Abstract
We have refined a medium-throughput assay to screen hit compounds for activity against N-myristoylation in intracellular amastigotes of Leishmania donovani. Using clinically-relevant stages of wild type parasites and an Alamar blue-based detection method, parasite survival following drug treatment of infected macrophages is monitored after macrophage lysis and transformation of freed amastigotes into replicative extracellular promastigotes. The latter transformation step is essential to amplify the signal for determination of parasite burden, a factor dependent on equivalent proliferation rate between samples. Validation of the assay has been achieved using the anti-leishmanial gold standard drugs, amphotericin B and miltefosine, with EC50 values correlating well with published values. This assay has been used, in parallel with enzyme activity data and direct assay on isolated extracellular amastigotes, to test lead-like and hit-like inhibitors of Leishmania N-myristoyl transferase (NMT). These were derived both from validated in vivo inhibitors of Trypanosoma brucei NMT and a recent high-throughput screen against L. donovani NMT. Despite being a potent inhibitor of L. donovani NMT, the activity of the lead T. brucei NMT inhibitor (DDD85646) against L. donovani amastigotes is relatively poor. Encouragingly, analogues of DDD85646 show improved translation of enzyme to cellular activity. In testing the high-throughput L. donovani hits, we observed macrophage cytotoxicity with compounds from two of the four NMT-selective series identified, while all four series displayed low enzyme to cellular translation, also seen here with the T. brucei NMT inhibitors. Improvements in potency and physicochemical properties will be required to deliver attractive lead-like Leishmania NMT inhibitors.
Author Summary
We have developed an assay for screening test compounds for their ability to kill intracellular amastigotes of Leishmania parasites, causative agents of human leishmaniasis. The assay is based on freeing amastigotes from infected macrophages by mild detergent lysis and measuring the number of parasites following their extracellular replication by a fluorescence-based method. The validity of the assay has been confirmed using the gold standard drugs, Amphotericin B and Miltefosine, which kill parasites at highly reproducible concentrations. Our results show that this assay is easily transferable between laboratories, can be adapted to specific applications and used to test any parasite species or strain, and does not rely on genetically-modified parasites. These features will enable its use in screening isolates taken directly from patients, vectors or reservoir hosts. We used this assay, in parallel with enzyme activity data, to test lead-like and hit-like inhibitors of a validated target enzyme, Leishmania N-myristoyltransferase (NMT). Compounds from two of four newly-identified Leishmania NMT-selective hit series displayed host cell cytotoxicity, while all four series displayed low translation of enzyme to cellular activity in analysis of intracellular parasite viability. Improvements in potency and physicochemical properties will be required to deliver attractive lead-like Leishmania NMT inhibitors.
Introduction
The Leishmaniases, together with Human African Trypanosomiasis (HAT) and Chagas disease, are caused by kinetoplastid parasites of the TriTryp group (Leishmania spp., Trypanosoma brucei and T. cruzi respectively [1]–[3] and described as neglected tropical diseases [4]. All of these infections are diseases of poverty and cause severe impact, as measured in disability adjusted life years (DALY), in endemic countries (91 in total for the Leishmaniases, including countries in east and northern Africa, the Middle East, the Indian sub-continent and Central and South America [3]). They have also received limited funding for research and development of new drugs [4] although there are currently “repurposed” drugs in clinical or preclinical trials for all three disease groups e.g. fexinidazole for visceral leishmaniasis (VL) and HAT (acute and CNS stages) [5], [6]; the antifungal lanosterol-14 alpha-demethylase inhibitors, posaconazole [7] and E1224 (a prodrug of ravuconazole [8], [9]), for Chagas disease (see [4] and www.dndi.org/ for further details). Despite this recent encouraging progress, there is an urgent need to develop more potential therapeutics and especially, to identify new chemical entities which are orally available and fast acting for the treatment of these diseases. The aim is to cure with a single oral daily dose over a 10 day course in the case of VL and HAT, a challenging target-product profile particularly for the intracellular (amastigote) Leishmania parasite in the host.
