Abstract
Objective: This study is to investigate the hepatitis B virus (HBV)-induced tubular epithelial-myofibroblast transdifferentiation (TEMT) in human renal tubular epithelial HK-2 cells. Methods: Human proximal tubular epithelial HK-2 cells were cultured. These HK-2 cells were divided into 4 groups: the blank control group, the vector control group, the HBV-transfected group, and the inhibitor-treated group. Transfection was performed with lipofectamine. Measurements of hepatitis B e antigen (HBeAg) and hepatitis B surface antigen (HBsAg) in culture supernatant were determined by electrochemiluminescence immunoassay. Immunocytochemical staining, reverse transcription PCR (RT-PCR), and Western blot analysis were performed to detect the mRNA and protein expression levels, respectively. Results: The immunocytochemical staining showed that, the expression level of E-cadherin was dramatically decreased, while the α-SMA expression level was significantly elevated, in HBV-transfected HK-2 cells. The mRNA level of TGF-β1 and the protein level of p-p38 mitogen-activated protein kinase (MAPK) were elevated in HK-2 cells transfected with HBV. When treated with the p38 MAPK-specific inhibitor, the activation of p38 MAPK was eliminated in HBV-transfected HK-2 cells. In addition, the altered expression levels of E-cadherin and α-SMA, the increased contents of HBeAg and HBsAg in the culture supernatant, as well as the morphological changes of TEMT in HBV-transfected HK-2 cells, were all reversed by the inhibiter treatment. Conclusion: HBV transfection could induce TEMT in HK-2 cells, which was mediated by the TGF-β1/p38 MAPK pathway. These findings provide new insights into the prevention and treatment of HBV-associated glomerulonephritis.
Keywords: Hepatitis B virus (HBV), tubular epithelial-myofibroblast transdifferentiation (TEMT), human tubular epithelial cells, TGF-β1/p38 MAPK pathway
Introduction
Hepatitis B virus-associated glomerulonephritis (HBV-GN) is one of the common hepatic injuries induced by HBV infection [1]. The mechanisms for HBV-GN have not yet been fully elucidated, which might involve immune complex deposition injury, autoimmune damage, and other kidney damages directly induced by HBV-related pathways [2]. HBV-GN might lead to renal interstitial fibrosis (RIF) in the late stage, including tubular cell loss and excessive extracellular matrix (ECM) accumulation. Tubular epithelial-myofibroblast transdifferentiation (TEMT) has been reported to play an important role in RIF pathogenesis [3].
Under pathological conditions, renal tubular epithelial cells would lose their epithelial phenotypes, and transdifferentiate into myofibroblasts (referred to as TEMT). The transdifferentiated cells would secrete large amounts of type I, III, and IV collagen and fibronectin, leading to excessive ECM accumulation and the subsequent fibrosis. Several cytokines have been shown to be involved in TEMT, including transforming growth factor (TGF)-β1. Increasing evidence supports the role of TGF-β1 and its downstream signal transduction pathways in the development and progression of renal fibrosis [4,5]. On the other hand, TGF-β1 also plays an important role in HBV-related liver diseases [6,7]. Our previous study has shown that HBV could replicate and express corresponding antigens in human tubular epithelial cells, resulting in cellular apoptosis [8]. However, whether or not HBV infection could induce TEMT in tubular epithelial cells and the related mechanism(s) have not yet been fully established.
In this study, human proximal tubular epithelial HK-2 cells were transfected with recombinant full-length HBV genotype C, and the process of HBV-induced TEMT, especially concerning the involvement of the TGF-β1/p38 mitogen-activated protein kinase (MAPK) pathway, was investigated. Our findings might contribute to understanding the mechanisms of HBV-related pathogenesis.
