Abstract
Lymphoepithelioma-like carcinoma (LELC) of salivary glands is a rare kind tumor. In this study, the authors evaluated 21 patients with LELC of salivary glands who had long-term follow-up. Clinical characteristics, Epstein-Barr virus (EBV) infection, immunohistochemical features, oncoprotein expression, treatments, and outcomes were analyzed. All patients were Chinese. Their ages ranged from 20 to 73 years. All tumors showed the typical syncytial growth pattern of undifferentiated epithelial cells with a significant lymphocyte reaction. All of patients were found by in situ hybridization to have the EBV genome. All tumors showed positive immunostaining of AE1/AE3, CK5/6 and p63. Nearly all cases had bcl-2 oncoprotein expression, but the detection rate of p53, and c-erb B-2 expression was extremely low. LELC of salivary glands is a distinct entity of salivary cancer. LELC of salivary glands can receive multimodality treatment and has a better prognosis similar to that of nasopharyngeal carcinoma.
Keywords: Lymphoepithial carcinoma, salivary gland, Epstein-Barr virus, oncogenes, bcl-2, prognosis
Introduction
Primary lymphoepithelioma-like carcinoma (LELC) of salivary glands is a rare kind of tumor with morphologic features similar to undifferentiated nasopharyngeal carcinoma (NPC) and most of the cases have been reported in South Chinese and Eskimos [1,2]. It involves mainly the parotid gland. Begin et al. first reported primary LELC in 1987 and observed that it is an Epstein-Barr virus (EBV)-associated carcinoma [3]. The following reports found that LELC had occurred in various organs such as thymus [4], skin [5], stomach [6], urinary bladder [7], lung [8], uterine cervix [9], endometrium [10], and so on. Epstein-Barr virus (EBV) has also been implicated to be closely association to the pathogenesis of LELC of some organs [2].
In this study we contributed 21 cases of primary LELCs of salivary glands and most of these patients had follow-up data. The objective of this study was to analyze the clinical characteristics, immunohistochemical profiles, treatments, and prognosis of primary LELCs of salivary glands.
Materials and methods
Patients
From the databases of the Pathology Divisions at the first people’s hospital of Changzhou, China, 21 cases of lymphoepithelioma-like carcinomas of salivary glands recorded over an 8-year period (January 2006 through December 2013) were collected retrospectively. The tumors were reviewed on the basis of H&E morphology according to the 2013 World Health Organization (WHO) criteria by 2 experienced pathologists in salivary gland tumor. We excluded undifferentiated carcinomas without dense lymphoid infiltrates and Epstein-Barr virus (EBV)-encoded RNA (EBER) staining in our study. All patients underwent endoscopic examination of the nasopharynx and systemic work-up to rule out metastatic LELC from the nasopharynx and other body sites.
Clinical data were collected from patients’ medical records and analyzed in conjunction with gross and microscopic findings. Pathologic or clinical staging were performed according to the International Union Against Cancer (UICC), which is based on tumor size (defined as the largest diameter) and the presence of lymph node or distant metastases. Clinical follow-ups were conducted through direct contact with patients or through their physicians.
In situ hybridization of EBV-related RNAs
The presence of EBV was examined by in situ hybridization using digoxigenin-labeled 30-base oligonucleotide probes targeted to EBER-1, as previously described [11]. Paraffin sections were deparaffinized, rehydrated, treated with proteinase K for 15 minutes at 37°C, and acetylated with 0.25% acetic acid in 0.1 mol/L triethanolamide, followed by prehybridization at 42°C for 1 hour. The sections were hybridization with the digoxigenin-labeled oligonucleotide probe at 42°C overnight. The slides were then washed in sodium chloride and sodium citrate and then incubated with antidigoxigenin antibody (Boehringer Mannheim, Biochemica, Germany) for 3 hours at room temperature. The signals were developed by the nitroblue tetrazolium chloride and X-phosphate (Boehringer digoxigenin kit) after washing and counterstained with nuclear fast red. A positive reaction was characterized by dark yellow coloration within the nucleus. A known EBV positive NPC and EBV-negative parotid tissue was included as positive and negative controls in each hybridization experiment.