Small molecule screens with Leishmania spp. have often been performed with the easily cultured but less clinically-relevant extracellular insect (promastigote) form of the parasite [10]–[13]. Axenic amastigotes, adapted to replicate at acidic pH and elevated temperature as extracellular parasites, are also used as a screening resource [14], [15] but are not ideal as they are not derived from the parasitophorous vacuole (PV), the intracellular compartment in which amastigotes reside within host cells [16]. Indeed, the differing gene expression and proteomic profiles presented by promastigotes, axenic amastigotes and intracellular amastigotes of several Leishmania species suggest differing molecular profiles during parasite stage differentiation. For example, the mRNA expression profiles of L. mexicana promastigotes and axenic amastigotes are remarkably similar whereas when either is compared to the mRNA profile of lesion-derived amastigotes, different mRNAs are significantly up- or down-regulated [17]. Similarly, analysis of global mRNA expression profiles of L. infantum axenic and intracellular amastigotes isolated from cultured human (THP-1) macrophages reveal very few differentially expressed genes in common between the stages [18]. At the protein level, comparison of promastigotes with intracellular amastigotes in L. mexicana reveals several proteins upregulated solely in amastigotes, including enzymes linked to respiration/energy metabolism, fatty acid metabolism and protein synthesis, and proteins involved in stress responses [19]. Upregulated fatty acid metabolism has also been described in comparisons of lesion-derived L. mexicana amastigotes and dividing promastigotes [20].
These observations confirm that small compound testing should ideally be focused on the clinically-relevant parasite stage, the intracellular amastigote. This conclusion is supported by a recent study comparing compound efficacy against extracellular promastigotes and intracellular amastigotes; only a small number of those compounds active against the extracellular life cycle stages were also active against the intracellular stage [21]. In addition, progressing a promastigote screening cascade towards intracellular screening has been shown to not only identify false positives but also exclude compounds that are active against the relevant intracellular stage [22]. It is possible that the study design may have selected for compounds active against differentiating parasites rather than true intracellular amastigotes in this case; compounds were added immediately after removing free parasites from macrophages infected over a period of 4 h with L. donovani promastigotes, a stage at which amastigote differentiation would not have been complete. As an additional complication, compounds that are active against one Leishmania species may not be active against another, if the observed differences in gene expression profiles [23] are reflected in their proteomes and metabolomes [24], [25].
High-throughput image-based phenotypic screens of intracellular amastigotes have gained support as the current preferred approach for drug discovery against Leishmania spp. [21], [22] and undoubtedly provide a superior approach for screening small compounds and conducting lead-optimization. However, sophisticated imaging resources are not readily available in many lab settings, preventing widespread adoption of these techniques. In addition, some image-based assays rely on genetically-modified parasites carrying a selectable marker, a feature that can compromise the identification of new compounds by conferring resistance itself, as reported for neomycin and paromomycin in Saccharomyces cerevisiae [26]. The generation of mutants without selectable markers should overcome this problem [27]. The expression levels of fluorescent or bioluminescent reporters encoded on episomes is dependent on their episomal copy number but this can also vary in a clonal population [28], potentially affecting the read-out to determine the compound efficacy. Similarly, genetic modification of parasites might change their biological properties and subsequently, their sensitivity to test compounds [29].
Jain and colleagues [30] recently approached the lack of a non–image based method for intracellular amastigote screening by developing an assay in which L. donovani promastigotes were used to infect cultured THP-1 macrophages, differentiated amastigotes subsequently released by SDS lysis and, following transformation back to promastigotes and growth for two days, quantified using Alamar blue. In parallel work, driven by the need to advance a specific drug development project for the leishmaniases, we have adopted a similar assay, although using clinically-relevant amastigotes for infection of bone marrow-derived macrophages together with saponin lysis, and subsequently validated the method using the gold standard drugs against VL, amphotericin B and miltefosine.
