Abstract
We sought to investigate the expression of EpCAM and Trop2 in Pituitary adenomas (PAs) and study the correlation of protein expression with invasiveness, proliferation, clinical functioning, recurrence/progression, and some other factors. We investigated the expression of EpCAM and Trop2 in 74 samples of PAs by immunohistochemistry and made correlative analysis of protein overexpression with clinicopathological parameters. Follow-up data was analyzed for recurrence/progression with Kaplan-Meier method and Multivariate Cox regression analysis. Immunohistochemistry results showed that overexpression rates of EpCAM and Trop2 were 51/74 (68.9%) and 43/74 (58.1%), respectively. For both EpCAM and Trop2, PAs with invasiveness showed a higher overexpression rate than PAs without invasiveness (PEpCAM = 0.001; PTrop2 = 0.006). Nonfunctional Pituitary adenomas (NFPAs) demonstrated a higher EpCAM overexpression than functional Pituitary adenomas (FPAs) (P = 0.026). Both EpCAM and Trop2 overexpression correlated significantly with expression of proliferation factor Ki-67 (PEpCAM = 0.011; PTrop2 = 0.000), but not with gender and age. Follow-up analysis revealed that Trop2 overexpression was a significantly predictive factor for recurrence/progression by means of Kaplan-Meier method d (P = 0.028) and Multivariate Cox regression analysis (P = 0.025). This study reveals that both EpCAM and Trop2 overexpression in PAs correlate significantly with invasiveness and proliferation. EpCAM presents a potential target for differential diagnosis and immunotherapy for NFPAs. Follow-up analysis shows that Trop2 is a predictive factor for recurrence/progression for PAs.
Keywords: Pituitary adenomas, EpCAM, Trop2
Introduction
Pituitary adenomas (PAs) are epithelial tumors arising from adenohypophysial cells and represent 10% to 15% of primary intracranial neoplasms [14]. From the clinical perspective, PAs are classified to nonfunctional Pituitary adenomas (NFPAs) and functional Pituitary adenomas (FPAs). Compared to FPAs, NFPAs are more difficult to treat because there are nearly no clinical signs until NFPAs reach enough mass to produce compression symptoms [12,14].
Among a wide range of clinical behaviors that PAs exhibit invasiveness related to the behavior of invading into adjacent structures, such as the cavernous sinus, skull base bone, and sphenoid sinus, attracts most clinical attentions because it makes clinical therapy tough to manage and causes lots of complications. Otherwise, invasiveness has been proven to correlate to poor prognosis [3].
The tumor-associated calcium signal transducer (TACSTD) gene family consists of two highly conserved and closely related genes, TACSTD1 and TACSTD2. The TACSD1 gene encodes TROP1, which was originally known as epithelial cell adhesion molecule (EpCAM). EpCAM is a glycosylated, type I transmembrane protein with an ectodomain, one transmembrane domain, and a cytoplasmic domain, and it is expressed in human epithelial tissues, cancers, progenitor and stem cells [25]. Since its coming to light, EpCAM has been found to play roles in cell adhesion, oncogenesis, inflammation, cell proliferation, and cell cycle regulation [25].
TROP2, encoded by TACSTD2 gene, is a type I, single transmembrane protein which was originally identified in human trophoblast and choriocarcinoma cell lines [6,13]. And it is overexpressed in many carcinomas, including colorectal cancer, gastric cancer, SCC of the oral cavity, and pancreatic cancer, but not expressed in normal tissue. Until now, there has been a good deal of TROP2-related studies focusing on its role in tumorigenesis [6,18,21,24]. Because of the tumor-specific expression, both EpCAM and TROP2 have been developed as attractive immunotherapeutic targets in cancer treatment [6,18-21,24].
In the previous studies, EpCAM and Trop2 have been demonstrated to contribute to tumors’ recurrence/progression [1,2,8,16,23] and invasion ability. Osta et al showed that silencing EpCAM gene expression decreased invasion potential of breast cancer cell lines in vitro [19]; Ohmachi T et al exhibited that TROP2 was a the cancer-related genes that correlated with invasion behavior of colorectal cancer [18].