Immunohistochemical staining
Immunohistochemical analysis was performed on 5-μm thick, formalin-fixed, paraffin-embedded tissue sections of each case and all slides were stained on a BENCHMARK Autostainer with the EnVision detection system. Monoclonal antibodies were used against AE1/AE3 (IS053, 1:100, DAKO), CK5/6 (D5/16B4, 1:50, Dako), p63 (4A4, 1:200, Dako), bcl-2 (124, 1:50, Dako), p53 (DO-7, 1:50, Dako), C-erb B-2 (10A7, 1:100, Novocastra), EGFR (H11, 1:200, Dako), CD20 (B-LY1, 1:200, Dako), CD3 (LN10, 1:100, Novocastra).
Results
Clinical features
The Clinical features of the 21 patients are tabulated in Table 1. Their ages ranged from 20 to 73 years with a median age of 43 years and a mean age of 47 years. Of the 21 Chinese patients in this study, fourteen patients were male and seven of them were female (M:F ratio 2:1). All patients had normal nasopharyngoscopic findings. Seventeen of the tumors were located in the parotid gland and four in the submandibular gland. Most of the patients received a multimodality treatment approach including surgery, radiotherapy and (or) chemotherapy in our series that resulted in encouraging results. The follow up period ranged from 15 to 113 months and most patients were alive without disease at the end of the follow up period, except for two who died of liver metastasis nine months and fourteen months after surgery. Two patients who died both had lymph node metastasis at the time of surgery. Case 6 developed a local recurrence 20 months after the initial surgery; the recurrences were successfully treated by a surgical resection and radiotherapy. The patient is alive with no residual disease more than three years after the last surgery.
Table 1.
Clinical features of lymphoepithelioma-like carcinoma of the salivary glands
| Case | Sex/age | Clinical presentation | Treatment | Clinical stage | Recurrence | Outcome |
|---|---|---|---|---|---|---|
| 1 | M/40 | Parotid gland | Surgery, RT, CT | T3N1M0 | None | AWD 15 m |
| 2 | F/57 | Parotid gland | Surgery, RT | T2N0M0 | None | AWD 15 m |
| 3 | F/30 | Parotid gland | Surgery, RT | T4aN0M0 | None | AWD 16 m |
| 4 | F/64 | Parotid gland | Surgery, RT, CT | T2N0M0 | None | AWD 38 m |
| 5 | M/63 | Parotid gland | Surgery, RT | T1N2M0 | None | AWD 56 m |
| 6 | M/73 | Parotid gland | Surgery, RT | T2N0M0 | Local (20 m postoperation) | AWD 63 m |
| 7 | M/59 | Parotid gland | Surgery, RT | T2N0M0 | None | AWD 67 m |
| 8 | M/54 | Parotid gland | Surgery, RT, CT | T2N1M0 | None | AWD 74 m |
| 9 | F/41 | Parotid gland | Surgery, RT | T2N0M0 | None | AWD 90 m |
| 10 | M/66 | Submandibular gland | Surgery, RT, CT | T2N2M0 | None | AWD49 m |
| 11 | M/52 | Submandibular gland | Surgery, RT | T2N1M0 | None | AWD 54 m |
| 12 | M/39 | Submandibular gland | Surgery, RT | T2N0M0 | None | AWD 66 m |
| 13 | M/55 | Parotid gland | Surgery, RT, CT | T3N2bM0 | Liver (14 m postoperation) | DOD 38 m |
| 14 | M/43 | Parotid gland | Surgery, RT | T2N0M0 | None | AWD 18 m |
| 15 | F/50 | Parotid gland | Surgery, RT, CT | T2N2M0 | None | AWD 25 m |
| 16 | M/32 | Parotid gland | Surgery, RT | T1N0M0 | None | AWD 37 m |
| 17 | M/20 | Parotid gland | Surgery, RT, CT | T3N1M0 | Liver (9 m postoperation) | DOD 65 m |
| 18 | F/30 | Submandibular gland | Surgery, RT, CT | T2N1M0 | None | AWD 113 m |
| 19 | M/43 | Parotid gland | Surgery, RT | T2N0M0 | NA | NA |
| 20 | F/38 | Parotid gland | Surgery, RT | T1N0M0 | NA | NA |
| 21 | M/32 | Parotid gland | Surgery, RT | T1N0M0 | NA | NA |
M, male; F, Female; RT, radiotherapy; CT, chemotherapy; AWD, alive with disease; DOD, died of disease; NA, not available; m, month.