Utilising this assay, we have extended work on the development of myristoyl-CoA:protein N-myristoyltransferase (NMT) as a promising candidate for the development of anti-kinetoplastid drugs [31]–[34]. In collaboration with Pfizer, we recently published the results of a high-throughput screen against L. donovani NMT that resulted in the identification of four chemically diverse and selective series [35]. Here, we present results generated with the novel NMT inhibitors identified in the Pfizer screen [35], together with several T. brucei NMT inhibitors [36], [37]. Several of the latter compounds were reported to have similar activity against L. major NMT and we have also found them to be potent inhibitors of L. donovani NMT. Structural biology studies on the compounds derived from the Pfizer screen have been published recently [38]; structure-activity-relationships and synthetic details are being published separately [39].
Materials and Methods
Animals and parasites
BALB/c mice were obtained from Charles River (Margate, UK). Rag-2−/− mice were bred in-house, housed under specific pathogen-free conditions and used at 6–12 weeks of age. The Ethiopian strain of Leishmania donovani (MHOM/ET/67/HU3, also known as LV9) was maintained by serial passage in Rag-2−/− mice. Amastigotes were isolated from infected spleens by homogenization and saponin lysis, as previously described [40]. Mice were infected with 3×107 L. donovani amastigotes intravenously (i.v.) via the tail vein in 200 µl of RPMI 1640 (GIBCO, Paisley, UK). All experiments were approved by the University of York Animal Procedures and Ethics Committee and performed under UK Home Office licence (‘Immunology and Immunopathology of Leishmaniasis’ Ref # PPL 60/4377).
In vitro infection
Macrophages (BMDM) were differentiated from bone marrow of 6–8 weeks old female BALB/c mice as described previously [41] and plated out at 4.2×104 cells/per well in 96-well plates. BMDMs were adhered for at least 4 h and infected overnight at a multiplicity of infection of 15 with freshly isolated L. donovani amastigotes (see above). BMDMs were maintained in DMEM supplemented with 4 mM L-Glutamine (both GIBCO, Paisley, UK) and 4% L929-cell conditioned medium. All experiments were performed at 37°C and 5% CO2.
Reference compounds and determination of parasite burden
The anti-leishmanial reference drugs amphotericin B (Fungizone, kindly provided by Vanessa Yardley, London School of Hygiene and Tropical Medicine) and miltefosine (Sigma, #M9198) were used, the former reconstituted according to the manufacturer's instructions. Miltefosine was dissolved in deionised water. Compounds were prepared at 2× concentration in a 7-point threefold dilution series and tested in triplicate. The highest concentrations used were 30 µM (miltefosine) and 1.2 µM (amphotericin B). Infected macrophages were incubated with the compounds for 72 h. Infected and uninfected cells were then washed twice with DMEM before lysis with 100 µl 2 mg·ml−1 saponin in DMEM for 5 min at room temperature (RT). Lysis was stopped by addition of 100 µl undiluted fetal calf serum (FCS).
Plates were centrifuged at 2200×g (Heraeus Multifuge 3SR Plus) for 5 min at RT and the medium carefully removed. For all manipulations of this type, as much as possible of the supernatant was carefully but swiftly removed with an 8-channel pipette, placing the pipette tips into the corners of the wells in an angled plate and quickly aspirating until virtually all liquid was removed. Fresh medium was quickly replaced to prevent cells drying out. In this case, 200 µl RPMI 1640 medium (#22409-015, GIBCO, Paisley, UK) supplemented with 20% heat-inactivated FCS, 100 µM adenine, 20 mM 2-[N-morpholino] ethanesulphonic acid (pH 5.5), 5 µM hemin, 3 µM biopterin, 1 µM biotin, penicillin (100 U/ml) and streptomycin (100 µg/ml) were then added, at final pH 6.7, and incubation continued at 26°C. After 96 h, 20 µl Alamar blue (Trek Diagnostics) was added and incubation continued at 26°C for a further 6 h or until the Alamar blue was reduced sufficiently (as determined visually by its conversion from oxidised state blue to reduced state pink). Further reduction was stopped by addition of 20 µl 8% formaldehyde solution. Fluorescence was measured using a POLARstar Optima (BMG Labtech) plate reader (ex. 544 nm, em. 590 nm) with the lid removed.
