Abstract
Dendritic cells (DCs) and natural killer (NK) cells initiate specific immune responses against tumor cells. The aim of the present study was to determine the cytotoxicity and the subsets of the DC and NK cells and the cytokines level of DC and NK cells from cancer tissue and peripheral blood in the gastric cancer patients. Cytotoxicity of DC and NK was determined using the Cytotox non-radioactive assay. The cytotoxic activity of DC or NK isolated from cancer tissue and peripheral blood was attenuated in gastric cancer patients. CD11c, CD80, CD83, CD16, CD57 and CD69 were decreased in the cancer tissue and peripheral blood in the gastric cancer patients. CD86, CCR7 and CD59 were no significance in the cancer tissue and peripheral blood from gastric cancer patients. Tumor necrosis factor (TNF)-α, interleukin (IL)-2, T-bet and IL-15Rβ levels were decreased in DC and NK from the gastric cancer tissue and peripheral blood in the gastric cancer patients. IL-15 and IL-15Rα level were no significance in DC and NK in the gastric cancer tissue and peripheral blood in the gastric cancer patients. These results indicate that the cytotoxic activity and subsets and cytokines of DC and NK cells in the cancer tissue and peripheral blood in the gastric cancer patients were decreased. The decrease of subsets content and cytokines of DC and NK may contribute to a decrease in the function of DC and NK in the tissue and peripheral blood in the gastric cancer patients.
Keywords: Dendritic cell, natural killer cell, cytotoxicity, cytokine
Introduction
Dendritic cells (DCs) are recognized as being specialized appropriate T-antigen presenting cells which play a key role in generating primary and secondary immune responses against specific antigens [1]. Two peripheral blood DC subsets have been described: myeloid-derived CD11c+CD123-DCs (DC1) and lymphoid-derived CD11c-CD123+ DCs (DC2) [2,3]. Natural killer (NK) cells contribute to the innate immune defense against tumors and microbial pathogens. Human NK cells make up approximately 10% to 15% of total blood lymphoid cells and perform two major functions: the first is recognizing and lysing tumor cells and virally infected cells [4,5]; the second is regulating the innate and adaptive immune responses [6,7].
It has been shown that DC vaccine transfected with gastric cancer cell total RNA carrying the 4-1BB/4-1BB ligand gene has a stronger ability to kill gastric cancer cells through promoting T cell proliferation and enhancing the ability of cytotoxic T lymphocytes to kill gastric carcinoma cells [8]. Circulating lymphocyte subsets in gastric cancer patients are significantly changed such as CD3+, CD8+ CD4+, CD19+, CD44+, CD25+, NK cells [9]. The levels of CD3+T, CD4+T, NK cell and CD4+T/CD8+T in gastric cancer patients were lower as compared to healthy volunteers [10]. CD81 mRNA levels were found to be low in primary tumors, and these low expression levels were found to correlate with the stage and grade of the tumors [11]. However, the cytotoxic activity and subsets content of DC and NK in the cancer tissue and peripheral blood in the gastric cancer patients are not very clear.
It has been demonstrated that impaired DC function contributes to the less effective innate and adaptive immune responses against H. pylori seen in gastric cancer patients and H. pylori can regulate interleukin (IL)-10 production [12]. The IL-12 and tumor necrosis factor (TNF)-alpha level was much higher in transgened DC cells than blank DC cells [13]. IL1β physiologically induced by helicobacter pylori infection enhanced gastric carcinogenesis by affecting both inflammatory and epithelial cells [14]. Mammalian IL-15 plays an important role in the activation of DC and NK cells along with its receptors named ILR [15]. However, the levels of inflammatory cytokines in DC and NK in the cancer tissue and peripheral blood in the gastric cancer patients are not very clear. The present study was designed to determine the cytotoxic activity and the subsets content of DC and NK and the levels of inflammatory cytokines in DC or NK isolated from cancer tissue and peripheral blood in the gastric cancer patients.
