Abstract
Background:
Wound healing is a complex process. Different types of skin cells, extracellular matrix and variety of growth factors are involved in wound healing. The use of recombinant growth factors in researches and production of skin substitutes are still a challenge.
Objectives:
Much research has been done on the effects of gene therapy and cell therapy on wound healing. In this experimental study, the effect of insulin-like growth factor (IGF-1) gene transfer in fibroblast cells was assessed on acute dermal wound healing.
Materials and Methods:
Fibroblasts were cultured and transfected with IGF-1. Lipofectamine 2000 was used as a reagent of transfection. Transgene expression levels were measured by the enzyme linked immunosorbent assay (ELISA). To study in vivo, rats (weighing 170-200 g) were randomly divided into three groups (five/group) and full-thickness wounds were created on the dorsum region. Suspensions of transfected fibroblast cells were injected into the wound and were compared with wounds treated with native fibroblast cells and normal saline. For the microscopic examination, biopsy was performed on day seven.
Results:
In vitro, the maximum expression of IGF1 (96.95 pg/mL) in transfected fibroblast cells was 24 hours after gene transfer. In vivo, it was clear that IGF-1 gene therapy caused an increase in the number of keratinocyte cells during the wound healing process (mean of group A vs. group B with P value = 0.01, mean of group A vs. group C with P value = 0.000). Granulation of tissue formation in the transfected fibroblast group was more organized when compared with the normal saline group and native fibroblast cells.
Conclusions:
This study indicated that the optimization of gene transfer increases the expression of IGF-1. High concentrations of IGF-1, in combination with cell therapy, have a significant effect on wound healing.
Keywords: IGF-1, Gene Therapy, Cell Transplantation, Fibroblast, Wound Healing
1. Background
Full-thickness wounds, as one of the main issues in medicine, cause significant morbidity and mortality. There have been several reported complications because of delay in wound closure (1). Thus, rapid wound closure seems to be a necessary factor in wound healing and patient recovery (2, 3). Today, for improving the wound healing processes, cultured epithelial autografts (CEAs) are directly transplanted to full-thickness wounds (4, 5).
Wound healing as an intricate and dynamic process includes three sequential overlapping phases. These phases are as follows: inflammatory, proliferative and remodeling (6). Many potential agents, such as blood cells, parenchymal cells, extracellular matrix and growth factors participate in the wound healing process (7-9). Fibroblast cells as the main cell of connective tissue are almost involved in the entire wound healing process. They do their roles by producing collagen fibers during the proliferation phase of wound healing (10).
Today, many studies on wound treatment have been done to improve the wound healing process. Cell therapy, the transfer of cells to injured skin, is one of the treatment options, which has been considered by previous studies. Cell therapy is a new clinical method, which is used in medicine for repair of damaged tissue. In this method, autologous fibroblasts and autologous keratinocytes are used for the treatment of full-thickness wounds (11, 12). Herein, the fibroblast cells, as signaling cells, produce growth factors, which are necessary for connecting all cells with each other during the wound healing process (13-15).
It has been revealed that the IGF-1, produced by fibroblast and other epithelial cells, has an important role in re-epithelialization and granulation tissue formation of epidermal tissue during wound healing processes (6, 10, 16, 17). Despite the low levels of IGF-1 in dermal and granular layers of normal skin, this amount is more significant in damaged skin layers (18). Previous studies on experimental skin wound models showed that the amount of IGF-1 is significantly increased during wound healing processes (17-19). The expression of proteins and mRNA IGF-1, were significantly decreased in diabetic patients (18, 20-22). These decreases in expression of mRNA and proteins IGF-1, which led to the prolongation of wound healing processes, are one of the main concerns of diabetic patients (23). In the study conducted by Kratz et al. in 1992, it was shown that IGF-1 has a major role in the proliferation of fibroblasts and keratinocytes in the skin (24).
Due to the short lifetime of growth factors, the efficiency of their use is still very low in wound healing (25). Additionally, it has been shown that many growth factors have positive effects on chronic and acute wound healing in diabetic and non-diabetic patients (26). Therefore, it seems that the transfer of the gene encoding the growth factor involved in re-epithelialization will be helpful and effective. The transfer of IGF-1 gene and protein, as well as other genes directly into the wound location has previously been investigated by others (16, 27).