Drug dilutions for the extracellular assays were prepared in the same way but in supplemented RPMI medium. Assays were performed in triplicate with 4×105 L. donovani amastigotes (freshly isolated as described above) per well per 96-well plate, plating 100 µl of parasite suspension per well first and then adding the drug dilution to be tested. Sealed 96 well plates were then placed at 26°C. After 72 h, 20 µl Alamar blue was added and the fluorescence measured 6 h later.
Host cell cytotoxicity was determined by seeding out as above; compounds were added at 2× concentration in a 7-point threefold dilution series and tested in duplicate. After 72 h, 1/10 vol Alamar blue was added and the fluorescence measured 6 h later. To determine LD50 values, the following highest concentrations were used based on the L. donovani NMT potency or, if necessary, lowered due to compound insolubility at a higher concentration: 150 µM – IMP-0000556; 90 µM - IMP-0000197, DDD100887 and DDD86211; 45 µM - DDD85646, IMP-0000083, IMP-0000195.
Drug activities were determined by calculating the percentage of the measured fluorescence values of treated compared to untreated cells. GraphPad Prism (ver. 5) was used to plot dose response curves, to calculate EC50 values by non-linear regression with variable slope, to calculate correlation coefficients of curves and for statistical analysis. Table 1 lists the mean EC50 values and standard deviation for each experiment, calculated using GraphPad Prism (ver. 5).
Table 1. Activity of gold standard drugs after 3-day treatment against intra- and extracellular L. donovani amastigotes.
| EC50 ± SD intracellular L. donovani amastigotes | EC50 ± SD extracellular L. donovani amastigotes* | |
| Amphotericin B | 30±9 nM | 50±11 nM |
| Miltefosine | 1.38±0.06 µM | 7.85±0.5 µM |
*Assay was set up with amastigotes isolated from spleens of infected Rag2−/− mice with subsequent incubation at 26°C. All assays were run in triplicate with biological repeats (intracellular amphotericin B, n = 4; intracellular miltefosine, n = 3; extracellular amphotericin B, n = 3; extracellular miltefosine, n = 2).
Results
Establishing the L. donovani amastigote assay
To avoid reliance on fluorescent/luminescent parasites, sophisticated microscopy equipment or counting parasites on Giemsa-stained slides, we developed an assay for drug-testing similar to that already described [30] in which surviving parasites are released from infected macrophages, allowed to differentiate into promastigotes and then enumerated. In our assay, bone marrow-derived macrophages are infected with L. donovani amastigotes and incubated for 72 h in the presence or absence of test or control compounds. Macrophages are then subject to conditional lysis with saponin, a milder treatment than the 0.05% SDS used by Jain et al. [30], followed by incubation for up to 4 days in promastigote medium at 26°C. During this time, the amastigotes trapped in the macrophage debris transform to promastigotes and these break free (due to flagellar motion) and commence replication. In the presence of Alamar blue, its active component, resazurin, is then reduced to resorufin by the metabolically active parasites. This reduction is directly dependent upon parasite numbers (see Fig. 1). The transformation from amastigote to promastigote and subsequent proliferation is necessary to amplify the signal obtained by the Alamar blue reduction. This allows determination of the parasite burden - that is, the respective parasite load on the day of lysis, assuming an equal proliferation rate irrespective of initial parasite numbers.
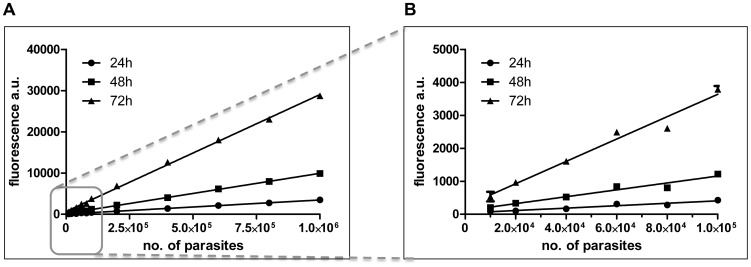
Figure 1. Linear correlation of the number of L. donovani amastigotes and the reduction of Alamar blue.
