Abstract
Abnormal expression of aquaporins (AQPs) has been reported in several human cancers. Epidermal growth factor receptor (EGFR)-extracellular signal-regulated kinases1/2 (ERK1/2) are associated with tumorigenesis and cancer progression and may upregulate AQPs expression. In this study, we investigated acquaporin-8 expression and signaling via epidermal growth factor receptor-extracellular signal-regulated kinases1/2 in human esophageal cancer Eca-109 cells by western blot, immunofluorescence and wound healing (scratch) assays. Our results showed that epidermal growth factor (EGF) induced both Eca-109 migration and AQP8 expression. Wound healing results showed that cell migration was increased by 1.23-1.10-fold at 24 h and 48 h after EGF treatment. AQP8 expression was significantly increased (1.19-fold) at 48 h after EGF treatment in Eca-109. The EGFR kinase inhibitor, PD153035, blocked EGF-induced AQP8 expression and cell migration. AQP8 expression was decreased from 3.65-fold (EGF-treated) to 0.55-fold (PD153035-treated) in Eca-109. Furthermore, the MEK [MAPK (mitogen-activated protein kinase)/Erk1/2]/Erk1/2 inhibitor U0126 also inhibited EGF-induced AQP8 expression and cell migration. AQP8 expression was decreased from 3.92-fold (EGF-treated) to 1.38-fold (U0126-treated) in Eca-109. In conclusions, EGF induces AQP8 expression and cell migration in Eca-109 cells via the EGFR/Erk1/2 signal transduction pathway.
Keywords: Eca-109, aquaporin 8, EGFR, Erk1/2, migration
Introduction
The cells of all living organisms are composed mainly of water, which is essential for to life. Aquaporins (AQPs) are small transmembrane proteins that are expressed in a variety of epithelial tissues where they regulate rapid water movement across epithelial barriers driven by osmotic gradients. Based on permeability, mammalian AQPs are divided into three groups: a) water-selective permeable aquaporins (AQP0, AQP1, AQP2, AQP4, AQP5, AQP6, and AQP8); b) aquaglyceroporins permeable to water, glycerol, urea, and other solutes (AQP3, AQP7, AQP9, and AQP10); c) subcellular aquaporins or super-aquaporins (AQP11 and AQP12), which have low homology with the other AQPs [1]. Tissue distribution and regulation studies have provided indirect evidence for the involvement of AQPs in a variety of physiological processes. Current theories associate AQP activity with increased osmotic pressure to form cell protrusions that are essential for migration. Malignant tumor cells have vigorous activities and active metabolism, and their demand for water is increased compared with normal cells. Furthermore, aquaporins may play a key role in tumor energy metabolism [2-4]. Recent evidence suggests that aquaporins are also involved in cell migration [5], angiogenesis [6], and tumor growth [7], and are strongly expressed in tumor cells of different origins, particularly aggressive tumors. A further, yet not sufficiently considered possibility for the pro-tumorigenic functions of AQPs is the direct or indirect regulation of other genes. For example stabilization of hypoxia-inducible factor is facilitated by AQP1 expression [8] and AQP5 interacts with the Ras/extracellular signal-regulated kinase (Erk1/2)/retinoblastoma protein signaling pathway [9]. It is important to elucidate their tumor-specific expression patterns and to reveal their potential functions besides water transport in the different organs and tumor entities.
The AQP8 gene located on chromosome 16 p12, encodes a 261 amino acid protein [10], which participates only in water metabolism. AQP8 is involved in the pathogenesis of inflammatory disease [11,12]. So far, however, the function of AQP8 function in carcinogenesis is controversial. Epidermal growth factor receptor (EGFR)-Erk1/2, which belongs to the mitogen-activated protein kinase (MAPK) family, forms part of a critically important signal transduction pathway that is closely associated with tumorigenesis and tumor progression [13].
In this study, we investigated the capacity of EGFR-Erk1/2 to induce AQP8 expression in human esophageal cancer Eca-109 cells and signaling via the epidermal growth factor receptor (EGFR)-extracellular signal-regulated kinases1/2 (Erk1/2) pathway.
Materials and methods
Cell lines and reagents
Human esophageal cancer Eca-109 cell line (purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China); TRIzol (Invitrogen, Carlsbad, California, USA); AQP8 primary rabbit polyclonal antibody (sc-28624, Santa Cruz), EGFR primary rabbit monoclonal antibody (sc-03, Santa Cruz, USA), p-EGFR (Tyr1068) (3777, Cell Signaling Technology, USA); β-actin (BA2305, Boster, China); Pierce secondary goat antibody (31430, Thermo Scientific, USA); EGFR kinase inhibitor PD153035 (S1079, Selleckchem, USA); Erk1/2 inhibitor U0126 (PHZ1283, Gibco, USA).