2. Objectives
Thus, we aimed to study the in vivo effects of cell therapy and gene therapy on acute wound healing by transferring IGF-1 genes into fibroblast cells and transplanting them to acute wound models in rats.
3. Materials and Methods
3.1. Skin Preparation
The hair of the abdominal region was shaved and the skin was thoroughly disinfected. A small piece of full thickness skin grafts measuring 2 × 2 cm was harvested by a scalpel. The created wounds were covered with Vaseline gauze and sterile bandages. The samples were placed in Dulbecco's Modified Eagle Medium (DMEM, Gibco, Cat.No.31966021) supplemented with 10% fetal bovine serum (FBS) (Invitrogen) and 10% penicillin/streptomycin (Sigma), and transferred to the culture room. All dermis separation steps were done using sterile material and reagents, under a laminar hood.
3.2. Fibroblasts Cultivation
Skin samples were washed once by 70% ethanol and phosphate buffered saline (PBS) three times. Next, the dermis part of skin samples were separated from the epidermis using 0.25% Trypsin-EDTA (1X) (Gibco®). The obtained dermis samples were cut into smaller than 1 mm pieces by sterile scissors and explanted to a 100 mm tissue culture dish (Corning®) with complete Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10% FBS, 1% penicillin/streptomycin and incubated at 37°C with 5% CO2. Cultivated cells were fed every three days. After multiplication of cells to 85-95% confluence, they were harvested by trypsinization. The obtained suspension was centrifuged and the fibroblasts were subcultured in a new cell culture flask (CELLSTAR®). The subcultured cells in the fifth passage were preserved at -80°C.
3.3. Transfection Procedure
Lipofectamine 2000 (Invitrogen-Cat. No. 11668-027) was used as a transfection reagent. For harvesting transfected cells with high efficiency and low cytotoxicity, the optimizing of transfection methods by different concentration of DNA, cell density and lipofectamine 2000 was necessary. For this purpose, the cells were transfected by two methods: reverse and direct transfection. Transfection was performed at ratios of 1: 1.5, 1: 2, 1: 3, 1: 4, 1: 5, 1: 6, 1: 7 and 1: 8, DNA (in µg): lipofectamine 2000 (in µL).
3.4. IGF-1 Gene Transfer
The fifth passage of fibroblasts at a density of 4 × 104 was seeded and cultured in a 96-well plate. When the cultured cells were proliferated to 85-95% confluence, they were transfected by 0.2 µg of IGF1 (NM-001082477) rat cDNA clone (Origene, Cat No. RN200508)/lipofectamine 2000 at different ratios. The complex was placed in serum-free medium, added to the cells, and incubated at 37°C and 5% CO2 for 4-6 hours. Then the transfection mixture was replaced with regular growth medium. The medium was collected 24 hours, 48 hours and 72 hours later for quantitative analysis of IGF-1 expression.
3.5. Measurements of IGF-1 Growth Factor
In order to measure IGF-1 protein levels produced by transfected and untransfected fibroblast cells in vitro, the culture supernatants were examined by the ELISA test. Samples were detected according to the manufacturer’s instructions for IGF-1 using the Quantikine ELISA Mouse/Rat IGF-1 Immunoassay kit (R and D Systems, Cat. No. MG100).
3.6. Animals
Prior to commencement of our experimental study, all animal procedures were approved by the Research Committee of Mazandaran University of Medical Sciences. Fifteen adult male Wistar rats, weighing 170-200 g, were allowed to acclimatize for 1 week before initiation of the experiment. They were kept in individual cages in a controlled temperature environment, at 21 ± 1°C, with a 12-hour light/dark cycle, and maintained on standard rodent chow and water ad libitum. The rats had free access to water and food during the research. The animals were randomly distributed into three groups (five/group) (27):
Wounds were treated with 5 × 105 transfected fibroblasts (study group)
Wounds were treated with 5×105 non-transfected fibroblasts (transplant control)
Wounds were treated with 0.9% sterile saline solution (wet non-transplanted control)
3.7. Wound Model and Surgical Procedure
For creating full-thickness skin wounds, the rats were anesthetized by intramuscular injection of ketamine (50 mg/kg) and xylazine (5 mg/kg) to prevent pain and suffering. The skin in the dorsum region was then shaved and thoroughly disinfected. Under sterile conditions, a square-like skin incision, measuring 1.5 × 1.5 cm, comprised of a full-thickness skin layer down to the panniculus carnosus muscle was created on the dorsum, using a scalpel blade.