L. donovani amastigotes were isolated from the spleen of an infected Rag-2 −/− mouse. Up to 106 (A) or 105 (B) parasites were suspended in 200 µl/well in supplemented RPMI, Alamar blue was added and the plate incubated at 26°C. At the indicated time points, the fluorescence per well was determined and plotted over the initial number of parasites/well. The correlations between Alamar blue conversion and the initial parasite numbers are highly significant: P<0.0001 as shown by linear regression analysis (R224h– 0.9979, R248h – 0.9991, R272h – 0.9984).
Firstly, a direct correlation was established between parasite numbers after transformation and the resulting fluorescence from the reduced Alamar blue. This was done by seeding freshly isolated splenic amastigotes in supplemented RPMI into a 96-well plate. Alamar blue was added and the parasites incubated at 26°C. Transformation occurred over time and the resulting promastigotes started to replicate, resulting in Alamar blue reduction. Fluorescence was measured every 24 h to monitor this reduction of the dye relative to the parasite numbers per well. As shown in Fig. 1, the reduction of Alamar blue was directly proportional to the numbers of viable cells over at least two orders of magnitude (initial cell numbers 104–106) for at least 72 h (Fig. 1). This finding confirms the assumption that the proliferation rate is equivalent between samples, irrespective of the initial number of parasites.
Linearity was tested with up to 106 L. donovani amastigotes (Fig. 1), which in our hands reflected an idealized condition as these cell numbers were not achieved upon conditional lysis of the macrophages. This correlation should however be verified prior to implementation of this assay as it is dependent on the parasite strain/s as well as the macrophage cell lines used. It is important to stress that the protocol described here is only evaluating the anti-leishmanial activity of compounds against the intracellular lifecycle stage. Upon lysis and freeing of amastigotes, the transformation to promastigotes is not affected by the compounds as these are no longer present at this stage of the protocol.
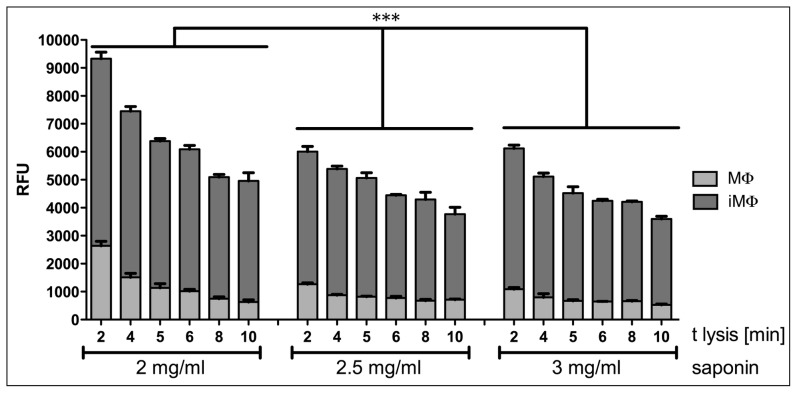
Secondly, uninfected and infected macrophages were lysed with varying amounts of saponin (2, 2.5 and 3 mg·ml−1), a milder detergent compared to SDS, dissolved in non-supplemented DMEM. As shown in Fig. 2, in our hands amastigotes were most effectively freed from macrophages, with limited survival of the latter, using 100 µl of 2 mg·ml−1 saponin and incubation for 5 min, prior to lysis termination by addition of 100 µl of undiluted FCS. Analysis using two-way ANOVA with Bonferoni post test on the fluorescence intensity difference between infected and uninfected macrophages showed that significantly more amastigotes were freed from macrophages when using 2 mg·ml−1 saponin compared to higher concentrations; there was no significant difference between 2.5 and 3 mg·ml−1 saponin lysis. Using this lysis method, a 5-fold greater signal from freed amastigotes over live macrophages was achieved. To determine the lysis efficiency (i.e. the background level) and to compensate for inter-experiment variation, uninfected macrophages were monitored in all experiments carried out using this method.