Materials and methods
Tissue and blood specimens
Forty-eight gastric cancer and corresponding normal gastric tissue samples (more than 10 cm away from the edge of the gastric cancer) were taken from gastric cancer patients (the Third Affiliated Hospital of Suzhou University and the Drum Tower Hospital). Blood samples were collected from cancer patients and control into a tube containing EDTA-K2. No patients had received chemotherapy or radiotherapy before surgery.
Measurement of cytokine production
DC and NK cells isolated from cancer tissue and blood were cultured for 5 days and the culture media were then collected. The total protein was extracted and measured using a protein assay kit (BCA; Pierce, Santa Cruz, CA, USA). The levels of cytokines release were determined by enzyme-linked immunoassay (ELISA) kit (R & D Systems; Oxfordshire, UK) according to the manufacturer’s instructions. Briefly, a 96-well microplate was coated with an antibody specific for TNF-α, T-bet, IL-2 or IL-15. We added 100 μl of sample and 100 μl of standard diluent buffer to each well in duplicate, incubated it for 90 min at 37°C, and then washed it five times. Subsequently, 100 μl of biotinylated anti-TNF-α, IL-2, IL-15, T-bet or antibody solution were added, incubated for 60 min at 37°C, and then washed. 100 μl of streptavidin-horseradish peroxidase conjugate solution were added, incubated for 30 min at 37°C, and washed. 100 μl of chromagen solution were added and incubated in the dark for 15 min at 37°C. The reactions were stopped with HCl and read at 450 nm using an ELISA plate reader. Standardization curves were made with known concentrations of TNF-α, IL-2, IL-15, T-bet.
Western blotting
NK cells and DC from cancer and control were cultured for 5 days and the culture media were then collected and lysed in modified RIPA or lysed directly in 1 × SDS loading buffer. After process of electrophoresis and trarsmembrane, proteins on nitrocellulose membrane were probed with the IL-15Rα or IL-15Rβ primary antibody (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by incubation with the secondary antibodies (1:5000; Immunology Consultants Lab, USA). The bands were visualized by enhanced chemiluminescence using ECL (Pierce Chemical) and captured on X-ray films. GAPDH (Bioworld Technology Inc., USA) protein was used as a loading control. The total IL-15Rα or IL-15Rβ protein level were normalized to the GAPDH protein level.
Flow cytometry
Cellular phenotyping was performed on a FACS CantoII flow cytometer (Becton Dickinson, San Jose, CA, USA) as described with minor modifications. The following fluorochrome labelled monoclonal antibodies conjugated to FITC, PE, PETxR, PeCy7, PerCPCy5.5, APC, APC-Cy7, Pacific Blue and appropriate isotype controls were used for surface staining according to the manufacturer’s instructions: CD11c, CD16, CD56, CD57, CD59, CD69, CD83, CD80, CD86, CCR7 (all mabs from Biolegend, Germany), Siglec-F (BD Biosciences, Germany), Langerin (CD207; 929F301, Imgenex, SanDiego, USA) and 120g8 (Dendritics, France). Absolute leukocyte numbers were determined by using trucount beads (Becton Dickinson, Germany) according to the manufacturer instructions.
Cytotoxicity of DC and NK
Cytotoxicity of DC and NK towards TRAMP-C1 target cells was determined using the Cytotox 96 non-radioactive assay. Briefly, after 5 days of culture, NK and DC cells were plated in 96-well plates. After 24 h, TRAMP-C1 target cells were added to the wells. After 24 h, plates were spun down and supernatant was collected for analysis of cytotoxicity using the Cytotox 96 non-radioactive assay (Promega) which determines release of lactate dehydrogenase.
Statistical analysis
Data were analyzed by using SPSS 18.0 (IBM, Armonk, NY, USA). Comparisons between 2 observations were assessed by Student’s paired t-test. One-way or two-way ANOVA was used followed by the Bonferroni test for post hoc analysis when multiple comparisons were made. All of the data were expressed as the mean ± SE. A value of P < 0.05 was considered statistically significant.