Materials and methods
Materials and reagents
Recombinant full-length HBV genotype C (PHY106-CHBV) plasmid was a kind gift from Prof. Jun Cheng at Beijing Ditan Hospital (Beijing, China). Human proximal tubular epithelial HK-2 cell line was preserved in the Laboratory of Biochemistry and Molecular Biology, School of Medicine, Shandong University. Plasmid extraction kit and LipofectamineTM2000 were purchased from Invitrogen (Grand Island, NY, USA). DMEM/F12 medium and fetal bovine serum was purchased from Gibco (Grand Island, NY, USA). Rabbit anti-human anti-α-smooth muscle actin (α-SMA) antibody and rabbit anti-human anti-E-cadherin antibody were purchased from Abcam (Cambridge, MA, USA). Rabbit anti-human anti-p-p38 MAPK and rabbit anti-human anti-p38 MAPK antibodies were purchased from Anbo (San Francisco, CA, USA). The p38 MAPK specific inhibitor SB20358 was purchased from Beyotime (Haimen, Jiangsu, China). Biotin-labeled goat anti-rabbit IgG and DAB kit were purchased from Boster Biological Engineering Co., Ltd. (Wuhan, Hubei, China). Reverse transcription kit and PVDF membrane were purchased from Ferments (Flambourough, Ontario, Canada). ECL developing kit was from Cwbio (Beijing, China). TE2000-U inverted fluorescence microscope was from Nikon (Tokyo, Japan).
Cell culture and grouping
Human proximal tubular epithelial HK-2 cells were cultured using DMEM/F12 medium containing 10% fetal calf serum, as well as penicillin and streptomycin, in a 37°C, 5% CO2 incubator. These HK-2 cells were divided into the following groups: (1) the blank control group that was free from intervention; (2) the vector control group that was transfected with empty plasmid (PHY106); (3) the HBV-transfected group in which HK-2 cells were transfected with PHY106-CHBV DNA; and (4) the inhibitor-treated group in which HK-2 cells were pre-treated with 10 µmol/L SB203580 for 30 min, and then transfected with PHY106-CHBV DNA.
Transient transfection
Cells in exponential growth phase were planted into 24-well plates at a density of 1×106 cell/ml. When 80-90% confluence was reached, transient transfection was performed. 0.8 µg plasmid and 2 µL liposome were added into 50 µL opti-MEM, respectively, and gently mixed. After incubated at room temperature for 5 min, opti-MEM medium containing plasmid and liposome was mixed together and incubated for another 20 min. For transfection, 400 µL fresh DMEM/F12 medium was added into each well. For the blank control group, another 100 µL DMEM/F12 medium was added; for the vector control group, 100 µL liposome-PHY106 complex was added; for the HBV-transfected group and the inhibitor-treated group, 100 µL liposome-PHY106-CHBV DNA was added. After 6 h, the transfection medium was replaced by complete medium for further incubation.
Electrochemiluminescence immunoassay (ECLIA)
The contents of hepatitis B e antigen (HBeAg) and hepatitis B surface antigen (HBsAg) in the culture supernatant were detected with the ECLIA method at 24 h, 48 h, and 72 h, respectively, after transfection.
Immunocytochemical staining
Cells were cultured on 10-mm cover-slips in a 24-well plate. For immunocytochemical staining, cells were fixed by 4% paraformaldehyde, and treated with 0.5% TritonX-100 at room temperature for 30 min. After incubated with 3% H2O2, the cells were blocked with rabbit serum at room temperature for 15 min. Then rabbit anti-human anti-E-cadherin antibody (1:100 dilution) and rabbit anti-human anti-α-SMA antibody (1:100 dilution) was added, respectively, to incubate the cells at 4°C overnight. Biotin-labeled secondary antibody was used for incubation at 37°C for 30 min before SP addition. After 8-min DAB incubation, cells were subjected to counter staining with hematoxylin and the subsequent gradient alcohol dehydration, xylene transparency, and gum sealing. LIMPS microscope was used for image capturing, and Image Pro Plus 6.0 software was used to analyze the optical density.
Reverse transcription PCR (RT-PCR)
Cells were seeded in 6-well plates, and the transfection was performed as mentioned above. After 72 h, total RNA was extracted with the Trizol agent, and RT-PCR was performed. The primer sequences for TGF-β1 were: forwards, 5’-TCCACCTGCAAGACTATCGAC-3’, and reverse, 5’-GAGGTATCGCCAGGAATTGTT-3’; β-actin primer sequences were: forward, 5’-AGTTGCGTTACACCCTTTC-3’, and reverse, 5’-CCTTCACCGTTCCAGTTT-3’. PCR conditions were as follows: denaturation at 95°C for 10 min; 95°C for 15 s, 66°C for 1 min, for totally 30 cycles. PCR products were subjected to 1.5% agarose gel electrophoresis. Bio-Rad gel imaging system was used for image capturing, and ImageJ software was used for optical density analysis. β-actin was used as control.