Cell culture
Eca-109 cells were cultured at 37°C in a humidified incubator 50 ml/L CO2 with Dulbecco’s modified Eagle medium (DMEM) supplemented with 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 100 ml/L fetal bovine serum (FBS).
In vitro wound healing (scratch) assay
The procedure was performed as described [14]. In brief, Eca-109 cells were cultured as confluent monolayers in 6-well plates, synchronized in 10 ml/L FBS for 24 h. The monolayer was wounded by removing a 300-500 μm-wide strip of cells across the well with a standard 10 μl pipette tip. Floating cells were removed by washing with PBS. Media containing 10 ml/L FBS with or without the indicated concentrations of EGF, PD153035 and U0126 were added to the wells and incubated for an additional 24 h or 48 h. Five representative images of the scratched areas were photographed under light microscopy (ZEISS, Observer, Z1, magnification: ×50) at 0 h, 24 h and 48 h. The average gap at these time-points was used to quantify the wound healing in each group.
Western blot analysis
Cultured human Eca-109 cells with or without treatment were washed with cold PBS and harvested by scraping into 500 µl RIPA buffer. Cell lysates were incubated at 4°C for 30 min. After centrifugation at 10000 ×g for 10 min at 4°C, the protein concentration was determined by BCA assay. Proteins were then denatured in 5× SDS-PAGE loading buffer for 5 min at 100°C.The proteins were separated by SDS-PAGE (100 g/L or 5 g/L gels) and transferred to PVDF membrane (IPVH00010, Millipore, USA) for 50 min at 4°C. Non-specific binding was blocked with 50 g/L dried skimmed milk. After blocking, membranes were incubated with specific antibodies against AQP8 (1:200), EGFR (1:800), phospho-EGFR (1:1000) and β-actin (1:300) in dilution buffer overnight at 4°C. The blots were incubated with secondary antibody at room temperature for 1 h. Antibody binding was detected using the ECL (enhanced chemiluminescence) detection system (PIERCE, Rockford, Illinois, USA). Relative expression value (integral optical density, IOD) = specific antibodies (IOD)/β-actin (IOD).
Immunofluorescence analysis
Following treatment, cells were fixed in cold acetone for 10 min at 4°C, washed three times with PBS (5 min/wash). Fixed cells were stained with anti-AQP8 primary antibody overnight at 4°C, followed by fluorescein isocyanate-labeled secondary antibody for 45 min at 37°C. PBS was used instead of the primary antibody in the negative control. After washing, AQP8 immunofluorescence was observed using a ZEISS fluorescence microscope.
Statistical analysis
Data are expressed as the mean ± standard deviation. Statistical differences among groups were compared using the one-ANOVA for measurement data. P < 0.05 was considered to be statistically significant.
Results
EGF induces cell migration in Eca-109 cells
Invasion of malignant tumors is correlated with cell migration; therefore, we tested the capacity of EGF to induce migration in Eca-109 cells. Eca-109 cells cultured in 6-well plates were treated with EGF at 50 ng/ml. Wound healing was monitored and digitized results showed that cell migration was increased by 1.23-1.10-fold at 24 h and 48 h after EGF treatment (Figure 1A-C, P < 0.05) when compared with untreated cells. These results clearly demonstrate that EGF induces human Eca-109 migration.
Figure 1.