Although the roles of EpCAM and TROP2 are not yet fully understood, both of them are thought to participate in growth and proliferation of carcinoma cells [20,24]. In this study, we explore the expression of EpCAM and Trop2 in PAs by immunohistochemistry. Our results revealed that both EpCAM and Trop2 significantly correlated with invasiveness and proliferation of PAs. Furthermore, EpCAM presented a higher overexpression rate in NFPAs than that in FPAs, and Trop2 was showed to be a predictive factor for recurrence/progression for PAs.
Materials and methods
Patients and samples
We analyzed the archival records from the collection of PAs files from Provincial Hospital Affiliated to Shandong University, Jinan, PR China, and selected 74 consecutive cases, who undergoing maximal surgical debulking (gross total resection judged from MRI) and were willing to contribute to this research, during from December 2008 to December 2009. The Clinical data (including age, gender, radiologic examination and clinical behaviors) were obtained from patients’ medical records in the hospital information systems (Table 1). The sections of all tumors were produced from paraffin-embedded tissue samples stored in Department of Pathology and reviewed by 2 pathologists to define the histological type. Informed consents have been obtained from the involved patients or their authorised relatives. The study was approved by the Ethics Committee in Jinan and is in accord with the Helsinki Declaration.
Table 1.
Expression of EpCAM and Trop2 in PAs and their correlation with clinicopathological parameters
| EpCAM overexpression | Trop2 overexpression | |||||
|---|---|---|---|---|---|---|
| Invasiveness | Yes | No | Yes | No | ||
| Yes | 34 | 6 | χ2 = 10.510 | 29 | 11 | χ2 = 7.408 |
| No | 17 | 17 | P = 0.001* | 14 | 20 | P = 0.006* |
| r = 0.377 | r = 0.316 | |||||
| P = 0.001* | P = 0.006* | |||||
| No Clinical functioning | ||||||
| Yes | 36 | 10 | χ2 = 4.953 | 29 | 17 | χ2 = 0.270 |
| No | 15 | 13 | P = 0.026* | 14 | 14 | P = 0.334 |
| r = 0.259 | r = 0.128 | |||||
| P = 0.026* | P = 0.276 | |||||
| Ki-67 | 4.6 ± 3.7 | 2.9 ± 2.7 | r = 0.294 | 5.1 ± 3.8 | 2.7 ± 2.3 | r = 0.405 |
| P = 0.011* | P = 0.000* | |||||
| Gender | ||||||
| Male | 36 | 12 | r = -0.179 | 28 | 20 | r = -0.006 |
| Female | 15 | 11 | P = 0.128 | 15 | 11 | P = 0.958 |
| Age | 40.8 ± 13.9 | 43.0 ± 15.2 | r = -0.059 | 43.3 ± 13.9 | 39.0 ± 14.7 | r = 0.159 |
| P = 0.619 | P = 0.176 | |||||
Chi-Square (X2) test is used to analyze differences of EpCAM and Trop2 expression in PAs of different classifications. Spearman rank correlation coefficient (r) is used to analyze the correlation of EpCAM and Trop2 with clinicopathological parameters.
P-values indicating significance when P < 0.05.
Immunohistochemistry
The immunohistochemical study was performed using the Envision PV-style two-step method (PV-9000/9003 Polymer Detection System, Zhongshan Goldenbridge Biotechnology, China). Sections of intestinal adenocarcinoma with known EpCAM positivity [25] were used as a positive control. For negative control, primary antibodies were replaced by the preparation solution for antibodies. 4-µM thickness of formalin-fixed, paraffin-embedded tissue sections were baked at 60°C for 30 minutes, deparaffinized in xylene, and rehydrated in graded concentrations of ethanol. Heat-induced antigen retrieval was carried out (10 mM citrate buffer [pH 6.0] at 98°C for 20 minutes in a thermostat-controlled waterbath), then quenching endogenous peroxidase activity by incubating in 0.3% hydrogen peroxide at 37°C for 30 minutes and blocking nonspecific staining by normal serum from the same species as that of secondary antibodies at 37°C for 30 minutes. Primary antibodies (anti-EpCAM, ab71916, Abcam, Cambridge, UK, 1/100; anti-Trop2, A F650, R&D Systems, Minneapolis, USA, 1:50; anti-Ki67, ab66155, Abcam, Cambridge, UK, 1/200) were applied at 4°C overnight, then rewarming at 37°C for 30 minutes, followed by Polymer Helper (Zhongshan Goldenbridge Biotechnology) incubation at 37°C for 20 minutes and polyperoxidase-anti-goat IgG (Zhongshan Goldenbridge Biotechnology) incubation at 37°C for 25 minutes. Diaminobenzidine was used as the substrate to observe the specific antibody localization, and hematoxylin was used as a nuclear counterstain. Sections were examined and scored for EpCAM, Trop2 and Ki-67 by two observers who were unaware of the histological types or clinical features. All the samples were stained at the same time.