Pathology
Grossly, the tumors were solitary, circumscribed or multinodular in all of the 21 resected specimens. The cut surfaces were white or tan and elastic with fish flesh appearance. Histologically, all tumors had similar morphology and were indistinguishable from lymphoepithelioma of the nasopharynx. The tumors showed either lobular or diffuse growth pattern and were characterized by a seemingly syncytial appearance, large vesicular nuclei, prominent eosinophilic nucleoli, and a heavy lymphocytic infiltration (Figure 1). They had predominantly pushing borders and grew in the form of anastomosing smooth-contoured islands or a jigsaw puzzle pattern appearance or in the form of diffuse sheets. Definite squamous differentiation was not seen. The prominent lymphoid reaction consisted of mature lymphocytes often admixed with plasma cells and histiocytes and with or without neutrophils and eosinophils. The tumor nests were infiltrated with lymphoid cells and follicles with germinal centers were frequently seen in the surrounding stroma.
Figure 1.
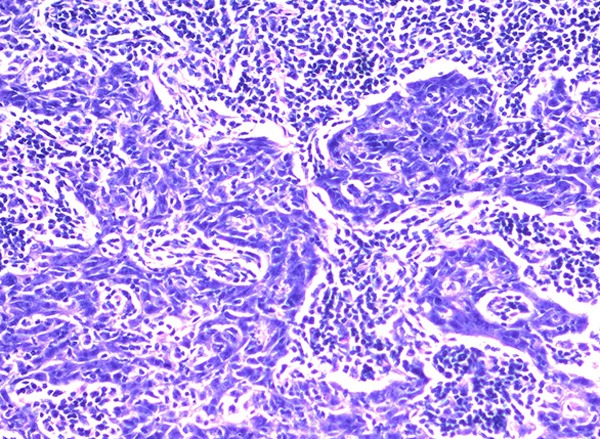
LELC is characterized by syncytial growth pattern of tumor cells with large vesicular nuclei, prominent nucleoli, and a heavy lymphocytic infiltration (hematoxylin and eosin ×200).
In situ hybridization
In situ hybridization for EBER-1 showed strong positive signals in all 21 cases of lymphoepithelial carcinomas of the parotid and submandibular glands studied. The EBER-1 RNA was shown to be present in the nuclei of the large tumor cells. All normal salivary glandular epithelium and the accompanying lymphocytes and plasma cells were negative (Figure 2).
Figure 2.
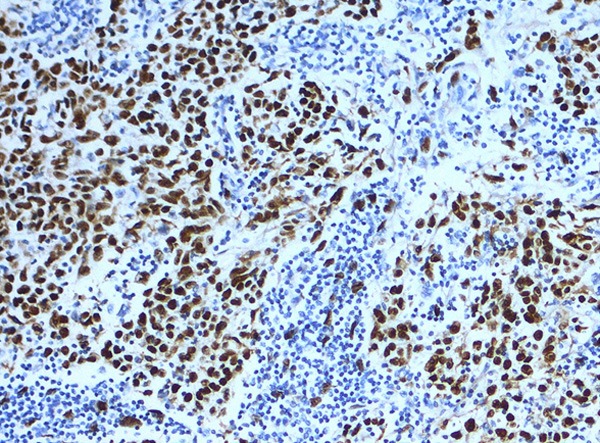
Positive EBER hybridization signals in the nuclei of large undifferentiated neoplastic cells but is absent in the surrounding lymphocytic infiltrate (in situ hybridization ×200).
Immunohistochemistry
All 21 (100%) cases of tumors were diffusely and strongly positive for AE1/AE3 (Figure 3A) and CK5/6 (Figure 3B) with staining of the cell membrane and cytoplasm. Staining for p63 (Figure 3C) was strongly positive in 100% (21/21) of cases, with positive nuclear staining in > 20% of cells. A bcl-2 immunostaining (Figure 3D) was detected in 20 of 21 tumors and the proportion of bcl-2-stained cells in these cases varied from 20% to 90%. Staining for p53 was positive (> 10% of tumor cells) in 9% (2/21) of cases. Staining of EGFR was 5% (1/21) of cases and C-erb B-2 immunostaining was negative. The accompanying lymphoid infiltrate was positive for CD20 and CD3 in all cases. Overall, the number of cells staining for CD3 seemed to exceed those stained for CD20.
Figure 3.