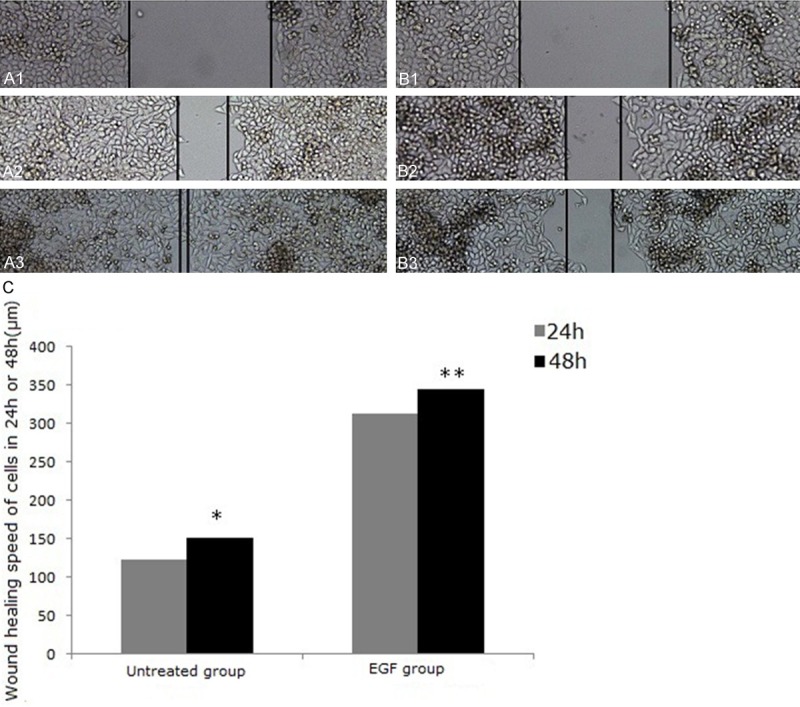
EGF induces cell migration and wound healing in human esophageal cancer Eca-109 cells. Human Eca-109 cells were treated with (A) or without (B) EGF (50 ng/ml) for 0 h (A1, B1), 24 h (A2, B2) and 48 h (A3, B3) and cell migration was detected in wound healing assays. The average wound gap is shown in (C). *P < 0.05 vs. untreated group (24 h), **P < 0.05 vs. untreated group (48 h). Magnification (A and B: ×50).
EGF induces AQP8 expression in Eca-109 cells
Recent studies have indicated upregulated AQP8 expression and increased apoptotic responsiveness in carcinoma [15,16], and thus decreased cell migration. We next investigated whether AQP8 is expressed in human Eca-109 and whether EGF induces AQP8 expression. Cells treated with EGF (50 ng/ml) were collected at 0 h, 24 h and 48 h and analyzed for AQP8 by western blotting and immunofluorescence. As shown in Figure 2, AQP8 was expressed in human Eca-109 cells and EGF induced AQP8 expression in a time-dependent manner. AQP8 expression was significantly increased (1.19-fold) at 48 h in Eca-109 (Figure 2A, 2B, P < 0.05). This effect was confirmed by immunofluorescence analysis (Figure 2C-E).
Figure 2.
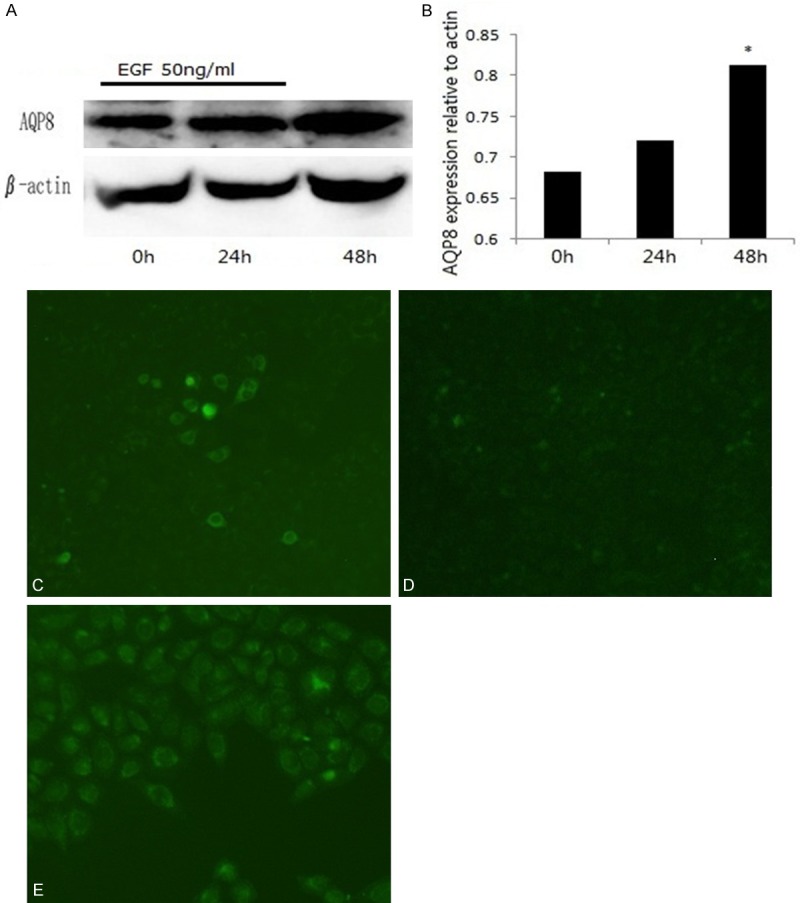
EGF induces AQP8 expression in human esophageal cancer Eca-109 cells. Human Eca-109 cells were treated with EGF (50 ng/ml) and harvested at 0 h, 24 h and 48 h). AQP8 expression was analyzed by Western blot (A) and quantified by AQP8 expression/β-actin expression (B). AQP8 expression was also detected by immunofluoresence at 0 h (C), 24 h (D) and 48 h (E) after EGF treatment. *P < 0.05 vs. 0 h. Magnification (C-E: ×100).
