds of cell’s function, such as growth, differentiation, migration, gene expression, protein synthesis and apoptosis. The skeletal system, from growth and development to bone remodeling after maturation, is also under regulation of mechanical stimulation. The skeletal system makes itself to the best state by the function of calcium regulating hormoneand the adaptive response of mechanical load in the environment. So the correct understanding of how the bone cells and osteoblast feeling mechanical stimulation and how to response it, is the key to reveal the transduction mechanism of mechanical signal and understand the development of bone metabolic disease, like osteoporosis.
Nowadays the researches have showed that the extracellular matrix-integrin-cytoskeleton system was playing an important role in signal transduction process. Integrin, a glycoprotein trans-membrane receptor in cell membrane, is a heterodimer which is combined with α (120~185KD) subunit and β (90~110KD) subunit through noncovalent binding. It mainly mediates the intercellular adhesion and the adhesion between cell and extracellular matrix, it also plays an important role in cell migration, differentiation and mechanical signal transduction process [11-15]. Nowadays, the vertebrate receptor family has been found out at least 17 α subunit and 9 β subunit, which composed 25 different kind of integrin [16]. Some studies have proved that the α-subunit might relate to substrate specific adhesion and the β subunit might relate to cell signal transduction function. Most of integrin ligand is ECM, the recognition site is RGD sequence of ligand (arginine-glycine-aspartate). They can mediate intercellular reaction by signal transduction after combination. Integrin is transmembrane glycoprotein, which includes extracellular fragment, transmembrane fragment, intracellular fragment. Extracellular fragment is integrin ligand binding portion, and intracellular fragment is the portion which connects with cytoskeleton and transduction signal.
Focal adhesion kinase (FAK) is a kind of non-receptor tyrosine kinase which plays an important role in cell cycle regulation, cytoskeleton assembly adhesion migration action, growth regulation survival and angiogenesis through multiple signaling pathway. FAK is located on human chromosome 8q24, full length of cDNA is 4285bp encoding 1052 amino acid. The FAK gene is very special without SH2 and SH3 domain, it consists of three functional areas: central kinase area, N-terminal FERM (protein 4.1, ezrin, radixin and moesin homology) and C-terminal, each area obtains about 400 amino acid [17]. FAK is known as having 6 phosphorylated tyrosines: Tyr 397, Tyr 407, Tyr 576, Tyr 577, Tyr 861, Tyr 925 [18]. The Tyr 397 and Tyr 407 are in amino terminal, Tyr 576 and Tyr 577 are in activating cyclic of kinase domain, Tyr 861 and Tyr 925 are located in amino terminal. These areas are the key points of FAK performing signal transduction function. Tyr 397 is the main self-phosphorylated part which combines with various protein molecules including SH2 domain, such as Scr family, phospholipase Cγ, cell signal inhibitors, growth factor connecting protein 7, She adaptor protein, p120RasGAP, P13K p85 subunit [19]. Tyr 576 and Tyr 577 are the main part of Src family tyrosine phosphorylation, the second are Tyr 861 and Tyr 925. The phosphorylation Tyr925 may combines with a kind of adaptor protein, namely growth receptor bound protein 2 (Grb2), the Tyr407 and Tyr861 might be the binding sites of other SH2 protein. And the two areas locating on carboxyl terminal with Pro are the protein combing sites obtaining SH3, like Crk associated substrate (Cas) [20]. After combing integrin and ligand, the formation of FAP would be promoted, which need integrin-β subunit intracellular region involving, so β subuint might related to the cell signal transduction function. FAP includes many cytoskeletal proteins such as actin, vinculin [21] and some important signal transduction protein like FAK. After FAP was formed, the conformation of FAK has changed and the kinase domain is in the active state. The above phosphorylation sites have phosphorylated, then they combines with downstream signal molecules so that the signal transduction could happen. FAK was considered to a basic molecule for integrin-dependent signal transduction pathway, exerts key roles in integrin signal transduction process [22].
Integrin plays an important role in recognition, growth and differentiation of cells. Because of its extracellular and intracellular domain respectively connected to the ECM and the cytoskeleton, it is responsible for tansducing signals from the extracellular matrix into cells through the complex pathways, playing an important role in regulating the shape, movement, survival and proliferation of cells. Our study had found that integrin-β1 in proliferation differentiation of MG-63 osteoblast-like cells showed a notable bimodel distribution. It highly expressed in mineralization period and the end of proliferation period, which suggested that it may play an important role in osteoblast proliferation differentiation process. Moreover we found that stress stimulation significantly promoted integrin-β1 expression. Carvalho RS had found that mechanical stimulation effected integrin-β1 distribution of human osteosarcoma cell TE-85 and promoted its expression. That means stress stimulation may regulate osteoblast function through effecting integrin-β1 expression and signal transduction [4].