Results
Cytotoxic activity of DC and NK
The cytotoxic activity of DC and NK isolated from cancer tissue was attenuated in gastric cancer patients compared with controls. The cytoxicity of DC and NK isolated from peripheral blood was also attenuated in cancer patients (Figure 1).
Figure 1.
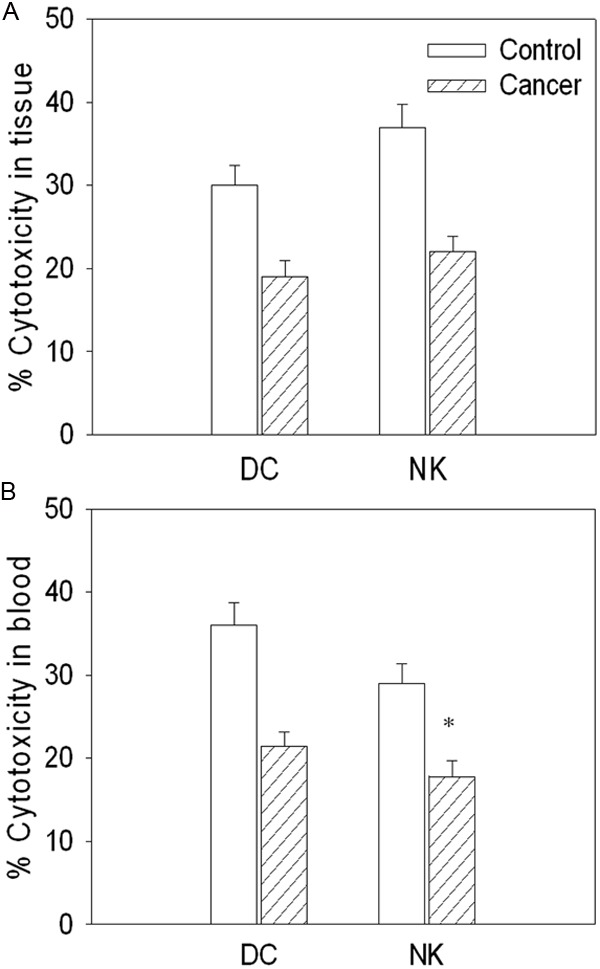
Cytotoxicity of dendritic cells (DCs) and natural killer (NK) cells isolated from cancer tissue and peripheral blood in gastric cancer patients. A. The cytotoxicity of DC and NK isolated from cancer tissue; B. The cytotoxicity of DC and NK isolated from peripheral blood. Values are mean ± SE. *P < 0.05 versus Control. N = 8 for each group.
DC subsets in tissue and blood
CD11c content was decreased in the cancer tissue and peripheral blood in the gastric cancer patients compared with controls. CD80 and CD83 were also decreased in the cancer tissue and peripheral blood in the gastric cancer patients compared with controls. In the cancer tissue and peripheral blood, CD86 and CCR7 were no significance in the gastric cancer patients compared with controls (Figure 2).
Figure 2.