Western blot analysis
Cells were collected and lysed with lysis buffer on ice. After centrifugation, protein concentration was determined with the BCA method. 50 μg protein samples were subjected to SDS-PAGE, and then electrically transferred to a PVDF membrane. The blot was blocked with 5% non-fat milk at 37°C for 2 h. The following primary antibodies were used for incubation at 4°C overnight: α-SMA (1:500 dilution), E-cadherin (1:500 dilution), p-p38 MAPK (1:500 dilution), p38 MAPK (1:1000 dilution), and GAPDH (1:3000 dilution). Then horseradish peroxidase-conjugated secondary antibody (1:5000 dilution) was used for incubation at 37°C for another 1 h. ECL developing exposure was performed. Bio-Rad gel imaging system was used for image capturing, and ImageJ software was used for optical density analysis. GAPDH was used as control.
Statistical analysis
Data are expressed as mean ± SD. SPSS19.0 software was used for statistical analysis. F analysis and SNK-t test were used for comparison. P < 0.05 was considered statistically significant.
Results
Detection of HBeAg and HBsAg in culture supernatant of HK-2 cells
HK-2 cells were transfected with the HBV-containing plasmids (PHY106-CHBV DNA), and the contents of HBeAg and HBsAg in cell culture supernatants were determined with the ECLIA method at 24 h, 48 h, and 72 h, respectively, after transfection. HK-2 cells without transfection and HK-2 cells transfected with empty vector were used as the blank control and the vector control. Our results showed that, the cell culture supernatant was negative for HBsAg and HBeAg in the blank and vector control groups at all indicated time points. On the other hand, HBsAg and HBeAg was positive in the culture supernatant from HK-2 cells transfected with HBV, and the contents of these two antigens were increased from 24 h to 72 h post-transfection (Table 1). The results suggest that HBV could efficiently replicate and express corresponding antigens in HK-2 cells. According to these results, the following measurements in HK-2 cells were carried out at 72 h after HBV transfection.
Table 1.
Measurements of HBsAg and HBeAg in culture supernatant of HK-2 cells
| Groups | n | 24 h post-transfection | 48 h post-transfection | 72 h post-transfection | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| HBsAg (ng/ml) | HBeAg (PEIU/ml) | HBsAg (ng/ml) | HBeAg (PEIU/ml) | HBsAg (ng/ml) | HBeAg (PEIU/ml) | ||
| Blank control | 5 | 0.10±0.02 | 0.21±0.02 | 0.11±0.02 | 0.15±0.02 | 0.12±0.02 | 0.17±0.03 |
| Vector control | 5 | 0.10±0.01 | 0.14±0.02 | 0.16±0.02 | 0.19±0.03 | 0.10±0.02 | 0.21±0.03 |
| HBV transfection | 5 | 0.16±0.02 | 0.12±0.02 | 0.51±0.03* | 1.02±0.04* | 2.62±0.02* | 1.93±0.06* |
| Inhibitor treatment | 5 | 0.10±0.03 | 0.20±0.01 | 0.13±0.02 | 0.17±0.02 | 0.11±0.02 | 0.18±0.03 |
Note: Compared with the control groups,
P < 0.05.
HBsAg and HBeAg quantification > 1.0 was considered as positive, while < 1.0 was considered as negative.
Morphological observation and immunocytochemical staining of HK-2 cells
To investigate whether HBV infection could induce TEMT in HK-2 cells, morphological observation and immunocytochemical staining for E-cadherin and α-smooth muscle actin (α-SMA) were performed. Under inverted phase-contrast microscope, the HK-2 cells in the blank and vector control groups exhibited cobblestone-shaped morphology, which was typical of epithelial cells. HBV-transfected HK-2 cells, however, exhibited spindle-shaped fibroblast-like morphology (Figure 1A). E-cadherin is an epithelial cell-specific adhesion molecule, and α-SMA is a specific marker for myofibroblasts. Our results from immunocytochemical staining showed that, compared with the blank and vector control groups, the expression level of E-cadherin was dramatically decreased in HBV-transfected HK-2 cells (Figure 1A, 1B; P < 0.05). On the other hand, the expression level of α-SMA was significantly elevated in HBV-transfected HK-2 cells than the control groups (Figure 1A, 1C; P < 0.05). These results suggest that HBV infection could induce the transdifferentiation from renal tubular epithelial cells into myofibroblasts.