3.8. Transplantation of Allogenic Fibroblasts
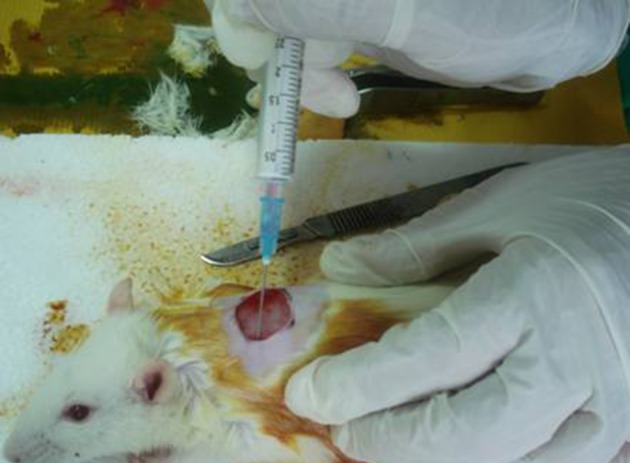
After reaching 85-95% confluence of the fibroblasts fifth passage, half of them were transfected. After cell count, using the trypan blue exclusion test, the cells were transferred into two individual syringes with serum and antibiotic free DMEM (one per group) and transferred to the operation theater. In groups A and B, the transfected and untransfected cells were re-suspended and injected intradermally around the wound at four different sites (Figure 1). In group C, normal saline (NS) solution with same volume was injected. After the operation, the rats were kept in separate cages.
Figure 1. Injection of Cells Around The Wound.
3.9. Histological Analysis
The skin tissue samples derived by biopsy were examined for pathohistological changes with a small excision containing part of the wound area and normal area. Tissues were fixed in 10% formalin. Paraffin-embedded sections (5 μm thick) were serially prepared with a 15 μm interval between each consecutive section and stained with hematoxylin and eosin. The characters of tissue repair including: epithelialization, granulation tissue formation, severity of inflammation in healed areas was evaluated by a blinded pathologist. The numbers of fibroblasts, keratinocyte and neutrophil cells were counted with the Bio report OLYSIA Soft Imaging System GmbH, version 3.2 (Build 670).
3.10. Statistics
Data were evaluated using the SPSS version 15 and all data were expressed as ANOVA, One-Way ANOVA and Tukey tests. Significant differences were calculated between the groups. Significance was accepted at P < 0.05.
4. Results
4.1. Fibroblast Cell Culture Findings
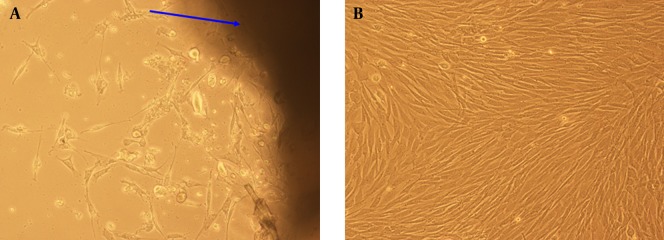
After separating the desired dermis from the abdominal part of rats and cultivating them in the culture plates, the fibroblasts began to migrate and grow radially around the cultured dermis. These growths were especially remarkable during the first 72 hours of cultivation. These cultivated fibroblast cells multiplied to 90-95% of confluence after 19 days of culture (Figure 2).
Figure 2. A) Growth of Fibroblasts in the Implanted Dermis After 72 Hours (× 100 Magnification); B) The Cultured Fibroblasts After 19 Days.
4.2. Optimization of in Vitro Gene Transfer to Rat Fibroblasts
In the current study, fibroblast cells at density of about 5 × 105 from the fifth passage were cultured in flasks (T-75). After multiplication of cells to 90-95% confluence, they were transfected at ratio of 1 to 5 by 0.2 μg of IGF1 (NM-001082477) rat cDNA clone (Origene, Cat No. RN200508) complex with lipofectamine 2000 (Invitrogen-Cat. No. 11668-027) in 96-well plates.