EpCAM and Trop2 expression for PAs staining was evaluated by calculating a total immunostaining score (TIS) as the product of a proportion score (PS) and an intensity score (IS). The PS describes the estimated fraction of positively stained cells (0, none; 1, < 10%; 2, 10-50%; 3, 51-80%; 4, > 80%). The IS represents the estimated staining intensity as compared with control cells (0, no staining; 1, weak; 2, moderate; 3, strong). The TIS (TIS = PS × IS) ranges from 0 to 12 with only nine possible values (that is, 0, 1, 2, 3, 4, 6, 8, 9 and 12). The ‘overexpression’ has been defined as a TIS > 4 [10].
Scores for Ki-67 were recorded as the number of immunopositive cells per high-power microscope (×400). The number of immunopositive cells under 5 microscopes per section with the highest cell counts was counted and the average was recorded.
Figures were prepared with Photoshop 12.0 software for Windows.
Follow-up analysis
Recurrence/progression was defined as the discovery of an elevated hormone level at any time in the postoperative surveillance period after an initial remission or a re-emerging tumor in patients without evidence of residual tumor after surgical therapy.
All the patients underwent preoperative and postoperative MRI and followed 3 or 6 months interval within the first 1 year. Then, for assessing tumor recurrence, serial MRI scans were performed at a 1-year interval in asymptomatic patients, but if mass-related symptoms or hormonal alterations developed, a MRI scan was performed immediately.
The follow-up analysis of patients with or without EpCAM/Trop2 overexpression was calculated with the Kaplan-Meier method and the difference was analyzed using the two-sided log-rank test in SPSS 21.0 for windows. Multivariate Cox regression analysis in a forward stepwise method to evaluate the effect of multiple independent prognostic factors on follow-up outcome.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 for windows. Chi-Square (X2) Tests were used to analyze differences of EpCAM and Trop2 expression in PAs of different classifications. Spearman rank correlation (r) analysis was used to analyze the correlations of EpCAM and Trop2 expression with clinicopathological parameters. Results were reported as being statistically significant if P-values < 0.05 (2-sided).
Results
Immunohistochemistry results showed that EpCAM was widely overexpressed in PAs with the ratio of 51/74 (68.9%) and Trop2 was overexpressed in PAs with the ratio of 43/74 (58.1%). Both of the proteins presented a membranous and cytoplasmic mixed pattern (Figures 1, 2). For both EpCAM and Trop2, PAs with invasiveness showed a higher overexpression rate than PAs without invasiveness (X2EpCAM = 10.510, P = 0.001; X2Trop2 = 7.408, P = 0.006). NFPAs demonstrated a higher EpCAM overexpression than FPAs (X2EpCAM = 4.953, P = 0.026). Otherwise, the overexpression of EpCAM and Trop2 was correlated significantly with expression of proliferation factor Ki-67 (rEpCAM = 0.294, P = 0.011; rTrop2 = 0.405, P = 0.000), but not with gender (rEpCAM = -0.179, P = 0.128; rTrop2 = -0.006, P = 0.958) and age (rEpCAM = -0.059, P = 0.619; rTrop2 = 0.159, P = 0.176) (Table 1).
Figure 1.
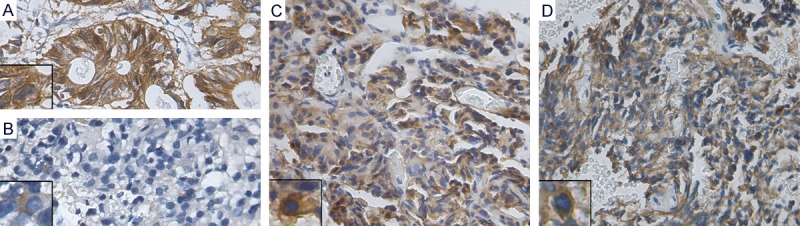
Representative immunohistochemical staining for EpCAM (400×). Membranous and cytoplasmic staining in PAs cells was observed in (A-D). (A) Intestinal adenocarcinoma with strong EpCAM expression (overexpression, TIS = 12); (B) The non-invasive and functioning PA with weak EpCAM expression (no overexpression, TIS = 4, predominantly cytoplasmic staining); (C) The invasive PA with strong EpCAM expression(overexpression, TIS = 12, predominantly membranous staining); (D) The NFPA with strong EpCAM expression (overexpression, TIS = 9, predominantly cytoplasmic staining). Inserts show representative staining.