EGFR mediates EGF-induced AQP8 expression and cell migration in Eca-109 cells
We used an EGFR kinase inhibitor, PD153035 (5 μM), to further investigate the role of EGFR in EGF-induced AQP8 expression and cell migration. Western blot data showed that pretreatment with PD153035 inhibited EGF-induced AQP8 expression. AQP8 expression was decreased from 3.65-fold (EGF-treated) to 0.55-fold (PD153035-treated) in Eca-109 (Figure 3A, 3B). This effect was confirmed by immunofluorescence analysis (Figure 3C-F). Moreover, EGF-induced cell migration was inhibited also by PD153035 (Figure 3G). EGF induced EGFR phosphorylation in a time-dependent manner, reaching a peak at 15 min post-EGF treatment and remaining elevated for 1 h (Figure 3H, 3I).
Figure 3.
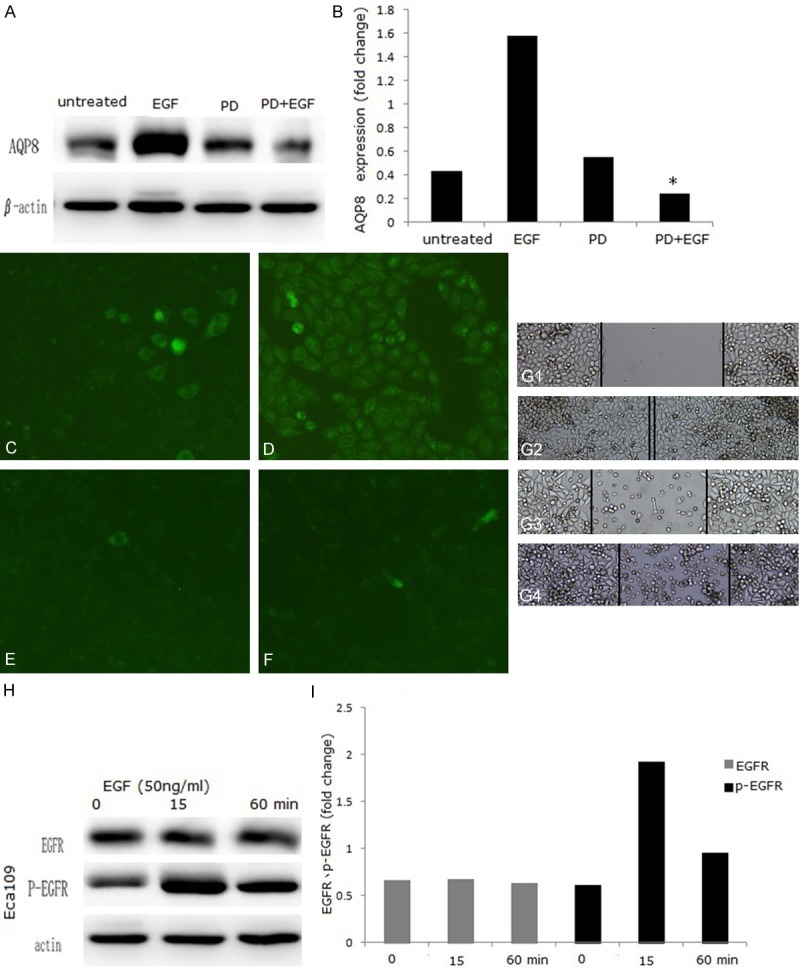
EGF-induced AQP8 expression and cell migration are blocked by PD153035. Eca-109 cells were treated with or without PD153035 for 48 h. AQP8 expression in Eca-109 cells was analyzed by Western blot (A) and quantified (B). Immunofluorescence was used to detect AQP8 expression: (C) untreated group; (D) EGF group; (E) PD153035 group; (F) EGF+PD153035 group. Cell migration was detected by wound healing assays: (G1) untreated group; (G2) EGF group; (G3) PD153035 group; (G4) EGF+PD153035 group. For EGFR phosphorylation analysis, cells were treated with EGF (50 ng/ml) and harvested at 0 min, 15 min and 60 min. p-EGFR and EGFR expression was analyzed by Western blot (H) and quantified in (I). *P < 0.05 vs. untreated group. Magnification: (C-F: ×100; G: ×50).