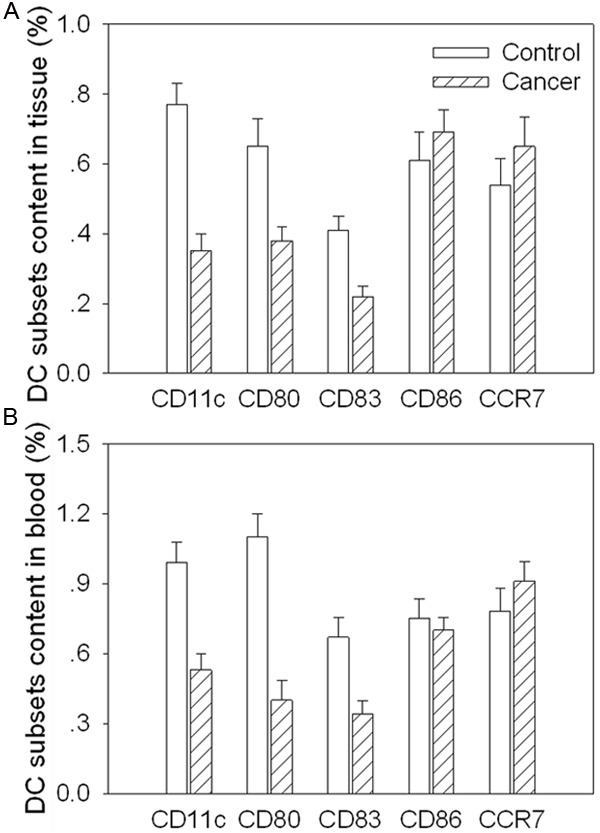
Subsets content of dendritic cells (DCs) isolated from cancer tissue and peripheral blood in gastric cancer patients. A. The subsets content of DC isolated from cancer tissue; B. The subsets content of DC isolated from peripheral blood. Values are mean ± SE. *P < 0.05 versus Control. N = 8 for each group.
NK subsets in tissue and blood
CD16 was decreased in the cancer tissue and peripheral blood in the gastric cancer patients compared with controls. CD56, CD57 and CD69 were also decreased in the cancer tissue and peripheral blood in the gastric cancer patients. However, CD59 was no significance in the cancer tissue and peripheral blood in the gastric cancer patients compared with controls (Figure 3).
Figure 3.
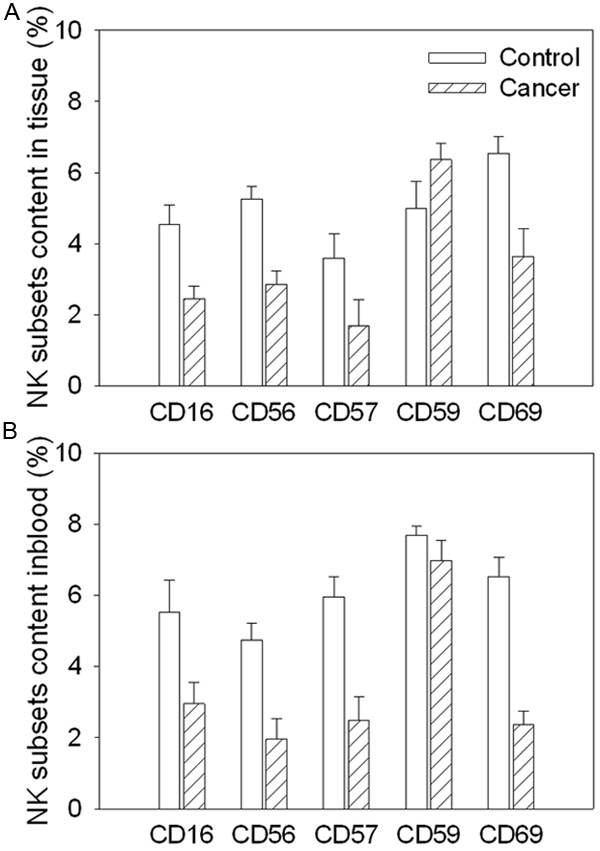
Subsets content of natural killer (NK) cells isolated from cancer tissue and peripheral blood in gastric cancer patients. A. The subsets content of NK isolated from cancer tissue; B. The subsets content of NK isolated from peripheral blood. Values are mean ± SE. *P < 0.05 versus Control. N = 8 for each group.
Cytokines production in DC and NK in the tissue
TNF-α level was decreased in DC and NK in the gastric cancer tissue compared with controls. IL-2 and T-bet levels were also decreased in DC and NK isolated from gastric cancer tissue. IL-15 and IL-15Rα level were no significance in DC and NK in the gastric cancer tissue compared with controls. However, IL-15Rβ protein level was decreased in the DC and NK isolated from cancer tissue in the gastric cancer patients (Figure 4).