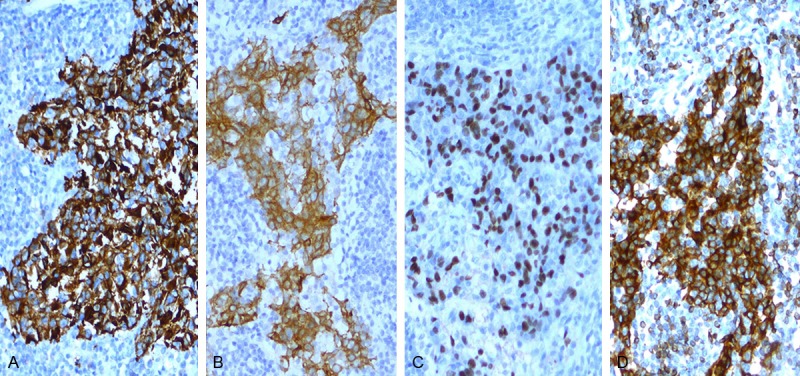
Immunoreactivity for AE1/AE3, CK5/6, p63 and bcl-2 in LELC (hematoxylin and eosin ×200). A. IHC for AE1/AE3 highlighting the epithelial component; B. IHC for CK5/6 highlighting the epithelial component; C. IHC for p63, showing intense nuclear staining in the majority of the tumor cells; D. Bcl-2 immunostain showed diffuse positivity with strong intensity.
Discussion
Primary LELC of salivary glands is a rare and clinicopathologically distinctive neoplasm. Over the past 4 decades, < 150 cases have been reported in the literature, and to our knowledge, the cohort reported here is the largest to date. Our experience supported the notion that primary LELC of salivary gland was more prevalent in South Chinese and Eskimos. In our study, there were 14 male patients and 7 female patients. The male/female ratio (2:1) was different from the observation of Zhang et al. (3:5) [12], but was consistent with the results reported by Leung et al (7:3) [13]. The patients range in age from 20 to 73 years (mean 47 years), which is similar to the reports of Zhang et al and Leung et al. As reported previously [12,13], the parotid glands were more often affected than the submandibular glands in our series.
The diagnosis of LELC depends mainly on morphologic characteristics, which exhibit well-circumscribed nodules characterized by nests, sheets, and cords of syncytial-like growth pattern of anaplastic cells with prominent eosinophilic nucleoli surrounded by moderate to heavy lymphocyte infiltrates. Primary LELC of salivary glands is histologically indistinguishable from the prototypical nasopharyngeal lymphoepithelioma, so the major differential diagnosis for LELC of salivary glands is metastatic NPC. Endoscopic examination and random biopsies of nasopharynx, combined with computed tomography is indicated in patients with salivary gland LELC to exclude the possibility of a coexisting NPC because in the latter situation different treatment is required.
Because nasopharyngeal lymphoepithelioma has been demonstrated to be strongly associated with EBV infection [14], the role of EBV in LELC of salivary glands has also been investigated [15]. At present the analysis of EBER in situ hybridization is the most reliable, specific, and highly sensitive method for latent EBV detection in paraffin sections. The most abundant transcription of EBER can be detected during latent EBV infection. The secondary structure of EBER is very stable and the majority of EBER are located within the nucleus where they are conjugated with the cellular protein La [16]. In our study, all 21 patients had positive results from in situ hybridization for EBER, indicating latent EBV infection in the tumor cells and suggesting that EBV infection may be a factor influencing the tumorigenesis of LELCs of salivary glands.
All immunohistochemical staining in our study was performed not to determine the diagnosis of LELC but to help us define LELC. Our study showed that the tumor cells were consistently positive for AE1/AE3, CK5/6 and P63. This immunostaining pattern was similar to that reported for NPC [17] and indicates that LELC of salivary gland originates from epithelial tissue and belongs to the category of squamous carcinoma.
Regulators of apoptosis may be cell-specific and the first gene identified as part of the apoptotic process was bcl-2. The bcl-2 gene is located on chromosome, and it encodes for the bcl-2 protein. Bcl-2 is unique among the proto-oncogenes, as it is localized on the mitochondrial membranes and interferes with programmed cell death independently of its ability to promote cell division. In our series, we found that 20 of 21 cases showed positive bcl-2 immunostaining with moderate to strong intensity. Our result was consistent with the early report which revealed that overproduction of bcl-2 protein conferred a selective growth enhancement to the EBV-infected B cells [18]. Some studies showed that bcl-2 expression might correlate with a better prognosis [19], which seemed to be similar to our result.