Erk1/2 mediates EGF-induced AQP8 expression and cell migration in Eca-109 cells
Abundant studies have shown that activation of EGFR results in phosphorylation and activation of various effector proteins [17], including MAPK (mitogen-activated protein kinase) [18,19]. The data presented here indicated that activation of EGFR is required for EGF-induced AQP8 expression and cell migration. The MEK (MAPK/Erk)/Erk inhibitor U0126 (30 nM) was used to further clarify the cell signaling pathway leading to EGF-induced AQP8 expression and cell migration. Western blot data showed that pretreatment with U0126 partially inhibited EGF-induced AQP8 expression. AQP8 expression was decreased from 3.92-fold (EGF-treated) to 1.38-fold (U0126-treated) in Eca-109 (Figure 4A, 4B). Immunofluorescence analysis confirmed that the Erk1/2 inhibitors inhibited EGF-induced AQP8 expression (Figure 4C). Moreover, cell migration induced by EGF was also inhibited by U0126 (Figure 4D).
Figure 4.
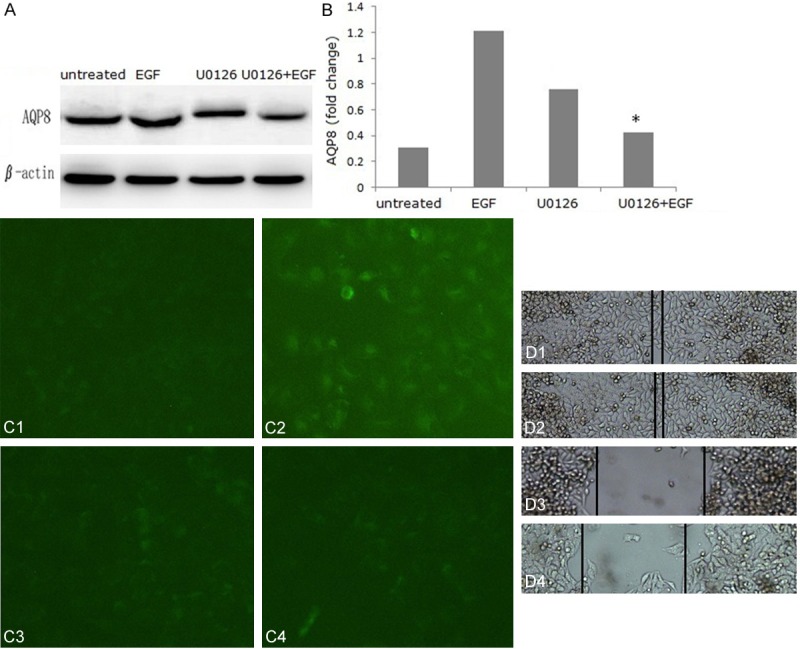
EGF-induced AQP8 expression and cells migration are blocked by U0126. Eca-109 cells were treated with or without U0126 for 48 h. AQP8 expression in Eca-109 cells was analyzed by Western blot (A) and quantified (B). Immunofluorescence was used to detect AQP8 expression: (C1) untreated group; (C2) EGF group; (C3) U0126 group; (C4) EGF+U0126 group. Cell migration was detected in wound healing assays: (D1) untreated group; (D2) EGF group; (D3) U0126 group; (D4) EGF+U0126 group. *P < 0.05 vs. untreated group. Magnification: (C: ×100; D: ×50).
Discussion
Invasion and metastasis of malignant tumors depends on cell migration, which is a dynamic, well-organized and complex process. Numerous studies have demonstrated that EGF activates its receptor, resulting in increased cell migration in human tumor cells [20-22]; thus EGF, via its receptor, plays an important role in cell migration and tumor invasion. Our study clearly showed that EGF induces migration of human esophageal cancer Eca-109 cells using an in vitro wound healing assay (Figure 1).