Figure 2.
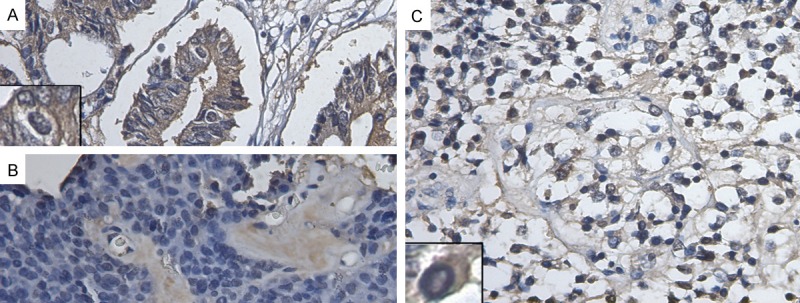
Representative immunohistochemical staining for Trop2 (400×). Membranous and Cytoplasmic staining in PAs cells was observed in (A-C). (A) Intestinal adenocarcinoma with strong Trop2 expression (overexpression, TIS = 12); (B) The non-invasive PA with no Trop2 expression; (C) The invasive PA with strong Trop2 expression (overexpression, TIS = 12). Inserts show representative staining.
During follow-up, 8 (10.8%) of the 74 patients showed recurrence and 2 (2.7%) patients showed progression. For Trop2, patients with overexpression showed a more significant propensity for recurrence or progression in Kaplan-Meier analysis (X2Trop2 = 4.844, P = 0.028), but for EpCAM, the situation is not the same (X2EpCAM = 0.018, P = 0.892) (Figure 3). Multivariate Cox regression analysis estimated Trop2 overexpression to be a significantly predictive factor for recurrence/progression (RR = 11.060, P = 0.025) (Table 2).
Figure 3.
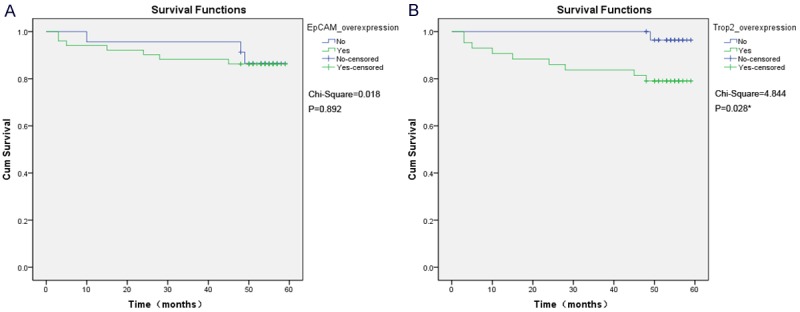
Kaplan-Meier analysis for recurrence/progression-free survival according to expression of EpCAM and Trop2. A. EpCAM overexpression didn’t show prognostic value for recurrence/progression-free survival (X2EpCAM = 0.018, P = 0.892); B. Patients with Trop2 overexpression demonstrated a significantly lower recurrence/progression-free survival as compared with the cases lacking Trop2 overexpression (X2Trop2 = 4.844, P = 0.028).
Table 2.
Cox regression analysis of variables in PAs patients for Recurrence/Progression
| Variables1 | RR | 95% CI | P |
|---|---|---|---|
| Trop2 overexpression | 11.060 | 1.353--90.387 | 0.025* |
| Invasiveness | 0.291 | 0.080--1.053 | 0.060 |
EpCAM overexpression, Ki67, Clinical Functioning, age and gender have been excluded in the equation by the forward stepwise method of SPSS 21.0.
Discussion
To our knowledge, this is the first study to investigate Trop2 expression in PAs and reveal the significant correlation between TACSTD family proteins (EpCAM and Trop2) overexpression and PAs invasiveness. Otherwise, this study showed that EpCAM and Trop2 overexpression in PAs was correlated significantly with proliferative factor Ki-67, and EpCAM overexpression related significantly to clinical functioning of PAs. Trop2 overexpression was showed to be a significantly predictive factor for recurrence/progression for PAs.