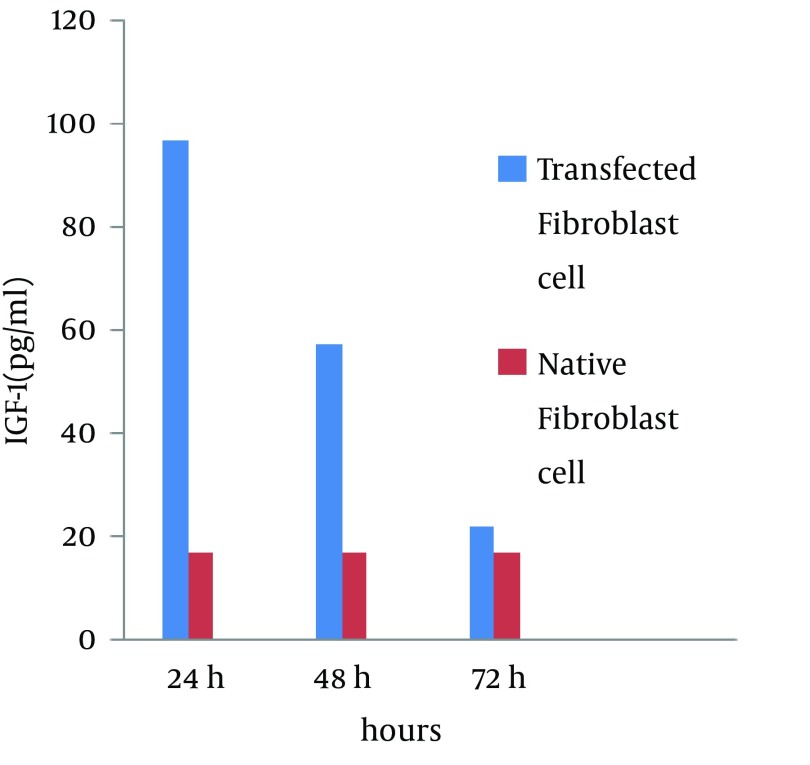
To optimize the transfection method, different concentrations of reagents were used by both direct and reverse methods. The results showed that the IGF1 expression via the reverse method was more than the direct method. We suggest that the use of 0.2 µg IGF-1 with 1 µL lipofectamine 2000 in the reverse method after 24 hours causes a significant increase in IGF-1 expression of fibroblasts (approximately 5.7 fold (96.95 pg/mL)) in comparison to the control groups (16.9 pg/mL) while at 48 and 72 hours after transfection, this rate was gradually reduced (3.4-time and 1.3-time, respectively) (Figure 3).
Figure 3. Quantitative Analysis of IGF-1 Protein by the Reverse Method at 24, 48 and 72 Hours After Transfection: A Comparison Between Transfected Fibroblasts and Native Fibroblasts.
4.3. Macroscopic Findings
Twenty-four hours after transfection of cells, the suspensions containing the native and transfected cells were transplanted to full thickness wounds. The healing process of the wounds was evaluated after seven days. In the experimental groups, the wound contracture, increase of content density and concentration and drying of the wounds were observed on the second day after transplantation, while, in the control group they were observed on the third and fourth day after transplantation (Figure 4). The results indicated that wound healing in terms of time was faster in the gene therapy group in comparison to the groups that received the native cells and normal saline.
Figure 4. Macroscopic Findings of Wound Healing After Seven Days in the Three Groups.
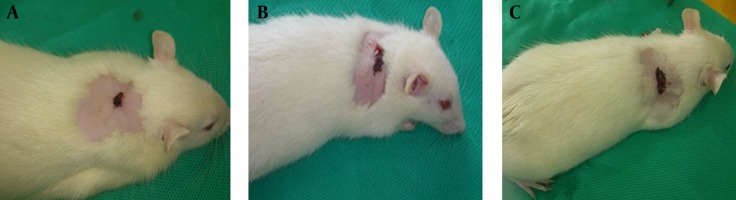
A: Wounds treated with transfected fibroblast cells. B; Wounds treated with native fibroblast cells. C; Wounds treated with normal saline.
Hair growth was also observed in the wound healing area. During a biopsy of healed area for histological evaluation, the abnormal and tightening fibrous tissue was well respected in the wound healing area of the control group.
4.4. Microscopic Findings
Seven days after the operation, a sample biopsy was taken from the wound area. The effects of gene and cell therapy on quantitative indexes of wound healing are presented in Table 1.