Cell migration is a multistep process involving numerous growth factors, cytokines and adhesion molecules as well as extracellular-matrix proteins. Recent studies have implicated another important process, namely, AQP-dependent cell migration, which involves AQP-facilitated water influx into the dynamic cellular protrusions (lamellapodia) at the leading edge of migrating cells [14,23]. To date, 13 mammalian homologs (AQP 0-12) have been identified [2]. Previous studies have shown that AQP8 is distributed predominantly in gastrointestinal epithelial cells (especially the colon), liver, pancreas, and the male and female reproductive systems. However, reports of the role of AQP8 in carcinogenesis are controversial. Some studies demonstrated that AQP8 contributes to tumor cell migration and proliferation [23,24], while others indicated that AQP8 induces tumor cell apoptosis and downregulated expression in carcinoma [16,25]. Here we provide evidence that AQP8 is expressed in human esophageal cancer Eca-109 cells and that EGF, which induced migration, also induced AQP8 expression in this cell line (Figure 2). Our findings support the hypothesis that AQP8 makes a positive contribution to carcinogenesis. This is consistent with reports of the roles of other AQP subtypes in tumorigenesis, such as AQP1 and AQP3. AQP1 increases the migration speed of B16F10 melanoma cells and 4T1 mammary gland tumor cells, while AQP3 is involved in FGF-2-induced migration of human breast cancer cells [14,26]. Our results suggest that upregulation of AQP8 in response to EGF accelerates Eca-109 cell migration by enhancing plasma-membrane water permeability, which in turn boosts transmembrane water fluxes that occur during cell migration.
The discovery of the AQP family has provided insights into the molecular mechanisms of membrane water permeability and tumor cell migration. However, the mechanisms by which EGF upregulates AQP8 expression in Eca-109 cells remain poorly understood. In this study, we have provided the evidence that EGFR and Erk1/2 mediate EGF-induced AQP8 expression and Eca-109 migration. Our study demonstrated that EGF induced transient EGFR phosphorylation, and that the EGFR kinase inhibitor, PD153035, inhibited cell migration induced by EGF in Eca-109 cells. EGFR is known to be an essential regulator in tumorigenesis, including in tumor cell migration [27-29]. Here we demonstrated that both EGFR and its kinase activity were required for EGF-induced AQP8 expression and cell migration in human esophageal cancer Eca-109 cells (Figure 3).
Erk1/2 is a member of EGFR signaling pathway. It has been estimated that Erk1/2 targets more than 180 different molecules that are responsible for cell growth, survival, and differentiation; thus, aberrant regulation greatly affects cell growth. Erk1/2 kinase couples the signals from cell surface receptors to molecules that transmit cell proliferative signals. Following a cascade reaction, Erk1 phosphorylates Erk1/2, leading to the upregulation of various transcription factors such as Ets-1, c-Jun, c-Myc, and HIF1α [30]. Our study also showed that the Erk1/2 inhibitor U0126 inhibited cell migration induced by EGF in human Eca-109 cells and that the Erk1/2 inhibitor U0126 effectively restrained EGF-induced AQP8 expression (Figure 4A-D) in human Eca-109 cells. These results clearly demonstrate that the EGFR/Erk1/2 signaling pathway is involved in EGF-induced AQP8 expression and cell migration in human Eca-109 cells. We contend that Erk1/2 is a critical signaling molecule in the selective regulation of AQP8 expression and/or the function in human Eca-109 cells.
In conclusion, our findings demonstrate for the first time that AQP8 is expressed in human esophageal cancer Eca-109 cells, and that EGF induces AQP8 expression and cell migration via the EGFR/Erk1/2 signal transduction pathway in these cells. Our findings also provide an explanation for the positive function of AQP8 in carcinogenesis. It is envisioned that development of agents that induced AQP8 opening or overexpression may be beneficial in the prevention of human malignant tumor cell invasion.
Acknowledgements
This study was supported by the Natural Science Foundation of Xinjiang Uyghur Autonomous Region (Grant Number: 2012211A041) and the Key Laboratory Open Issue of Xinjiang Uyghur Autonomous Region (Grant Number: XJDX0208-2011-05).
Disclosure of conflict of interest
None.