Figure 1.
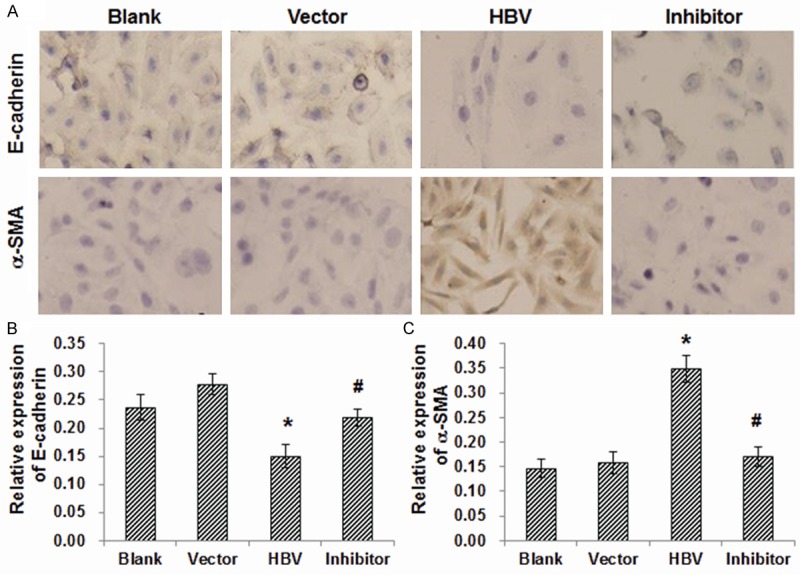
Immunocytochemical staining of E-cadherin and a-SMA in HK-2 cells. (A) HK-2 cells were subjected to immunocytochemical staining to detect the expression of E-cadherin (upper panel) and a-SMA (lower panel) (×200). The blank control group was free from intervention; the vector control group was transfected with empty plasmid (PHY106); the HBV-transfected group was transfected with PHY106-CHBV DNA; and the inhibitor-treated group was pre-treated with SB203580, and then transfected with PHY106-CHBV DNA. (B, C) Statistical analysis of the optical densities in the immunocytochemical staining of E-cadherin (B) and a-SMA (C). Compared with the blank and vector control groups, *P < 0.05; compared with the HBV-transfected group, #P < 0.05.
Involvement of TGF-β1/p38 MAPK pathway in TEMT of HK-2 cells
The TGF-β1/p38 MAPK pathway has been shown to be involved in fibrotic processes in various diseases [9,10]. Next, the involvement of the pathway in TEMT of HK-2 cells was investigated. The mRNA level of TGF-β1 and the protein level of p-p38 MAPK were detected with RT-PCR and Western blot analysis, respectively. The protein expression levels of E-cadherin and α-SMA in HK-2 cells were also detected. Our results from RT-PCR indicated that, compared with the blank and vector control groups, the mRNA expression level of TGF-β1 was significantly elevated in HK-2 cells transfected with HBV (Figure 2; P < 0.05). On the other hand, in line with our results from immunocytochemical staining, Western blot analysis indicated that the protein expression level of E-cadherin was dramatically decreased, while the α-SMA expression level was drastically increased, in HBV-transfected HK-2 cells (Figure 3). Moreover, the protein level of p-p38 MAPK was obviously elevated in HK-2 cells transfected with HBV, indicating the kinase activation (Figure 3). These results suggest that the TGF-β1/p38 MAPK pathway is involved in TEMT of HK-2 cells induced by HBV transfection.
Figure 2.