Figure 4.
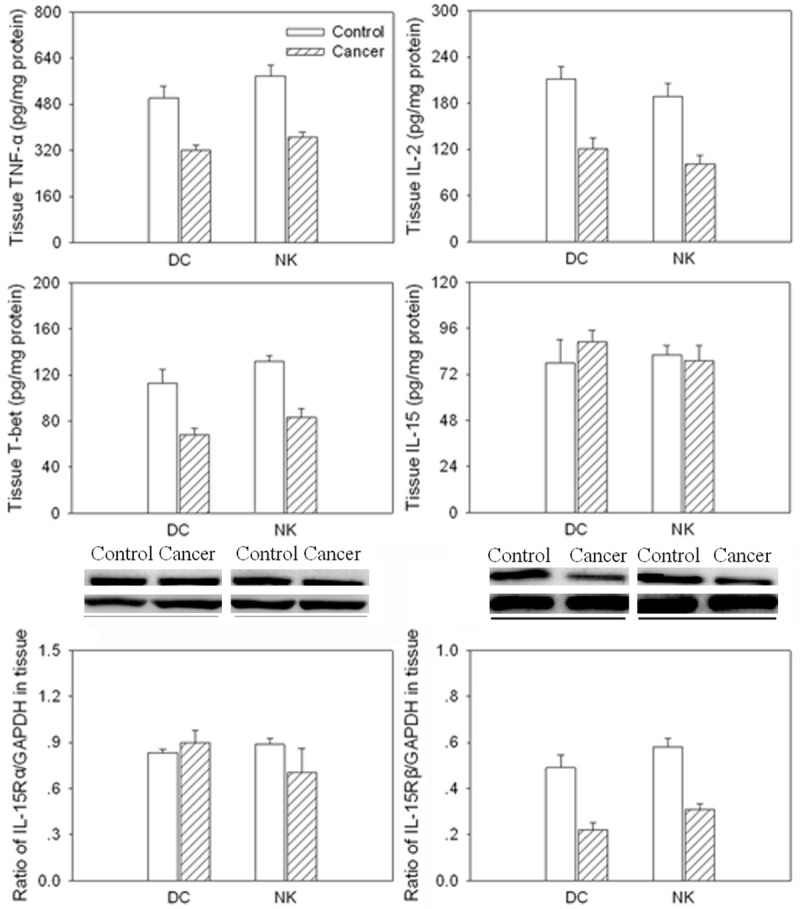
Levels of tumor necrosis factor (TNF)-α, interleukin (IL)-2, T-bet, IL-15, IL-15Rα and IL-15Rβ in dendritic cells (DCs) and natural killer (NK) cells isolated from cancer tissue in gastric cancer patients. Values are mean ± SE. *P < 0.05 versus Control. N = 8 for each group.
Cytokines production in DC and NK in blood
TNF-α level was decreased in DC and NK in the peripheral blood from gastric cancer patients compared with controls. IL-2 and T-bet levels were also decreased in DC and NK isolated from blood in the gastric cancer patients. IL-15 and IL-15Rα was no significance in DC and NK in blood from the gastric cancer patients compared with controls. However, IL-15Rβ protein level was decreased in the DC and NK isolated from blood in the gastric cancer patients (Figure 5).
Figure 5.