Chang et al reported that only 17.4% of patients with lung LELCs harbored EGFR mutations [20], and Tam et al observed that EGFR mutations were uncommon in LELC (1 of 11 patients were positive) [21]. In our study, there was only one EGFR mutation in the 21 cases of tumors. Thus, the relatively low rate of EGFR mutation in LELCs indicates that it might correlate with a better prognosis.
It is well known that specific genetic mutation and aberrant gene expression in tumor cells may change the biologic behaviors of a tumor. P53 is a tumor suppressor gene, involved in regulating cell proliferation, inducing apoptosis, and promoting chromosomal stability. Disruption of these functions appears to have an important role in carcinogenesis [22]. C-erb B-2 is a normal cellular gene that encodes a membrane protein (p185), which is tyrosine phosphorylated after interaction with its ligands. Overexpression of c-erb B-2 occurs either through changes in amplification or through mRNA overexpression and has been correlated with poor clinical outcome in several tumors [23]. To further define LELC of salivary glands we investigated its oncoprotein expression. Using immunohistochemical method, there were fewer, or an absence of, p53 protein and c-erb B-2 oncoprotein in this tumors. Our results indicated that fewer, or an absence of, p53 and c-erb B-2 oncoproteins in these cases was closely related to a better prognosis in LELCs of salivary glands. Overall, there is no report in the literature of oncoprotein expression in the LELC arising in the salivary glands.
All of our cases showed prominent infiltration of CD20+B lymphocytes and CD3+T lymphocytes in the tumor cell nests and the surrounding stroma and germinal centers were frequently observed in most tumors. LELC of salivary glands seemed to be able to induce a strong host immune response, which probably conferred to them relatively better prognosis.
Our experience suggested that LELC of salivary gland may be curable by resection, which is the recommended treatment of choice. In some cases adjuvant radiotherapy and chemotherapy were also given and showed a good partial response.
In our series, LELC of salivary gland showed a better prognosis than ordinary undifferentiated carcinoma. A strong host immune response, an absence of, p53 and c-erb B-2 oncoproteins, and bcl-2 expression may be closely related to a better prognosis in this tumor.
In conclusion, LELC of salivary glands has unique clinical and pathologic features. Our study confirms that primary LELC of salivary glands is strongly associated with EBV infection, with a male predominance, has low frequency of oncoprotein expressions. According to its better prognosis, LELC of salivary gland should be treated with multimodality approaches such as surgery, radiotherapy and chemotherapy.
Disclosure of conflict of interest
None.
References
- 1.Saw D, Lau WH, Ho JH, Chan JK, Ng CS. Malignant lymphoepithelial lesion of the salivary gland. Hum Pathol. 1986;17:914–923. doi: 10.1016/s0046-8177(86)80641-4. [DOI] [PubMed] [Google Scholar]
- 2.Iezzoni JC, Gaffey MJ, Weiss LM. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am J Clin Pathol. 1995;103:308–315. doi: 10.1093/ajcp/103.3.308. [DOI] [PubMed] [Google Scholar]
- 3.Begin LR, Eskandari J, Joncas J, Panasci L. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol. 1987;36:280–283. doi: 10.1002/jso.2930360413. [DOI] [PubMed] [Google Scholar]
- 4.Leyvraz S, Henle W, Chahinian AP, Perlmann C, Klein G, Gordon RE, Rosenblum M, Holland JF. Association of Epstein-Barr virus with thymic carcinoma. N Engl J Med. 1985;312:1296–1299. doi: 10.1056/NEJM198505163122006. [DOI] [PubMed] [Google Scholar]
- 5.Swanson SA, Cooper PH, Mills SE, Wick MR. Lymphoepithelioma-like carcinoma of the skin. Mod Pathol. 1988;1:359–365. [PubMed] [Google Scholar]