Table 1. The Wound Healing Rate of the Experimental and Control Groups, According to Quantity Indices a.
| Groups | Width of Epiderm, µm | Fibroblast b | Keratinocyte b | Neutrophil b |
|---|---|---|---|---|
| A c | 86.8 ± 6.76 | 37.4 ± 3.36 | 40.6 ± 1.14 | 1.6 ± 0.54 |
| B d | 76.2 ± 2.28 | 36.6 ± 1.14 | 35.4 ± 3.57 | 3.4 ± 1.14 |
| C e | 54.4 ± 3.5 | 30 ± 1.58 | 19.8 ± 1.3 | 6.4 ± 1.14 |
| A and B | 0.009 | 0.841 | 0.01 | 0.033 |
| A and C | 0.000 | 0.001 | 0.000 | 0.000 |
| B and C | 0.000 | 0.001 | 0.000 | 0.001 |
a Data are presented as Mean ± SD and P value.
b Data are presented in 40 × 10 magnification.
c Wounds treated with transfected fibroblasts (n = 5).
d Wounds treated with native fibroblasts (n = 5).
e Wounds treated with normal saline solution (n = 5).
Pathohistological assessment of the wound biopsy showed that wound epithelialization was achieved completely in the gene therapy group without any significant clinical signs, inflammation or epithelial defects. Moreover, a newly formed epithelium with rather well organized layers was observed. Comparative light microscope analysis proved that the population of fibroblast cells in this group was more than the control group. Additionally, the granulation tissue was denser, more fibrotic and less acute (Figure 5 A). In the treated wounds by native cells, the keratinocyte cells were in the migration phase from the edge to the center of wounds, and the newly formed epithelium were less organized in comparison with the gene therapy group (Figure 5 B). The normal saline groups showed a significant reduction in the number of fibroblast and keratinocyte cells and significant increase in neutrophils in comparison with the other groups (Figure 5 C).
Figure 5. The Wound Area With Healthy Skin at Day Seven.
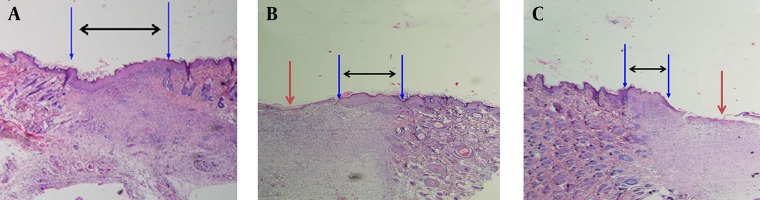
The black arrows indicate the extent of the formed new epithelium. The red arrows indicate the ulcer area. A: Histology of wounds treated with transfected allogeneic fibroblasts with the IGF-1 gene (without ulcer). B: Wounds treated with native fibroblasts. C: Wounds treated with normal saline. (H and E staining; 4 × 10 magnification).
5. Discussion
Although great advances in recent years have been seen in gene transfer to tissue, it is difficult to achieve high concentrations of transgene in vivo. Successful gene transfer, specifically in skin, is still a challenge; high levels of gene expression in order to obtain the correct concentration in wound healing is vital (28). Thus, we evaluated and optimized non-viral gene transfer in allogeneic fibroblast cells and measured the expression of IGF-1 in vitro. Lipofectamine 2000 as a transfection reagent with high transgene expression was mixed with the cDNA. We found that the ratio of 1 to 5 for cDNA/Lipofectamine 2000, with a cell density of 4 × 104 has high efficiency in transfection. Finally, in vitro, IGF-1 transgene expression reached 96.95 pg/mL after 24 hours.
The beneficial effects of transplantation of fibroblast and keratinocyte cell suspension were demonstrated in wound healing (12). Fibroblasts produce many cytokines and growth factors, which stimulate the proliferation and differentiation of cells in the wound bed (29). Also, a number of researchers have reported that wound healing, with skin substitutes containing living cells, occurs faster, better and with less fibrosis (12). In the present study, wounds treated with allogeneic fibroblasts compared with wounds treated with normal saline, showed significant improvement in epidermal repair. Therefore, it is necessary to always have available cultured cells for the treatment of wounds in the clinic.