The above have stated FAK was the hinge in integrin signal transduction process. Our study have found that the phosphorylation of FAK Tyr397 sites in Mg-63 osteoblast-like cells were caused by stress stimulation, which significantly activated in 30 min and continued to 60~120 min. Boutahar N had discovered that cyclic biaxial mechanical stimulation can significantly phosphorylated the Tyr397 and Tyr925 sites of FAK and they thought the phosphorylation of FAK Tyr397 sites was related to the phosphorylation of ERK Tyr925 sites [23]. After blocking the phosphorylation of FAK Tyr397 sites, the phosphorylation of ERK Tyr925 sites was significantly inhibited, which meant the phosphorylation of FAK Tyr397 sites can induced the transduction of Ras/Raf/MEK signal pathway. That may be due to the activation of some downstream signal molecules like Src and Grb2 caused by FAK phosphorylation, and the activation of MAPK (including ERK) signal pathway through Ras after activation [24]. This consistent with our study, that was because we found out stress stimulation not only activated FAK but also activated ERK, and the time curves of them were almost the same. That means the phosphorylation of FAK Tyr397 sites might relates to ERK activation. In addition, because of the importance of FAK in integrin signal transduction, we guessed the activation of FAK and ERK which were caused by stress stimulation were regulated by integrin-β1. That meant mechanical stimulation may be passed through integrin-β1/FAK/ERK signal pathway. Our study discovered that the activating function of stress stimulation of FAK and ERK was significantly inhibited to basal level while we used integrin-β1 blocking antibody to block the combination of MG-63 oesteoblast-like cells and the components of ECM. This confirms of speculation that integrin-β1 indeed regulates the activation of FAK and ERK caused by stress stimulation. In fact, integrin-β1/FAK/ERK (MAPK) signal pathway is widely existed in stromal cells [25,26]. Some studies had discovered that the stimulation of integrin activated the ERK signal pathway in fibroblasts [26,27]. We found that stretch-induced connective tissue growth factor (CTGF) expression is mediated through the PI3K-JNK-dependent pathway, not by p38 MAP kinase and ERK pathways [28].
In addition, we tested the interactions between integrin-β1 and FAK, and the affect of stress stimulation on their interactions by using co-immunoprecipitation. We discovered that there really existed interactions between integrin-β1 and FAK, and stress stimulation promoted combination of integrin-β1 with FAK. Many studies had found that there existed interactions between integrin-β1 and FAK [29-31]. But the stress stimulation on the interaction between osteoblast integrin-β1 and FAK, and the aspect of signal transduction process was little reported.
In a word, our study had found that integrin-β1 in proliferation differentiation of MG-63 osteoblast-like cells showed a notable bimodel distribution. It highly expressed in mineralization period and the end of proliferation period, which suggested that it may play an important role in osteoblast proliferation differentiation process. Stress stimulation significantly promoted integrin-β1 expression. The results of co-immunoprecipitation showed that stress stimulation promoted the combination of integrin-β1 with FAK. In addition, the phosphorylation of FAK Tyr397 sites and ERK were caused by strain stimulation. The phosphorylation of FAK Tyr397 sites and ERK were all significantly inhibited after we used integrin-β1 blocking antibody to block its function. The results of our study show that integrin-β1 might feel external strain stimulation through ECM composition and makes FAK phosphated through the interaction with FAK, thus causing a series of activation of signal molecules. Finally it reduces MAPK (ERK) activation and cellular responses to finish mechanical signal transduction.
Disclosure of conflict of interest
None.