Figure 2. Release of parasites from infected macrophages in 96-well plates.
Bone marrow derived macrophages were infected overnight with L. donovani amastigotes. Cells were incubated for a further 3 days and then treated with DMEM containing the indicated concentration of saponin. Supplemented RPMI was added to the released amastigotes and plates were further incubated for 4 days at 26°C before addition of Alamar blue and subsequent measurement of fluorescence in a plate reader. The survival of freed amastigotes is dependent on the concentration of saponin used and the time of lysis. A 5-fold greater signal of reduced Alamar blue from freed amastigotes over uninfected macrophages was achieved with 2 mg·ml-1 saponin for 5 min; this concentration was used for all other experiments performed. All samples were run in triplicate; one representative experiment is shown. MΦ, uninfected macrophages; iMΦ, infected macrophages. *** denotes P<0.0001 as determined by a two-way ANOVA.
Validating the amastigote assay with known drugs
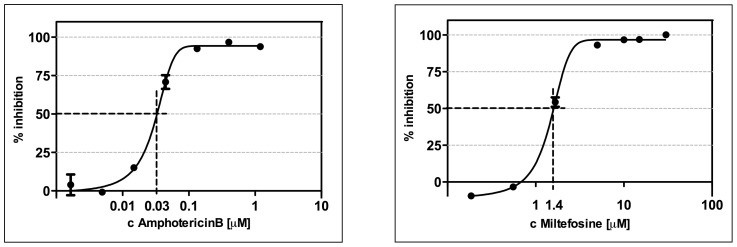
To confirm the efficacy of this assay, that uses primary macrophages infected with freshly isolated amastigotes rather than a macrophage-like cell line infected with promastigotes [30], dose response curves and EC50 values were generated for amphotericin B and miltefosine (the gold standard drugs for the treatment of VL [4]). The EC50 values were 30 nM and 1.38 µM respectively, correlating well with the data described by other groups [42]–[44]; see Fig. 3 and Table 1.
Figure 3. Dose response curves for amphotericin B and miltefosine against intracellular L. donovani amastigotes.
Parasites were released by conditional lysis with saponin from bone marrow derived macrophages of BALB/c mice after 72 h incubation with anti-leishmanial standard drugs. Using this method, the measured activities of amphotericin B (EC50 29 nM) and miltefosine EC50 (1.45 µM) were reproducible and comparable to reported values against intracellular L. donovani (43–45). Representative plots for amphotericin B (n = 4) and miltefosine (n = 3) are shown. (R2 AmphoB – 0.9905, R2 Mil – 0.9963).
Amphotericin B and miltefosine were also tested on amastigotes freshly isolated from mouse spleens, thereby avoiding any in vitro macrophage infection. These assays, referred to as the “extracellular assays” hereafter, resulted in EC50 values of 50 nM and 7.85 µM respectively (dose response curves not shown, Table 1) and were also in good alignment with values described elsewhere [43], [45].