Fibroblasts have a critical role in the proliferation phase of acute wound healing. They migrate into the wound and synthesize collagen and other essential proteins for the extracellular matrix (30). Unlike some researchers (12), Svensjo et al. showed that injection of autologous fibroblasts into the wounds of healthy pigs has little effect and suggested that exogenous fibroblasts are not involved in the promotion of wound healing (11). However, our results showed that allogeneic fibroblasts culture might play a role in increasing epidermal thickness.
Growth factors play an important role in the wound healing process. Moreover, a decrease in the concentration of growth factors in diabetic wounds has been observed and abnormal concentrations of growth factors are thought to have an effect on the wound-healing cascade (15, 31, 32). Due to the low lifetime of growth factors in the wound environment (25, 33), gene therapy along side cell therapy is important. With IGF-1 gene transfer, expression of growth factors and type 4 collagen increases and epithelialization is facilitated. Exogenous IGF-1 gene, as well as the endogenous, has a similar intracellular response (16). IGF-1 in plasma (circulating) does not play a role in wound healing and local IGF1 plays a very effective role in the healing process (16). Gene therapy is a technique that is used today for healing and tissue regeneration. Gene transfer to cells and then cell transplantation in the wound, reduces the need for re-transplantation of cells and thus reduces the cost of treatment.
It has been shown that IGF-1 enhances keratinocyte cell migration on ECM (Extracellular matrix) proteins (34). We found that with transient transfection of allogeneic fibroblasts and 5.7-fold IGF-1 gene expression in fibroblast cells and then transplantion into the wound, the epidermal thickness significantly increased, compared to wounds treated with native fibroblasts and normal saline. Transfected fibroblasts increased the number of fibroblasts in the ECM, which accelerated wound healing. Histopathological examinations of microscopic slides revealed that the wounds treated with transfected fibroblasts had fewer inflammatory cells, compared to the other two groups. In our study, the extent of lesions and microscopic examination indicated efficacy of gene therapy and cell therapy in the treatment of wounds. In addition, fibroblasts, such as keratinocytes, are an effective vehicle for gene transfer in the wound environment (23). In this experiment it was evident that transfected fibroblast cells with higher expression are more effective in facilitating healing compared to native fibroblast cells with lower expression. Our results indicate that IGF-1 gene therapy with cell therapy facilitates wound healing and shortens recovery time.
To achieve better valuable statistical results in association with cell therapy and gene therapy, studies need to be performed on a larger sample of animals. Since the skin of the pig is physiologically more similar to humans (35) and also several wounds can be created on one animal model, thus research is recommended to be carried out on pigs.
Acknowledgments
We wish to thank the Vice Chancellor of research of Mazandaran University of Medical Sciences, Sari, IR Iran and the Chancellor of research of Skin Research Center, Shahid Beheshti University of Medical Sciences, Tehran, IR Iran for their financial support.
Footnotes
Authors’ Contributions:Mahnaz Mahmoudi Rad: conception and design of the experiments, analysis and interpretation of data, drafting of the article, revision and final approval of the version to be published. Fereshteh Talebpour Amiri: conception and design of the experiments, analysis and interpretation of data, drafting of the article, revision and final approval of the version to be published. Fatemeh Fadaei Fathabadi: acquisition of data, statistical analysis and interpretation of data, drafting and revising the article. Nariman Mosaffa: substantial contributions to conception and design of the experiments, acquisition, analysis and interpretation of the data, and drafting of the article and revision. Azar Ghasemi: as a pathologist she contributed to the study of microscopic slides. Abbas Piryae: as a histologist he contributed to the study of microscopic slides. Farshid Yeganeh: as an immunologist he assisted with the transformation and gene transfer to cells. Alireza Khalilian: statistical analysis and contribution to details.
Funding/Support:This research was supported by research grants from Mazandaran University of Medical Sciences (Grant code: 91.51; date: 2012.8.17), Sari, IR Iran and Skin Research Center, Shahid Beheshti University of Medical Sciences, Tehran, IR Iran.