- 6.Rowlands DC, Ito M, Mangham DC, Reynolds G, Herbst H, Hallissey MT, Fielding JW, Newbold KM, Jones EL, Young LS, et al. Epstein-Barr virus and carcinomas: rare association of the virus with gastric adenocarcinomas. Br J Cancer. 1993;68:1014–1019. doi: 10.1038/bjc.1993.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinney CP, Ro JY, Babaian RJ, Johnson DE. Lymphoepithelioma of the bladder: a clinicopathological study of 3 cases. J Urol. 1993;149:840–841. doi: 10.1016/s0022-5347(17)36228-6. [DOI] [PubMed] [Google Scholar]
- 8.Butler AE, Colby TV, Weiss L, Lombard C. Lymphoepithelioma-like carcinoma of the lung. Am J Surg Pathol. 1989;13:632–639. doi: 10.1097/00000478-198908000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Mills SE, Austin MB, Randall ME. Lymphoepithelioma-like carcinoma of the uterine cervix. A distinctive, undifferentiated carcinoma with inflammatory stroma. Am J Surg Pathol. 1985;9:883–889. doi: 10.1097/00000478-198512000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Vargas MP, Merino MJ. Lymphoepitheliomalike carcinoma: an unusual variant of endometrial cancer. A report of two cases. Int J Gynecol Pathol. 1998;17:272–276. [PubMed] [Google Scholar]
- 11.Hsu HC, Chen CC, Huang GT, Lee PH. Clonal Epstein-Barr virus associated cholangiocarcinoma with lymphoepithelioma-like component. Hum Pathol. 1996;27:848–850. doi: 10.1016/s0046-8177(96)90460-8. [DOI] [PubMed] [Google Scholar]
- 12.Zhang G, Tang J, Pan Y, Zhuang Q, Wu C. CT features and pathologic characteristics of lymphoepithelial carcinoma of salivary glands. Int J Clin Exp Pathol. 2014;7:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- 13.Leung SY, Chung LP, Yuen ST, Ho CM, Wong MP, Chan SY. Lymphoepithelial carcinoma of the salivary gland: in situ detection of Epstein-Barr virus. J Clin Pathol. 1995;48:1022–1027. doi: 10.1136/jcp.48.11.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raab-Traub N. Epstein-Barr virus and nasopharyngeal carcinoma. Semin Cancer Biol. 1992;3:297–307. [PubMed] [Google Scholar]
- 15.Hamilton-Dutoit SJ, Therkildsen MH, Neilsen NH, Jensen H, Hansen JP, Pallesen G. Undifferentiated carcinoma of the salivary gland in Greenlandic Eskimos: demonstration of Epstein-Barr virus DNA by in situ nucleic acid hybridization. Hum Pathol. 1991;22:811–815. doi: 10.1016/0046-8177(91)90210-g. [DOI] [PubMed] [Google Scholar]
- 16.Howe JG, Steitz JA. Localization of Epstein-Barr virus-encoded small RNAs by in situ hybridization. Proc Natl Acad Sci U S A. 1986;83:9006–9010. doi: 10.1073/pnas.83.23.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi SR, Goodman ML, Bhan AK, Pilch BZ, Chen LB, Sun TT. Immunohistochemical study of nasopharyngeal carcinoma using monoclonal keratin antibodies. Am J Pathol. 1984;117:53–63. [PMC free article] [PubMed] [Google Scholar]
- 18.Tsujimoto Y. Overexpression of the human BCL-2 gene product results in growth enhancement of Epstein-Barr virus-immortalized B cells. Proc Natl Acad Sci U S A. 1989;86:1958–1962. doi: 10.1073/pnas.86.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pezzella F, Turley H, Kuzu I, Tungekar MF, Dunnill MS, Pierce CB, Harris A, Gatter KC, Mason DY. bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med. 1993;329:690–694. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]
- 20.Chang YL, Wu CT, Shih JY, Lee YC. Unique p53 and epidermal growth factor receptor gene mutation status in 46 pulmonary lymphoepithelioma- like carcinomas. Cancer Sci. 2011;102:282–287. doi: 10.1111/j.1349-7006.2010.01768.x. [DOI] [PubMed] [Google Scholar]
- 21.Tam IY, Chung LP, Suen WS, Wang E, Wong MC, Ho KK, Lam WK, Chiu SW, Girard L, Minna JD, Gazdar AF, Wong MP. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res. 2006;12:1647–1653. doi: 10.1158/1078-0432.CCR-05-1981. [DOI] [PubMed] [Google Scholar]
- 22.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 23.Giatromanolaki A, Gorgoulis V, Chetty R, Koukourakis MI, Whitehouse R, Kittas C, Veslemes M, Gatter KC, Iordanoglou I. C-erbB-2 oncoprotein expression in operable non-small cell lung cancer. Anticancer Res. 1996;16:987–993. [PubMed] [Google Scholar]