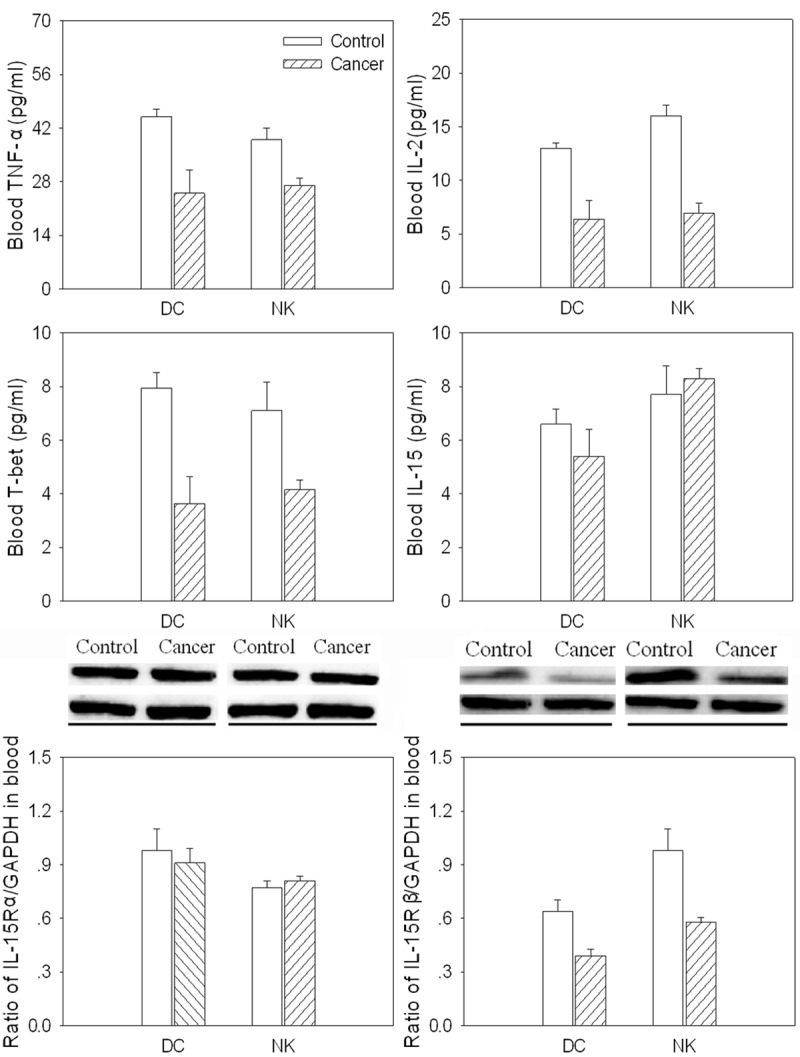
Levels of tumor necrosis factor (TNF)-α, interleukin (IL)-2, T-bet, IL-15, IL-15Rα and IL-15Rβ in dendritic cells (DCs) and natural killer (NK) cells isolated from peripheral blood in gastric cancer patients. Values are mean ± SE. *P < 0.05 versus Control. N = 8 for each group.
Discussion
Gastric cancer is the second most common cancer worldwide and the second most common cause of cancer-related deaths [16] and has a strong link with chronic inflammation [17,18]. Immune dysfunction may contribute to the tumor progression in the gastric cancer [19]. Impaired function of immune cells such as CD8(+) T cells, NK cells, and DCs results in tumor progression in cancer patients. The present study demonstrates new findings that the cytotoxic activity of DC or NK isolated from cancer tissue and peripheral blood was decreased in gastric cancer patients. The subsets content and inflammatory cytokines of DC and NK cells in the cancer tissue and peripheral blood in the gastric cancer patients were decreased. The decrease of subsets content and cytokines of DC and NK may contribute to a decrease in the function of DC and NK in the tissue and peripheral blood in the gastric cancer patients.