References
- 1.Vuorisalo S, Venermo M, Lepantalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50(3):275–91. [PubMed] [Google Scholar]
- 2.Brusselaers N, Pirayesh A, Hoeksema H, Richters CD, Verbelen J, Beele H, et al. Skin replacement in burn wounds. J Trauma. 2010;68(2):490–501. doi: 10.1097/TA.0b013e3181c9c074. [DOI] [PubMed] [Google Scholar]
- 3.Holavanahalli RK, Helm PA, Kowalske KJ. Long-term outcomes in patients surviving large burns: the skin. J Burn Care Res. 2010;31(4):631–9. doi: 10.1097/BCR.0b013e3181e4ca62. [DOI] [PubMed] [Google Scholar]
- 4.Fredriksson C, Kratz G, Huss F. Transplantation of cultured human keratinocytes in single cell suspension: a comparative in vitro study of different application techniques. Burns. 2008;34(2):212–9. doi: 10.1016/j.burns.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Munster AM. Use of cultured epidermal autograft in ten patients. J Burn Care Rehabil. 1992;13(1):124–6. doi: 10.1097/00004630-199201000-00028. [DOI] [PubMed] [Google Scholar]
- 6.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 7.Nassar D, Blanpain C. Epidermal development and homeostasis. Semin Cell Dev Biol. 2012;23(8):883. doi: 10.1016/j.semcdb.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Kanzler MH, Gorsulowsky DC, Swanson NA. Basic mechanisms in the healing cutaneous wound. J Dermatol Surg Oncol. 1986;12(11):1156–64. doi: 10.1111/j.1524-4725.1986.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 9.Kopecki Z, Luchetti MM, Adams DH, Strudwick X, Mantamadiotis T, Stoppacciaro A, et al. Collagen loss and impaired wound healing is associated with c-Myb deficiency. J Pathol. 2007;211(3):351–61. doi: 10.1002/path.2113. [DOI] [PubMed] [Google Scholar]
- 10.Lee SW, Kim SH, Kim JY, Lee Y. The effect of growth hormone on fibroblast proliferation and keratinocyte migration. J Plast Reconstr Aesthet Surg. 2010;63(4):e364–9. doi: 10.1016/j.bjps.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Svensjo T, Yao F, Pomahac B, Winkler T, Eriksson E. Cultured autologous fibroblasts augment epidermal repair. Transplantation. 2002;73(7):1033–41. doi: 10.1097/00007890-200204150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Velander P, Theopold C, Bleiziffer O, Bergmann J, Svensson H, Feng Y, et al. Cell suspensions of autologous keratinocytes or autologous fibroblasts accelerate the healing of full thickness skin wounds in a diabetic porcine wound healing model. J Surg Res. 2009;157(1):14–20. doi: 10.1016/j.jss.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Mansbridge JN, Liu K, Pinney RE, Patch R, Ratcliffe A, Naughton GK. Growth factors secreted by fibroblasts: role in healing diabetic foot ulcers. Diabetes Obes Metab. 1999;1(5):265–79. doi: 10.1046/j.1463-1326.1999.00032.x. [DOI] [PubMed] [Google Scholar]
- 14.Takehara K. Growth regulation of skin fibroblasts. J Dermatol Sci. 2000;24 Suppl 1:S70–7. doi: 10.1016/s0923-1811(00)00144-4. [DOI] [PubMed] [Google Scholar]
- 15.Bitar MS, Al-Mulla F. ROS constitute a convergence nexus in the development of IGF1 resistance and impaired wound healing in a rat model of type 2 diabetes. Dis Model Mech. 2012;5(3):375–88. doi: 10.1242/dmm.007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeschke MG, Schubert T, Krickhahn M, Polykandriotis E, Klein D, Perez-Polo JR, et al. Interaction of exogenous liposomal insulin-like growth factor-I cDNA gene transfer with growth factors on collagen expression in acute wounds. Wound Repair Regen. 2005;13(3):269–77. doi: 10.1111/j.1067-1927.2005.130309.x. [DOI] [PubMed] [Google Scholar]