References
- 1.Pommerenke H, Schmidt C, Durr F, Nebe B, Luthen F, Muller P, Rychly J. The mode of mechanical integrin stressing controls intracellular signaling in osteoblasts. J Bone Miner Res. 2002;17:603–611. doi: 10.1359/jbmr.2002.17.4.603. [DOI] [PubMed] [Google Scholar]
- 2.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 3.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho RS, Scott JE, Yen EH. The effects of mechanical stimulation on the distribution of beta 1 integrin and expression of beta 1-integrin mRNA in TE-85 human osteosarcoma cells. Arch Oral Biol. 1995;40:257–264. doi: 10.1016/0003-9969(95)98814-f. [DOI] [PubMed] [Google Scholar]
- 5.Clover J, Dodds RA, Gowen M. Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci. 1992;103:267–271. doi: 10.1242/jcs.103.1.267. [DOI] [PubMed] [Google Scholar]
- 6.Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. J Bone Miner Res. 1997;12:1189–1197. doi: 10.1359/jbmr.1997.12.8.1189. [DOI] [PubMed] [Google Scholar]
- 7.Bennett JH, Moffatt S, Horton M. Cell adhesion molecules in human osteoblasts: structure and function. Histol Histopathol. 2001;16:603–611. doi: 10.14670/HH-16.603. [DOI] [PubMed] [Google Scholar]
- 8.Aarden EM, Nijweide PJ, van der Plas A, Alblas MJ, Mackie EJ, Horton MA, Helfrich MH. Adhesive properties of isolated chick osteocytes in vitro. Bone. 1996;18:305–313. doi: 10.1016/8756-3282(96)00010-5. [DOI] [PubMed] [Google Scholar]
- 9.Gohel AR, Hand AR, Gronowicz GA. Immunogold localization of beta 1-integrin in bone: effect of glucocorticoids and insulin-like growth factor I on integrins and osteocyte formation. J Histochem Cytochem. 1995;43:1085–1096. doi: 10.1177/43.11.7560891. [DOI] [PubMed] [Google Scholar]
- 10.Hughes DE, Salter DM, Dedhar S, Simpson R. Integrin expression in human bone. J Bone Miner Res. 1993;8:527–533. doi: 10.1002/jbmr.5650080503. [DOI] [PubMed] [Google Scholar]
- 11.Damsky CH, Ilic D. Integrin signaling: it’s where the action is. Curr Opin Cell Biol. 2002;14:594–602. doi: 10.1016/s0955-0674(02)00368-x. [DOI] [PubMed] [Google Scholar]
- 12.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 13.Ingber D. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3:841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- 14.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 15.Martin KH, Slack JK, Boerner SA, Martin CC, Parsons JT. Integrin connections map: to infinity and beyond. Science. 2002;296:1652–1653. doi: 10.1126/science.296.5573.1652. [DOI] [PubMed] [Google Scholar]
- 16.Humphries MJ. Integrin structure. Biochem Soc Trans. 2000;28:311–339. [PubMed] [Google Scholar]
- 17.Schaller MD, Parsons JT. Focal adhesion kinase: an integrin-linked protein tyrosine kinase. Trends Cell Biol. 1993;3:258–262. doi: 10.1016/0962-8924(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 18.Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 20.Roy S, Ruest PJ, Hanks SK. FAK regulates tyrosine phosphorylation of CAS, paxillin, and PYK2 in cells expressing v-Src, but is not a critical determinant of v-Src transformation. J Cell Biochem. 2002;84:377–388. doi: 10.1002/jcb.10025. [DOI] [PubMed] [Google Scholar]
- 21.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 22.Alahari SK, Reddig PJ, Juliano RL. Biological aspects of signal transduction by cell adhesion receptors. Int Rev Cytol. 2002;220:145–184. doi: 10.1016/s0074-7696(02)20005-4. [DOI] [PubMed] [Google Scholar]
- 23.Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem. 2004;279:30588–30599. doi: 10.1074/jbc.M313244200. [DOI] [PubMed] [Google Scholar]
- 24.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morino N, Mimura T, Hamasaki K, Tobe K, Ueki K, Kikuchi K, Takehara K, Kadowaki T, Yazaki Y, Nojima Y. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44erk-1 and p42erk-2. J Biol Chem. 1995;270:269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- 27.Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao LW, Yang M, Dong J, Xie H, Sui GL, He YL, Lei JX, Liao EY, Yuan X. Stretch-inducible expression of connective tissue growth factor (CTGF) in human osteoblasts-like cells is mediated by PI3K-JNK pathway. Cell Physiol Biochem. 2011;28:297–304. doi: 10.1159/000331743. [DOI] [PubMed] [Google Scholar]
- 29.Chen LM, Bailey D, Fernandez-Valle C. Association of beta 1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J Neurosci. 2000;20:3776–3784. doi: 10.1523/JNEUROSCI.20-10-03776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Kyle E, Lieberman R, Crowell J, Kellof G, Bergan RC. Focal adhesion kinase (FAK) phosphorylation is not required for genistein-induced FAK-beta-1-integrin complex formation. Clin Exp Metastasis. 2000;18:203–212. doi: 10.1023/a:1006729106034. [DOI] [PubMed] [Google Scholar]
- 31.Bergan R, Kyle E, Nguyen P, Trepel J, Ingui C, Neckers L. Genistein-stimulated adherence of prostate cancer cells is associated with the binding of focal adhesion kinase to beta-1-integrin. Clin Exp Metastasis. 1996;14:389–398. doi: 10.1007/BF00123398. [DOI] [PubMed] [Google Scholar]