Testing small compounds inhibitory to NMT
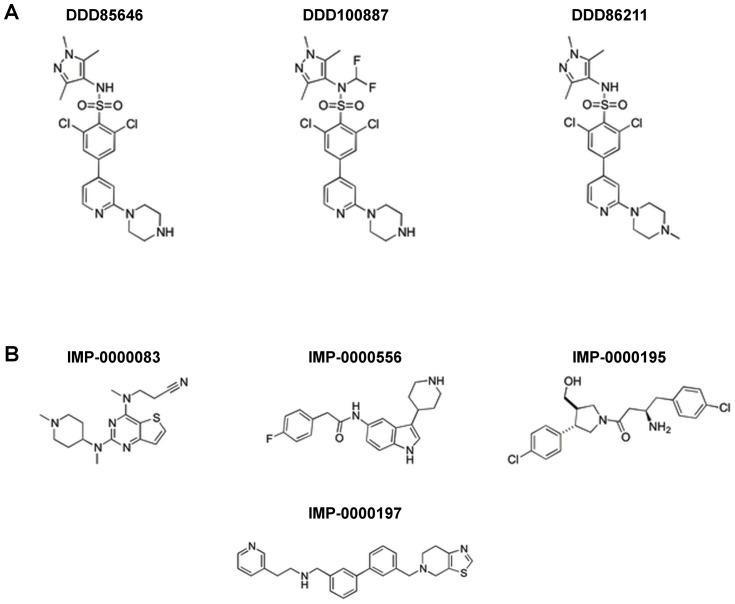
NMT inhibitory compounds were tested against L. donovani amastigotes in both the extracellular and intracellular assays described above. The focus was on the small compounds identified by high throughput screening against L. donovani NMT and found to have selectivity over the human NMTs (Fig. 4B) [35]. In addition, we profiled several examples from a series of T. brucei NMT inhibitors identified by the Dundee Drug Discovery Unit, since this series has been reported to also inhibit L. major NMT [36]. The original small molecule showing the highest activity against the NMT of bloodstream stages of T. brucei in vivo (DDD85646; Fig. 4A), identified by Frearson et al. [31], and two further compounds derived from this TbNMT inhibitor [37] (Fig. 4A, Fig. 5 and Table 2) were resynthesized and tested. The activities of all of these resynthesised compounds were confirmed by testing on purified L. donovani NMT (IC50 values in Tables 2 and 3), using the assay described by Goncalves and colleagues [46]. The figures generated were in agreement with those reported previously [35], [36]. The compounds were then progressed to evaluation of their cellular activity.
Figure 4. Chemical structures of NMT inhibitors.
(A) Resynthesised T. brucei inhibitors identified by the Dundee Drug Discovery Unit as also inhibiting L. major NMT [31], [37]. Results summarized in Table 2. (B) Selective inhibitors identified by high throughput screening against L. donovani NMT [35]. Results summarised in Table 3.
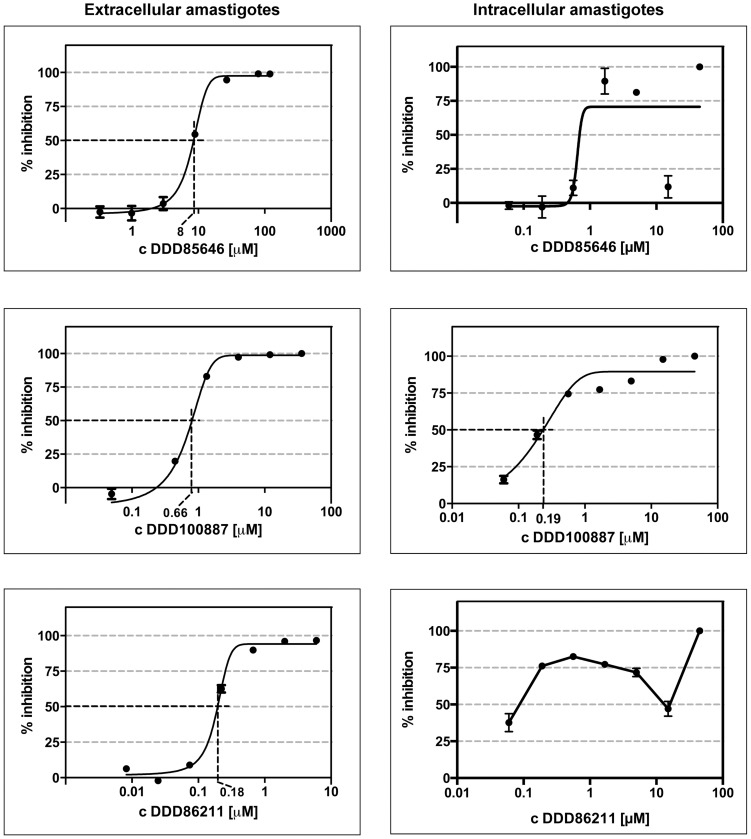
Figure 5. Cellular activity of compounds with good potency in the extracellular but varying potency in the intracellular model.