- 17.Yu DH, Mace KA, Hansen SL, Boudreau N, Young DM. Effects of decreased insulin-like growth factor-1 stimulation on hypoxia inducible factor 1-alpha protein synthesis and function during cutaneous repair in diabetic mice. Wound Repair Regen. 2007;15(5):628–35. doi: 10.1111/j.1524-475X.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 18.Blakytny R, Jude EB, Martin Gibson J, Boulton AJ, Ferguson MW. Lack of insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J Pathol. 2000;190(5):589–94. doi: 10.1002/(SICI)1096-9896(200004)190:5<589::AID-PATH553>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 19.Hassmann-Poznanska E, Taranta A, Bialuk I, Poznanska M, Zajaczkiewicz H, Winnicka MM. Analysis of gene expression profiles in tympanic membrane following perforation using PCR Array in rats--preliminary investigation. Int J Pediatr Otorhinolaryngol. 2013;77(10):1753–9. doi: 10.1016/j.ijporl.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Bitar MS. Insulin and glucocorticoid-dependent suppression of the IGF-I system in diabetic wounds. Surgery. 2000;127(6):687–95. doi: 10.1067/msy.2000.105869. [DOI] [PubMed] [Google Scholar]
- 21.Todorovic V, Pesko P, Micev M, Bjelovic M, Budec M, Micic M, et al. Insulin-like growth factor-I in wound healing of rat skin. Regul Pept. 2008;150(1-3):7–13. doi: 10.1016/j.regpep.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Peplow PV, Baxter GD. Gene expression and release of growth factors during delayed wound healing: a review of studies in diabetic animals and possible combined laser phototherapy and growth factor treatment to enhance healing. Photomed Laser Surg. 2012;30(11):617–36. doi: 10.1089/pho.2012.3312. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch T, Spielmann M, Velander P, Zuhaili B, Bleiziffer O, Fossum M, et al. Insulin-like growth factor-1 gene therapy and cell transplantation in diabetic wounds. J Gene Med. 2008;10(11):1247–52. doi: 10.1002/jgm.1251. [DOI] [PubMed] [Google Scholar]
- 24.Kratz G, Lake M, Ljungstrom K, Forsberg G, Haegerstrand A, Gidlund M. Effect of recombinant IGF binding protein-1 on primary cultures of human keratinocytes and fibroblasts: selective enhancement of IGF-1 but not IGF-2-induced cell proliferation. Exp Cell Res. 1992;202(2):381–5. doi: 10.1016/0014-4827(92)90089-q. [DOI] [PubMed] [Google Scholar]
- 25.Lynch SE, Nixon JC, Colvin RB, Antoniades HN. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987;84(21):7696–700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira CT, Herndon DN, Rocker R, Jeschke MG. Liposomal gene transfer of keratinocyte growth factor improves wound healing by altering growth factor and collagen expression. J Surg Res. 2007;139(2):222–8. doi: 10.1016/j.jss.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Jazwa A, Kucharzewska P, Leja J, Zagorska A, Sierpniowska A, Stepniewski J, et al. Combined vascular endothelial growth factor-A and fibroblast growth factor 4 gene transfer improves wound healing in diabetic mice. Genet Vaccines Ther. 2010;8:6. doi: 10.1186/1479-0556-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch T, Spielmann M, Yao F, Eriksson E. Gene therapy in cutaneous wound healing. Front Biosci. 2007;12:2507–18. doi: 10.2741/2251. [DOI] [PubMed] [Google Scholar]
- 29.Streit M, Braathen LR. Apligraf--a living human skin equivalent for the treatment of chronic wounds. Int J Artif Organs. 2000;23(12):831–3. [PubMed] [Google Scholar]
- 30.Loots MA, Lamme EN, Mekkes JR, Bos JD, Middelkoop E. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch Dermatol Res. 1999;291(2-3):93–9. doi: 10.1007/s004030050389. [DOI] [PubMed] [Google Scholar]
- 31.Greenhalgh DG. Wound healing and diabetes mellitus. Clin Plast Surg. 2003;30(1):37–45. doi: 10.1016/s0094-1298(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 32.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med. 2006;23(6):594–608. doi: 10.1111/j.1464-5491.2006.01773.x. [DOI] [PubMed] [Google Scholar]
- 33.Cross SE, Roberts MS. Defining a model to predict the distribution of topically applied growth factors and other solutes in excisional full-thickness wounds. J Invest Dermatol. 1999;112(1):36–41. doi: 10.1046/j.1523-1747.1999.00473.x. [DOI] [PubMed] [Google Scholar]
- 34.Peplow PV, Chatterjee MP. A review of the influence of growth factors and cytokines in in vitro human keratinocyte migration. Cytokine. 2013;62(1):1–21. doi: 10.1016/j.cyto.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Montagna W, Yun JS. The Skin of the Domestic Pig. J Invest Dermatol. 1964;42:11–21. [PubMed] [Google Scholar]