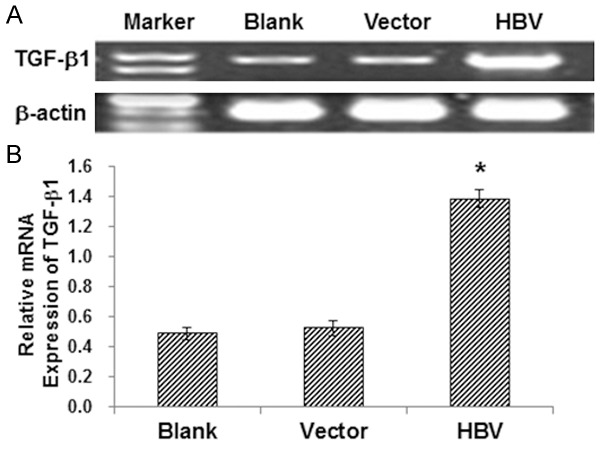
The mRNA expression levels of TGF-β1 in HK-2 cells. A. The TGF-β1 mRNA expression levels in HK-2 cells were detected by RT-PCR. B. Statistical analysis of the expression levels of TGF-β1 in HK-2 cells. Compared with the blank and vector control groups, *P < 0.05.
Figure 3.
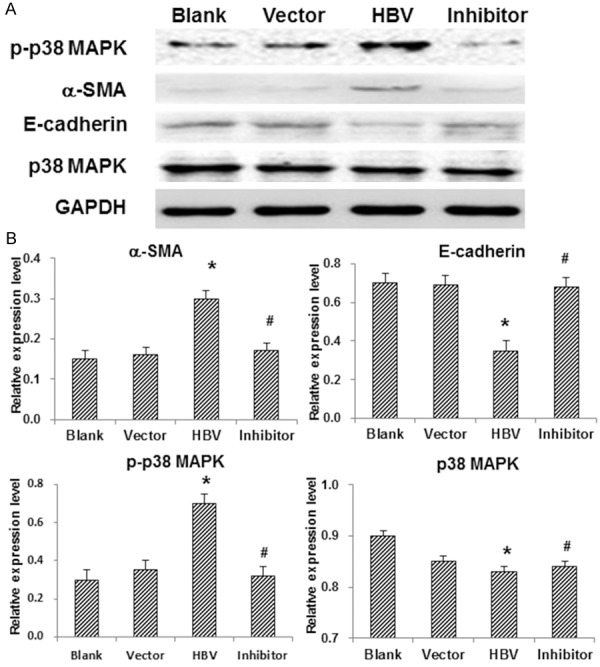
Involvement of the TGF-β1/p38 MAPK pathway in TEMT of HK-2 cells. A. The protein expression levels of p-p38 MAPK, E-cadherin, a-SMA, and p38 MAPK in HK-2 cells were detected by Western blot analysis. B. Statistical analysis of the expression levels of the proteins. Compared with the blank and vector control groups, *P < 0.05; compared with the HBV-transfected group, #P < 0.05.
To further investigate whether the signal transduction pathway was necessary for the TEMT of HK-2 cells, these cells were pre-treated with the p38 MAPK-specific inhibitor, SB20358, and then transfected with HBV-containing vectors. Our results indicated that the inhibitor treatment could significantly eliminate the activation of p38 MAPK (Figure 3). Furthermore, the decreased expression of E-cadherin and the increased expression of α-SMA in HBV-infected HK-2 cells were all reversed by SB20358 (Figures 1, 2 and 3). In addition, the contents of HBeAg and HBsAg in culture supernatant of HK-2 cells transfected with HBV were decreased (Table 1), and the morphological changes of TEMT were abolished (Figure 1A), after the inhibitor treatment, indicating that HBV-induced TEMT of HK-2 cells could be reversed by SB20358. Taken together, these results suggest that HBV-induced TEMT of HK-2 cells is mediated by the TGF-β1/p38 MAPK pathway.
Discussion
HBV infection can not only cause pathological changes in liver structure and function, but also induce a variety of extrahepatic damages, including HBV-GN. The mechanism for HBV-GN has not yet been fully elucidated, and the end-stage renal disease is mainly characterized by renal RIF, in which TEMT is an important determinant. In the present study, in HK-2 cells transfected with HBV-containing plasmids, whether HBV could promote the transdifferentiation of these renal tubular epithelial cells, as well as the possible involved mechanisms, were investigated.