Our results demonstrated high EpCAM overexpression rate in PAs, which was 51/74 (68.9%), just as in other epithelial-derived tumors [25], and exhibited the over expression of Trop2 in PAs for the first time, which rate was 43/74 (58.1%). We observed that both EpCAM and Trop2 were expressed in PAs cells with a membranous and cytoplasmic mixed pattern. As membrane proteins, EpCAM and Trop2 have been found to be involved in the intracellular signal transduction in cancer cells [13,25], and it is confirmed that both the enhanced membranous expression and cytoplasmic expression (shift expression) were positively related to the tumorigenesis in the previous studies for EpCAM and Trop2 [17,20,22,26]. So we tentatively put forward that EpCAM and Trop2 take part in the tumorigenesis in the overexpression-related PAs cases, but this viewpoint needs to be verified by further and meticulous research.
The invasive behavior of pituitary adenomas, which is related to invasion into the cavernous sinus, skull base bone and sphenoid sinus, prevents curative radical surgery and causes poor prognosis [3,15]. Although several potential biomarkers, such as FGFR4, MMP, PTTG, Ki-67 and p53, have been investigated [15], the molecular mechanisms underlying invasive behavior of PAs remain poorly understood.
As tumor-associated antigens, the roles of EpCAM and Trop2 in tumorigenesis have been extensively explored in some human tumors [18-21,24] and some of them have proved the positive correlation between TACSTD family proteins and tumor invasiveness. Previous studies have proved that EpCAM siRNA treatment significantly decreased cell invasion in the breast and tongue cancer cell lines [19,26] and Trop2 has been demonstrated to correlate positively to invasiveness in colorectal and pancreatic cancer [9,18].
Our results showed that both the overexpression of EpCAM and Trop2 was significantly correlated with the invasiveness of Pas (X2EpCAM = 10.510, P = 0.001; X2Trop2 = 7.408, P = 0.006), suggesting EpCAM and Trop2 promising biomarkers for PAs invasiveness.
Nucleus protein Ki-67 is a splendid marker for measuring the proliferative activity of tumor cells. Because it exists in all active phases of the cell cycle [G(1), S, G(2), and mitosis] and is absent from resting cells [G(0)], Ki67 is strongly associated with the percentage of growth fraction in a given cell population [11]. Our results showed that, in PAs, both EpCAM and Trop2 overexpression correlated significantly with Ki-67 expression (rEpCAM = 0.294, P = 0.011; rTrop2 = 0.405, P = p=0.000), which indicated the positive correlation between overexpression of these two proteins and PAs cells proliferation.
Nonfunctional PAs (NFPAs) account for approximately 25% of PAs [4]. Because of the hormonal inactivity, NFPAs are always not diagnosed until they became large enough to cause tumor mass effects, such as hypopituitarism, visual field defects, or headaches. In surgeries, complete resection is scarcely possible because of NFPAs’ invading to the adjacent structures, and other treatments, such as postoperative radiotherapy and chemotherapy, are always unsatisfactory [4]. As a result, there is an urgent need to determine new therapeutic biomarkers and develop novel antitumor agents against these refractory nonfunctional adenomas. Recently, due to the tumor specific expression, both EpCAM and Trop2 have been explored as immunotherapeutic targets of carcinoma and exhibited promising prospects [7,20,24,25].
In this study, our findings demonstrated that the EpCAM overexpression in NFPAs was significantly higher than that in FPAs (X2EpCAM = 4.953, P = 0.026). We propose EpCAM to be a helpful tool to distinguish NFPAs from FPAs and a potential target of immunotherapy for NFPAs.
For both EpCAM and Trop2, there were no differential expression related to gender (rEpCAM = -0.179, P = 0.128; rTrop2 = -0.006, P = 0.958) and age (rEpCAM = -0.059, P = 0.619; rTrop2 = 0.159, P = 0.176), which is in accord with previous results in other tumors [16,26].
To date, although a number of oncogenes, tumor suppressor genes, cell cycle mediators, microRNA (miRNAs), and long noncoding RNAs (lncRNAs) have been identified to be involved in the tumorigenesis of pituitary adenomas [14], there are still no reliable histological markers predictive of recurrence for Pituitary Adenomas [5]. Recently, both Trop2 [8,9,16] and EpCAM [1,2,23] have been showed to significantly related to disease recurrence/ progression.