References
- 1.Murai-Hatano M, Kuwagata T, Sakurai J, Nonami H, Ahamed A, Nagasuga K, Matsunami T, Fukushi K, Maeshima M, Okada M. Effect of low root temperature on hydraulic conductivity of rice plants and the possible role of aquaporins. Plant Cell Physiol. 2008;49:1294–305. doi: 10.1093/pcp/pcn104. [DOI] [PubMed] [Google Scholar]
- 2.Verkman AS, Hara-Chikuma M, Papadopoulos MC. Aquaporins-new players in cancer biology. J Mol Med. 2008;86:523–9. doi: 10.1007/s00109-008-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hara-Chikuma M, Verkman AS. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol. 2008;28:326–32. doi: 10.1128/MCB.01482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekine S, Shimada Y, Nagata T, Sawada S, Yoshioka I, Matsui K, Moriyama M, Omura T, Osawa S, Shibuya K, Hashimoto I, Watanabe T, Hojo S, Hori R, Okumura T, Yoshida T, Tsukada K. Role of aquaporin-5 in gallbladder carcinoma. Eur Surg Res. 2013;51:108–17. doi: 10.1159/000355675. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2008;456:693–700. doi: 10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou LB, Shi S, Zhan g RJ, Wang TT, Tan YJ, Zhang D, Fei XY, Ding GL, Gao Q, Chen C, Hu XL, Huang HF, Sheng JZ. Aquaporin-1 plays a crucial role in estrogen-induced tubulogenesis of vascular endothelial cells. J Clin Endocrinol Metab. 2013;98:E672–82. doi: 10.1210/jc.2012-4081. [DOI] [PubMed] [Google Scholar]
- 7.Nico B, Ribatti D. Role of aquaporins in cell migration and edema formation in human brain tumors. Exp Cell Res. 2011;317:2391–6. doi: 10.1016/j.yexcr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Echevarria M, Munoz-Cabello AM, Sanchez-Silva R, Toledo-Aral JJ, Lopez-Barneo J. Development of cytosolic hypoxia and hypoxia -inducible factor stabilization are facilitated by aquaporin-1 expression. J Biol Chem. 2007;282:30207–15. doi: 10.1074/jbc.M702639200. [DOI] [PubMed] [Google Scholar]
- 9.Kang SK, Chae YK, Woo J, Kim MS, Park JC, Lee J, Soria JC, Jang SJ, Sidransky D, Moon C. Role of human aquaporin 5 in colorectal carcinogenesis. Am J Pathol. 2008;173:518–25. doi: 10.2353/ajpath.2008.071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viggiano L, Rocchi M, Svelto M, Calamita G. Assignment of the aquaporin-8 water channel gene (AQP8) to human chromosome 16p12. Cytogenet Cell Genet. 1999;84:208–10. doi: 10.1159/000015260. [DOI] [PubMed] [Google Scholar]
- 11.Zhao G, Li J, Wang J, Shen X, Sun J. Aquaporin 3 and 8 are down-regulated in TNBS-induced rat colitis. Biochem Biophys Res Commun. 2014;443:161–6. doi: 10.1016/j.bbrc.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 12.Wang JQ, Zhang L, Tao XG, Wei L, Liu B, Huang LL, Chen YG. Tetramethylpyrazine upregulates the aquaporin 8 expression of hepatocellular mitochondria in septic rats. J Surg Res. 2013;185:286–93. doi: 10.1016/j.jss.2013.05.106. [DOI] [PubMed] [Google Scholar]
- 13.Tasioudi KE, Saetta AA, Sakellariou S, Levidou G, Michalopoulos NV, Theodorou D, Patsouris E, Korkolopoulou P. pERK activation in esophageal carcinomas: clinicopathological associations. Pathol Res Pract. 2012;208:398–404. doi: 10.1016/j.prp.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006;20:1892–4. doi: 10.1096/fj.06-5930fje. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Li Q, Yang T, Bai G, Li D, Li Q, Sun H. Expression of AQP5 and AQP8 in human colorectal carcinoma and their clinical significance. World J Surg Oncol. 2012;10:242. doi: 10.1186/1477-7819-10-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jablonski EM, Mattocks MA, Sokolov E, Koniaris LG, Hughes FM Jr, Fausto N, Pierce RH, McKillop IH. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007;250:36–46. doi: 10.1016/j.canlet.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang CY, Chao TT, Tai WT, Chang FY, Su WP, Chen YL, Chen PT, Weng CY, Yuan A, Shiau CW, Yu CJ, Chen KF. Signal transducer and activator of transcription 3 as molecular therapy for non-small-cell lung cancer. J Thorac Oncol. 