TEMT refers to the process that, under pathological conditions, renal tubular epithelial cells would lose the epithelial phenotype, and transdifferentiate into myofibroblasts. Myofibroblasts hardly exist in normal kidney tissues, and they are mainly derived from the differentiation of fibroblasts, the migration or partial proliferation of smooth muscle cells, and the transdifferentiation of renal tubular epithelial cells [11,12]. The α-SMA is a specific marker for myofibroblast [13], and in normal kidney tissues, α-SMA can only be found in vascular media [14]. It has been shown that the expression level of α-SMA in kidney tissues can partially reflect the amount of myofibroblasts [15,16], making α-SMA a widely used indicator for the transdifferentiation from renal intrinsic cells to myofibroblasts. E-cadherin is an epithelial cell-specific adhesion molecule, which is the main constituent of the adhesion belt. E-cadherin located in the epithelial cell membrane can connect with various ligandins and cytoskeleton components, contributing to the maintenance of normal cytoskeletal structures and the formation of epithelial cell polarity, which are very important for the structural integrity of epithelial cells [17]. The down-regulated expression of E-cadherin would affect the interactions between epithelial cells, resulting in loss of epithelial cell polarity. Zhong et al. [18] show that, in normal human kidney tissues, E-cadherin was abundantly expressed. On the other hand, in patients with type 2 diabetes, the expression of E-cadherin was dramatically declined, while the expression of α-SMA was drastically increased, along with the increasing clinical stages. In this study, HK-2 cells were transfected with HBV-containing plasmids. 72 h after transfection, elevated levels of HBsAg and HBeAg were detected in the culture supernatant, indicating that HBV could efficiently replicate and express corresponding antigens in these cells. Moreover, the observation of cellular morphology showed that, with the transfection, HK-2 cells changed from cobblestone-shaped epithelial cells into spindle-shaped myofibroblast-like cells. Furthermore, the expression of E-cadherin was declined, while the expression of α-SMA was elevated, in HK-2 cells after transfection. Taken together, these results suggest that HBV infection could induce the transdifferentiation of renal tubular epithelial HK-2 cells into myofibroblasts.
So far, the molecular mechanisms of TEMT are not entirely clear. Studies have shown that a variety of cytokines and/or signaling pathways might participate in regulating the transdifferentiation of renal tubular epithelial cells, including TGF-β1, hepatocyte growth factor, interleukin-l, and BMP-7 [19,20]. Among these cytokines, TGF-β1 is the most important factor in initiating and regulating TEMT [21]. TGF-β1-induced TEMT has been confirmed in various clinical studies and animal experiments. But whether or not HBV infection could increase the expression of TGF-β1 in tubular epithelial cells has not yet been reported. Our results showed that the mRNA expression level of TGF-β1 was significantly increased in HK-2 cells at 72 h after HBV transfection. HBV infection-caused cell injury could stimulate the secretion of TGF-β1, which could greatly contribute to the transdifferentiation of HK-2 cells. TGF-β1-assocaited signal transduction pathways involved in TEMT might include the Smad-dependent, p38 MAPK, PI3k/Akt, and RhoA/ROCK pathways. There would be also interactions between these signal transduction pathways. TGF-β1 binds to the receptor tyrosine kinases on the cell membrane, promoting the formation of receptor dimers and activating the tyrosine kinase inside the membrane. The autophosphorylated receptors connect with the SH2 domain within connexin GRB2, which further connect to GEF/NETl through SH3 domain. The interaction promotes the dissociation of Ras from GDP, resulting in the activated GTP-bound state. Activated Ras can bind to and activate the Raf sites on Ras proteins, stimulating the cascade amplification of MAPK pathway. MAPKs are serine/threonine