In this study, Kaplan-Meier analysis on determinants of recurrence/progression demonstrated significantly lower recurrence/progression-free survival with Trop2 overexpression (X2Trop2 = 4.844, P = 0.028), but not with EpCAM overexpression (X2EpCAM = 0.018, P = 0.892). Multivariate Cox regression analysis estimated Trop2 overexpression to be a significantly predictive factor of recurrence/progression (RR = 11.060, P = 0.025).
More studies for signal pathway are needed to show the mechanism underlying the significant results we showed in this study.
In conclusion, this study firstly shows that TACSTD family proteins (EpCAM and Trop2) overexpression in PAs relate significantly to invasiveness and proliferative factor Ki67, suggesting that EpCAM and Trop2 take part in the progress of PAs tumorigenesis. The differential EpCAM overexpression between NFPAs and FPAs indicates that EpCAM is a potential target for diagnosis and immunotherapy of NFPAs. Trop2 may be a significantly predictive factor for recurrence/progression for PAs. In the next moment, studies of signal pathway are needed to illuminate the mechanism of above findings and the feasibility of clinical application.
Acknowledgements
The authors thank the patients who contributed to this research. This study was supported by grant ZR2010HM013 from the Science and Technology Department of Shandong Province, 2014GSF118025 from Science and Technology Development Plan of Shandong Province, 81171062 and 81100836 from the National Natural Science Foundation of China.
Disclosure of conflict of interest
None.
References
- 1.Agboola AJ, Paish EC, Rakha EA, Powe DG, Macmillan RD, Ellis IO, Green AR. EpCAM expression is an indicator of recurrence in basal- like breast cancer. Breast Cancer Res Treat. 2012;133:575–582. doi: 10.1007/s10549-011-1813-7. [DOI] [PubMed] [Google Scholar]
- 2.Benko G, Spajić B, Krušlin B, Tomas D. Impact of the EpCAM expression on biochemical recurrence-free survival in clinically localized prostate cancer. Urol Oncol. 2013;31:468–474. doi: 10.1016/j.urolonc.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Buchfelder M. Management of aggressive pituitary adenomas: current treatment strategies. Pituitary. 2009;12:256–260. doi: 10.1007/s11102-008-0153-z. [DOI] [PubMed] [Google Scholar]
- 4.Colao A, Di Somma C, Pivonello R, Faggiano A, Lombardi G, Savastano S. Medical therapy for clinically non-functioning pituitary adenomas. Endocr Relat Cancer. 2008;15:905–915. doi: 10.1677/ERC-08-0181. [DOI] [PubMed] [Google Scholar]
- 5.Cornelius A, Cortet-Rudelli C, Assaker R, Kerdraon O, Gevaert MH, Prévot V, Lassalle P, Trouillas J, Delehedde M, Maurage CA. Endothelial expression of endocan is strongly associated with tumor progression in pituitary adenoma. Brain Pathol. 2012;22:757–764. doi: 10.1111/j.1750-3639.2012.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cubas R, Li M, Chen C, Yao Q. Trop2: a possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta. 2009;1796:309–314. doi: 10.1016/j.bbcan.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.El-Sahwi K, Bellone S, Cocco E, Casagrande F, Bellone M, Abu-Khalaf M, Buza N, Tavassoli FA, Hui P, Rüttinger D, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD. Overexpression of EpCAM in uterine serous papillary carcinoma: implications for EpCAM-specific immunotherapy with human monoclonal antibody adecatumumab (MT201) Mol Cancer Ther. 2010;9:57–66. doi: 10.1158/1535-7163.MCT-09-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang YJ, Lu ZH, Wang GQ, Pan ZZ, Zhou ZW, Yun JP, Zhang MF, Wan DS. Elevated expressions of MMP7, TROP2, and surviving are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis. 2009;24:875–884. doi: 10.1007/s00384-009-0725-z. [DOI] [PubMed] [Google Scholar]