2014;9:488–96. doi: 10.1097/JTO.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 18.Qu Y, Chen Q, Lai X, Zhu C, Chen C, Zhao X, Deng R, Xu M, Yuan H, Wang Y, Yu J, Huang J. SUMOylation of Grb2 enhances the ERK activity by increasing its binding with Sos1. Mol Cancer. 2014;1:95. doi: 10.1186/1476-4598-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan FT, Shen CS, Tao L, Tian C, Liu ZG, Zhu ZJ, Liu YP, Pei CS, Wu HY, Zhang L, Wang AY, Zheng SZ, Huang SL, Lu Y. PKM2 Regulates Hepatocellular Carcinoma Cell Epithelial-mesenchymal Transition and Migration upon EGFR Activation. Asian Pac J Cancer Prev. 2014;15:1961–70. doi: 10.7314/apjcp.2014.15.5.1961. [DOI] [PubMed] [Google Scholar]
- 20.Jeong YJ, Choi Y, Shin JM, Cho HJ, Kang JH, Park KK, Choe JY, Bae YS, Han SM, Kim CH, Chang HW, Chang YC. Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem Toxicol. 2014;68:218–25. doi: 10.1016/j.fct.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Cho KH, Choi MJ, Jeong KJ, Kim JJ, Hwang MH, Shin SC, Park CG, Lee HY. A ROS/STAT3/HIF-1α signaling cascade mediates EGF-induced TWIST1 expression and prostate cancer cell invasion. Prostate. 2014;74:528–36. doi: 10.1002/pros.22776. [DOI] [PubMed] [Google Scholar]
- 22.Magi S, Takemoto Y, Kobayashi H, Kasamatsu M, Akita T, Tanaka A, Takano K, Tashiro E, Igarashi Y, Imoto M. 5-Lipoxygenase and cysteinyl leukotriene receptor 1 regulate epidermal growth factor-induced cell migration through Tiam1 upregulation and Rac1 activation. Cancer Sci. 2014;105:290–96. doi: 10.1111/cas.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi YH, Rehemu N, Ma H, Tuokan T, Chen R, Suzuke L. Increased migration and local invasion potential of SiHa cervical cancer cells expressing Aquaporin 8. Asian Pac J Cancer Prev. 2013;14:1825–1828. doi: 10.7314/apjcp.2013.14.3.1825. [DOI] [PubMed] [Google Scholar]
- 24.Zhu SJ, Wang KJ, Gan SW, Xu J, Xu SY, Sun SQ. Expression of aquaporin8 in human astrocytomas: correlation with pathologic grade. Biochem Biophys Res Commun. 2013;440:168–72. doi: 10.1016/j.bbrc.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 25.Fischer H, Stenling R, Rubio C, Lindblom A. Differential expression of aquaporin 8 in human colonic epithelial cells and colorectal tumors. BMC Physiol. 2001;1:1. doi: 10.1186/1472-6793-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao XC, Zhang WR, Cao WF, Liu BW, Zhang F, Zhao HM, Meng R, Zhang L, Niu RF, Hao XS, Zhang B. Aquaporin3 is required for FGF-2-induced migration of human breast cancers. PLoS One. 2013;8:e56735. doi: 10.1371/journal.pone.0056735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gándola YB, Pérez SE, Irene PE, Sotelo AI, Miquet JG, Corradi GR, Carlucci AM, Gonzalez L. Mitogenic effects of phosphatidylcholine nanoparticles on mcf-7 breast cancer cells. Biomed Res Int. 2014;2014:687037. doi: 10.1155/2014/687037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Melo Maia B, Fontes AM, Lavorato-Rocha AM, Rodrigues IS, de Brot L, Baiocchi G, Stiepcich MM, Soares FA, Rocha RM. EGFR expression in vulvar cancer: clinical implications and tumor heterogeneity. Hum Pathol. 2014;45:917–25. doi: 10.1016/j.humpath.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Moerkens M, Zhang Y, Wester L, van de Water B, Meerman JH. Epidermal growth factor receptor signalling in human breast cancer cells operates parallel to estrogen receptor alpha signalling and results in tamoxifen insensitive proliferation. BMC Cancer. 2014;14:283. doi: 10.1186/1471-2407-14-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chetram MA, Hinton CV. PTEN regulation of Erk1/2 signaling in cancer. J Recept Signal Transduct Res. 2012;32:190–95. doi: 10.3109/10799893.2012.695798. [DOI] [PMC free article] [PubMed] [Google Scholar]