kinases which can phosphorylate other cytoplasmic proteins. MAPKs can translocate from the cytoplasm into the nuclei, regulating the activities of transcription factors. MAPK family consists of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK), p38, and big mitogen-activated protein kinase (ERK5). The TGF-β1/p38 MAPK pathway has been found to be involved in the fibrotic process in various diseases. Peinado et al. [9,10] have shown that TGF-β1 could induce TEMT via the MAPK signal transduction pathway in dog renal tubular epithelial cells. Moreover, it had been confirmed that Snail is involved in TEMT, and TGF-β1 could regulate the transcription and degradation of Snail [22,23]. Stambe et al. [24] show that, in renal interstitial fibrosis rats with unilateral ureteral obstruction, the levels of phosphorylated p38 MAPK are significantly increased in the renal interstitial fibroblasts and tubular epithelial cells. Furthermore, Chin et al. [25] report that TGF-β1 stimulates the synthesis of procollagen I via activating p38 MAPK in murine glomerular mesangial cells, and the specific inhibitor SB203580 could inhibit the expression of procollagen I, indicating that p38 MAPK was implicated in TGF-β1-induced ECM synthesis. Wang et al. [26] suggest that, in MKK3+/+ mice, TGF-β1 stimulation can readily activate p38 MAPK in glomerular mesangial cells, while TGF-β1 stimulus cannot induce p38 MAPK activation in MKK3-/- mice. These results suggest that TGF-β1-induced glomerular sclerosis might be mediated by the p38 MAPK pathway, and MKK3 is necessary for the activation of p38 MAPK by TGF-β1. Martin-Garrido et al. [27] show that TGF-β could up-regulate the expression of α-SMA in human vascular smooth muscle cells, which is mediated by the p38 MAPK pathway and serum response factors. Moreover, the p38 MAPK pathway is activated by the elevated ROS production induced by NADPH oxidase 4 (Nox4). Our results showed that, the protein expression levels of p38 MAPK and α-SMA were up-regulated, while the expression level of E-cadherin was down-regulated, in HBV-transfected HK-2 cells. SB203580, the specific inhibitor of p38 MAPK, could abolish the up-regulation of α-SMA and the down-regulation of E-cadherin. These results suggest that p38 MAPK might be involved in the transdifferentiation of HK-2 cells induced by HBV.
In conclusion, our results showed that HBV transfection could induce TEMT of HK-2 cells, which was mediated by the TGF-β1/p38 MAPK pathway. These findings contribute to the understanding of the pathogenesis of HBV infection, and provide new insights into the prevention and treatment of HBV-associated glomerulonephritis.
Acknowledgements
This work was supported by Shandong province science and technology development program (No. 2007GGWZ02054).
Disclosure of conflict of interest
None.
References
- 1.Mason A. Role of viral replication in extrahepatic syndromes related to hepatitis B virus infection. Minerva Gastroenterol Dietol. 2006;52:53–66. [PubMed] [Google Scholar]
- 2.Hong S, Wang YG, Liu CH, Zhang PJ, Liu Q. Expression of recombinant HBV in the NHMC cells and its influence on cell apoptosis. J Shandong Univ (Health Sci no date) 50:34–38. [Google Scholar]
- 3.Gu L, Gao Q, Ni L, Wang M, Shen F. Fasudil inhibits epithelial-myofibroblast transdifferentiation of human renal tubular epithelial HK-2 cells induced by high glucose. Chem Pharm Bull (Tokyo) 2013;61:688–694. doi: 10.1248/cpb.c13-00066. [DOI] [PubMed] [Google Scholar]
- 4.Hills CE, Squires PE. The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 2011;22:131–139. doi: 10.1016/j.cytogfr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Iwano M. EMT and TGF-beta in renal fibrosis. Front Biosci (Schol Ed) 2010;2:229–238. doi: 10.2741/s60. [DOI] [PubMed] [Google Scholar]
- 6.Saxena R, Chawla YK, Verma I, Kaur J. Effect of IL-12B, IL-2, TGF-β1, and IL-4 polymorphism and expression on hepatitis B progression. J Interferon Cytokine Res. 2014;34:117–128. doi: 10.1089/jir.2013.0043. [DOI] [PubMed] [Google Scholar]