DCs are potent professional antigen-presenting cells with the ability to prime naïve T cells, and play an important role in the initiation and regulation of immune responses. The NK cells play an important role in immune surveillance during tumorigenesis [20,21]. Gastric patients with low levels of circulating mature DCs had significantly lower values of T lymphocytes, T helper lymphocytes and NK cells than those with normal mature DC levels [22]. In the present study, we show that the cytotoxic activity of DC or NK isolated from cancer tissue and peripheral blood was attenuated in gastric cancer patients. The subsets of DC such as CD11c, CD80 and CD83 were decreased in the cancer tissue and peripheral blood in the gastric cancer patients compared with controls, CD86 and CCR7 were no significance in the gastric cancer patients compared with controls. The subsets of NK cell such as CD16, CD56, CD57 and CD69 were decreased in the cancer tissue and peripheral blood in the gastric cancer patients, CD59 was no significance in the tissue and peripheral blood in the gastric cancer patients compared with controls. These results show that the function of DC and NK cell was attenuated. The decrease of subsets content of DC and NK contribute to a decrease in the cytotoxic activity of DC and NK in the tissue and peripheral blood in the gastric cancer patients.
It has been shown that NK cells from peripheral blood of gastric cancer patients have a severely suppressed ability to produce IFN-γ after stimulation with H. pylori lysate [23]. T-bet, as a key marker for type 1 immune responses, was significant increases in T-bet(+) lymphocytes in tumor tissues as compared with normal stomach tissues, gastritis tissues or gastric polyp specimens and mainly consisted of CD4(+), CD8(+) and CD56(+) tumor-infiltrating lymphocytes [24]. In the present study, tumor necrosis factor (TNF)-α, interleukin (IL)-2, T-bet and IL-15Rβ levels were decreased in DC and NK in the gastric cancer tissue and peripheral blood in the gastric cancer patients. IL-15 and IL-15Rα level were no significance in DC and NK in the gastric cancer tissue and peripheral blood in the gastric cancer patients. The decrease of cytokine levels of DC and NK contribute to a decrease in the function of DC and NK in the tissue and peripheral blood in the gastric cancer patients.
In conclusion, the cytotoxic activity of DC and NK cell from cancer tissue and peripheral blood in the gastric cancer patients was attenuated. The decrease of subsets content and inflammatory cytokine levels of DC and NK may contribute to a decrease in the function of DC and NK in the tissue and peripheral blood in the gastric cancer patients.
Acknowledgements
This research project was supported by the National Natural Science Foundation of China (NSFC) (81171653).
Disclosure of conflict of interest
None.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Natural. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari S, Malugani F, Rovati B, Porta C, Riccardi A, Danova M. Flow cytometric analysis of circulating dendritic cell subsets and intracellular cytokine production in advanced breast cancer patients. Oncol Rep. 2005;14:113–120. [PubMed] [Google Scholar]
- 3.Hashizume H, Horibe T, Yagi H, Seo N, Takigawa M. Compartmental imbalance and aberrant immune function of blood CD123+ (plasmacytoid) and CD11c+ (myeloid) dendritic cells in atopic dermatitis. J Immunol. 2005;174:2396–2403. doi: 10.4049/jimmunol.174.4.2396. [DOI] [PubMed] [Google Scholar]
- 4.French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15:45–51. doi: 10.1016/s095279150200002x. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 6.Lee YC, Lin SJ. Neonatal Natural Killer Cell Function: Relevance to Antiviral Immune Defense. Clin Dev Immunol. 2013;2013:427–696. doi: 10.1155/2013/427696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol. 2013;34:573–82. doi: 10.1016/j.it.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Z, Guo C, Li Y, Tan B, Fan L, Xiao J. Enhanced antitumor effects of a dendritic cell vaccine transfected with gastric cancer cell total RNA carrying the 4-1BBL gene in vitro. Exp Ther Med. 2012;3:319–323. doi: 10.3892/etm.2011.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu QM, Yu CD, Ling ZQ. Elevated circulating CD19+ lymphocytes predict survival advantage in patients with gastric cancer. Asian Pac J Cancer Prev. 2012;13:2219–2224. doi: 10.7314/apjcp.2012.13.5.2219. [DOI] [PubMed] [Google Scholar]