- 9.Fong D, Moser P, Krammel C, Gostner JM, Margreiter R, Mitterer M, Gastl G, Spizzo G. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99:1290–1295. doi: 10.1038/sj.bjc.6604677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gastl G, Spizzo G, Obrist P, Dünser M, Mikuz G. EpCAM overexpression in breast cancer as a predictor of survival. Lancet. 2000;356:1981–1982. doi: 10.1016/S0140-6736(00)03312-2. [DOI] [PubMed] [Google Scholar]
- 11.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 12.Greenman Y, Stern N. Non-functioning pituitary adenomas. Best Pract Res Clin Endocrinol Metab. 2009;3:625–638. doi: 10.1016/j.beem.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Guerra E, Trerotola M, Aloisi AL, Tripaldi R, Vacca G, La Sorda R, Lattanzio R, Piantelli M, Alberti S. The Trop-2 signaling network in cancer growth. Oncogene. 2013;32:1594–1600. doi: 10.1038/onc.2012.151. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Zhang X. The molecular pathogenesis of pituitary adenomas: an update. Endocrinol Metab (Seoul) 2013;28:245–254. doi: 10.3803/EnM.2013.28.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mete O, Ezzat S, Asa SL. Biomarkers of aggressive pituitary adenomas. J Mol Endocrinol. 2012;49:R69–78. doi: 10.1530/JME-12-0113. [DOI] [PubMed] [Google Scholar]
- 16.Mühlmann G, Spizzo G, Gostner J, Zitt M, Maier H, Moser P, Gastl G, Zitt M, Müller HM, Margreiter R, Ofner D, Fong D. TROP2 expression as prognostic marker for gastric carcinoma. J Clin Pathol. 2009;62:152–158. doi: 10.1136/jcp.2008.060590. [DOI] [PubMed] [Google Scholar]
- 17.Ning S, Liang N, Liu B, Chen X, Pang Q, Xin T. TROP2 expression and its correlation with tumor proliferation and angiogenesis in human gliomas. Neurol Sci. 2013;34:1745–1750. doi: 10.1007/s10072-013-1326-8. [DOI] [PubMed] [Google Scholar]
- 18.Ohmachi T, Tanaka F, Mimori K, Inoue H, Yanaga K, Mori M. Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res. 2006;12:3057–3063. doi: 10.1158/1078-0432.CCR-05-1961. [DOI] [PubMed] [Google Scholar]
- 19.Osta WA, Chen Y, Mikhitarian K, Mitas M, Salem M, Hannun YA, Cole DJ, Gillanders WE. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64:5818–5824. doi: 10.1158/0008-5472.CAN-04-0754. [DOI] [PubMed] [Google Scholar]
- 20.Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev. 2012;38:68–75. doi: 10.1016/j.ctrv.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Sharkey RM, van Rij CM, Karacay H, Rossi EA, Frielink C, Regino C, Cardillo TM, McBride WJ, Chang CH, Boerman OC, Goldenberg DM. A new Tri-Fab bispecific antibody for pretargeting Trop-2-expressing epithelial cancers. J Nucl Med. 2012;53:1625–1632. doi: 10.2967/jnumed.112.104364. [DOI] [PubMed] [Google Scholar]
- 22.Stoyanova T, Goldstein AS, Cai H, Drake JM, Huang J, Witte ON. Regulated proteolysis of Trop2 drives epithelial hyperplasia and stem cell self-renewal via b-catenin signaling. Genes Dev. 2012;26:2271–2285. doi: 10.1101/gad.196451.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tena-Suck ML, Ortiz-Plata A, Galán F, Sánchez A. Expression of epithelial cell adhesion molecule and pituitary tumor transforming gene in adamantinomatous craniopharyngioma and its correlation with recurrence of the tumor. Ann Diagn Pathol. 2009;13:82–88. doi: 10.1016/j.anndiagpath.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Trerotola M, Cantanelli P, Guerra E, Tripaldi R, Aloisi AL, Bonasera V, Lattanzio R, de Lange R, Weidle UH, Piantelli M, Alberti S. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32:222–233. doi: 10.1038/onc.2012.36. [DOI] [PubMed] [Google Scholar]
- 25.Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171:386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanamoto S, Kawasaki G, Yoshitomi I, Iwamoto T, Hirata K, Mizuno A. Clinicopathologic significance of EpCAM expression in squamous cell carcinoma of the tongue and its possibility as a potential target for tongue cancer gene therapy. Oral Oncol. 2007;43:869–877. doi: 10.1016/j.oraloncology.2006.10.010. [DOI] [PubMed] [Google Scholar]