- 7.Karimi-Googheri M, Daneshvar H, Nosratabadi R, Zare-Bidaki M, Hassanshahi G, Ebrahim M, Arababadi MK, Kennedy D. Important roles played by TGF-β in hepatitis B infection. J Med Virol. 2014;86:102–108. doi: 10.1002/jmv.23727. [DOI] [PubMed] [Google Scholar]
- 8.Hong S, Wang YG. Pathogenesis of hepatitis B virus-associated glomerulonephritis. Guid China Med. 2011;9:39–41. [Google Scholar]
- 9.Alexopoulos E, Gionanlis L, Papayianni E, Kokolina E, Leontsini M, Memmos D. Predictors of outcome in idiopathic rapidly progressive glomerulonephritis (IRPGN) BMC Nephrol. 2006;7:16. doi: 10.1186/1471-2369-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin BY, Mohsenin A, Li SX, Choi AM, Choi ME. Stimulation of pro-alpha(1)(I) collagen by TGF-beta(1) in mesangial cells: role of the p38 MAPK pathway. Am J Physiol Renal Physiol. 2001;280:F495–504. doi: 10.1152/ajprenal.2001.280.3.F495. [DOI] [PubMed] [Google Scholar]
- 11.Durvasula RV, Shankland SJ. Podocyte injury and targeting therapy: an update. Curr Opin Nephrol Hypertens. 2006;15:1–7. doi: 10.1097/01.mnh.0000199012.79670.0b. [DOI] [PubMed] [Google Scholar]
- 12.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumarswamy R, Volkmann I, Jazbutyte V, Dangwal S, Park DH, Thum T. Transforming growth factor-β-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol. 2012;32:361–369. doi: 10.1161/ATVBAHA.111.234286. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Qu X, Yao J, Caruana G, Ricardo SD, Yamamoto Y, Yamamoto H, Bertram JF. Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:2612–2624. doi: 10.2337/db09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Q, Xing L, Wang L, Yao F, Liu S, Hao J, Liu W, Duan H. Therapeutic effects of suppressors of cytokine signaling in diabetic nephropathy. J Histochem Cytochem. 2014;62:119–128. doi: 10.1369/0022155413512493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Garrido A, Brown DI, Lyle AN, Dikalova A, Seidel-Rogol B, Lassègue B, San Martín A, Griendling KK. NADPH oxidase 4 mediates TGF-β-induced smooth muscle α-actin via p38MAPK and serum response factor. Free Radic Biol Med. 2011;50:354–362. doi: 10.1016/j.freeradbiomed.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medici D, Potenta S, Kalluri R. Transforming growth factor-β2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem J. 2011;437:515–520. doi: 10.1042/BJ20101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 20.Quinlan JM, Yu WY, Hornsey MA, Tosh D, Slack JM. In vitro culture of embryonic mouse intestinal epithelium: cell differentiation and introduction of reporter genes. BMC Dev Biol. 2006;6:24. doi: 10.1186/1471-213X-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, Flanders KC. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 2006;17:19–27. doi: 10.1016/j.cytogfr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Sommer M, Gerth J, Stein G, Wolf G. Transdifferentiation of endothelial and renal tubular epithelial cells into myofibroblast-like cells under in vitro conditions: a morphological analysis. Cells Tissues Organs. 2005;180:204–214. doi: 10.1159/000088937. [DOI] [PubMed] [Google Scholar]
- 23.Stambe C, Atkins RC, Tesch GH, Masaki T, Schreiner GF, Nikolic-Paterson DJ. The role of p38alpha mitogen-activated protein kinase activation in renal fibrosis. J Am Soc Nephrol. 2004;15:370–379. doi: 10.1097/01.asn.0000109669.23650.56. [DOI] [PubMed] [Google Scholar]
- 24.Strutz F, Zeisberg M, Renziehausen A, Raschke B, Becker V, Kooten C van, Müller G. TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2) Kidney Int. 2001;59:579–592. doi: 10.1046/j.1523-1755.2001.059002579.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Ma R, Flavell RA, Choi ME. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for activation of p38alpha and p38delta MAPK isoforms by TGF-beta 1 in murine mesangial cells. J Biol Chem. 2002;277:47257–47262. doi: 10.1074/jbc.M208573200. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshino J, Monkawa T, Tsuji M, Inukai M, Itoh H, Hayashi M. Snail1 is involved in the renal epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2007;362:63–68. doi: 10.1016/j.bbrc.2007.07.146. [DOI] [PubMed] [Google Scholar]