- 10.Liang J, Han F. [Role of NKG2D-Expressing NK Cells and sMICA in Immune Surveillance of Advanced Lung Cancer. ] . Zhongguo Fei Ai Za Zhi. 2009;12:59–63. doi: 10.3779/j.issn.1009-3419.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Yoo TH, Ryu BK, Lee MG, Chi SG. CD81 is a candidate tumor suppressor gene in human gastric cancer. Cell Oncol (Dordr) 2013;36:141–153. doi: 10.1007/s13402-012-0119-z. [DOI] [PubMed] [Google Scholar]
- 12.Chang LL, Wang SW, Wu IC, Yu FJ, Su YC, Chen YP, Wu DC, Kuo CH, Hung CH. Impaired dendritic cell maturation and IL-10 production following H. pylori stimulation in gastric cancer patients. Appl Microbiol Biotechnol. 2012;96:211–220. doi: 10.1007/s00253-012-4034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun HW, Tang C, Tang QB, Zou SQ, Qiu FZ. [Study of immunological effect of dendritic cell transfected with survivin gene on the specific anti-alimentary tract tumor] . Zhonghua Wai Ke Za Zhi. 2005;43:313–316. [PubMed] [Google Scholar]
- 14.Shigematsu Y, Niwa T, Rehnberg E, Toyoda T, Yoshida S, Mori A, Wakabayashi M, Iwakura Y, Ichinose M, Kim YJ, Ushijima T. Interleukin-1beta induced by Helicobacter pylori infection enhances mouse gastric carcinogenesis. Cancer Lett. 2013;340:141–7. doi: 10.1016/j.canlet.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Bae JS, Shim SH, Hwang SD, Kim JW, Park DW, Park CI. Molecular cloning and expression analysis of interleukin (IL)-15 and IL-15 receptor alpha from rock bream, Oplegnathus fasciatus. Fish Shellfish Immunol. 2013;35:1209–15. doi: 10.1016/j.fsi.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Gomceli I, Demiriz B, Tez M. Gastric carcinogenesis. World J Gastroenterol. 2012;18:5164–5170. doi: 10.3748/wjg.v18.i37.5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishigami S, Arigami T, Uchikado Y, Setoyama T, Kita Y, Sasaki K, Okumura H, Kurahara H, Kijima Y, Harada A, Ueno S, Natsugoe S. IL-32 expression is an independent prognostic marker for gastric cancer. Med Oncol. 2013;30:472. doi: 10.1007/s12032-013-0472-4. [DOI] [PubMed] [Google Scholar]
- 18.Tye H, Kennedy CL, Najdovska M, McLeod L, McCormack W, Hughes N, Dev A, Sievert W, Ooi CH, Ishikawa TO, Oshima H, Bhathal PS, Parker AE, Oshima M, Tan P, Jenkins BJ. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell. 2012;22:466–478. doi: 10.1016/j.ccr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol. 2013;190:794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- 20.Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28:491–496. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lissoni P, Brivio F, Ferrante R, Vigore L, Vaghi M, Fumagalli E, Bucovec R, Malugani F, Fumagalli L. Circulating immature and mature dendritic cells in relation to lymphocyte subsets in patients with gastrointestinal tract cancer. Int J Biol Markers. 2000;15:22–25. doi: 10.1177/172460080001500104. [DOI] [PubMed] [Google Scholar]
- 23.Lindgren A, Yun CH, Sjoling A, Berggren C, Sun JB, Jonsson E, Holmgren J, Svennerholm AM, Lundin SB. Impaired IFN-gamma production after stimulation with bacterial components by natural killer cells from gastric cancer patients. Exp Cell Res. 2011;317:849–858. doi: 10.1016/j.yexcr.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Chen LJ, Zheng X, Shen YP, Zhu YB, Li Q, Chen J, Xia R, Zhou SM, Wu CP, Zhang XG, Lu BF, Jiang JT. Higher numbers of T-bet(+) intratumoral lymphoid cells correlate with better survival in gastric cancer. Cancer Immunol Immunother. 2013;62:553–561. doi: 10.1007/s00262-012-1358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