All three compounds tested showed cellular activity on the intracellular and extracellular L. donovani amastigotes. When tested against intracellular amastigotes, only DDD100887 generated a reproducible dose-response curve. It was not possible to generate dose-response curves for DDD85646 and DDD86211 as both compounds resulted in an reproducible “activity valley” at higher concentrations before resulting in complete killing of the parasites at the highest concentration tested (see text for details). One representative experiment is shown for each compound. The correlation coefficients for the extracellular amastigotes were R2>0.98, with almost all very close to or >0.99. The correlation coefficient for intracellular DDD100887 is R2 0.9212.
Table 2. Activity values/properties of NMT inhibitors from DDD85646 series.* .
| IC50 L. donovani NMT | EC50 extracellular L. donovani amastigotes | EC50 intracellular L. donovani amastigotes | LD50 bone marrow derived macrophages | pKa | cLogP | Hydrogen bond donors | |
| DDD85646 1 | 3.6 nM | 8 µM | n.p. | >45 µM | 8.8 | 2.0 | 2 |
| DDD100887 2 | 20 nM | 660 nM | 190 nM | 23 µM | 8.8 | 4.2 | 1 |
| DDD86211 2 | 7.3 nM | 180 nM | n.p. | >45 µM | 6.5 | 2.4 | 1 |
Table 3. Activity values/properties of NMT inhibitors identified in Pfizer high-throughput screen.1 , 2 .
| IC50 L.don. NMT | EC50 extracellular L. donovani amastigotes | LD50 bone marrow derived macrophages | pKa | cLogP | Hydrogen bond donors | |
| IMP-0000083 (analogue of PF-00349412) | 281 nM | 24 µM | >45 µM | 8.4 | 2.6 | 0 |
| IMP-0000556 (PF-03393842) | 316 nM | no activity up to 30 µM | >45 µM | 10.0 | 3.1 | 2 |
| IMP-0000195 (PF-03402623) | 77 nM | 12 µM | 12 µM | 8.9 | 2.9 | 2 |
| IMP-0000197 (PF-00075634) | 914 nM | 16 µM | 50 µM | 9.8 | 4.5 | 1 |
Ref 35.
structures shown in Fig. 4B.
Hit compounds derived from the high-throughput screen were resynthesized and their enzyme activity confirmed in our assays. A range of different profiles were observed in the cellular assays with IMP-0000556 showing no cellular activity up to 30 µM whereas IMP-0000197 gave an EC50 of 16 µM in the extracellular assay. IMP-0000195, the most potent LdNMT inhibitor from the HTS screen, had a moderate cellular activity (EC50 12 µM) but was equally toxic to macrophages. Of the HTS-derived compounds, IMP-0000083 (a close analogue of the Pfizer hit) has the most promising profile. Although ∼4-fold less potent in its inhibition of LdNMT than IMP-0000195, the activity difference was reduced at the cellular level, while showing no toxicity to host cells (Table 3).
The re-synthesised version of the original T. brucei NMT inhibitor, DDD85646, as well as derivatives thereof (DDD86211 and DDD100887), were tested in both the extracellular assay and the intracellular assay (Fig. 5). DDD85646 was the most potent LdNMT inhibitor in the enzyme assay, followed by DDD86211 and DDD100887. However, at the cellular level, DDD85646 was the least active (EC50 8 µM in the extracellular amastigote assay). The cellular activity of DDD86211 against extracellular amastigotes (EC50 180 nM) was impressive, while DDD100887 was also effective (EC50 660 nM). All three compounds also had some activity against intracellular L. donovani amastigotes (Fig. 5 and Table 2). However, only compound DDD100887 generated a genuine dose-response curve with an EC50 value of 190 nM. In comparison, DDD85646 and DDD86211 caused an initial concentration-dependent increase in anti-leishmanial activity followed by a reproducible decrease at 15 µM. At the highest concentration tested, 45 µM, both compounds exerted a strong cytotoxic effect and all amastigotes were cleared from the macrophages (Fig. 5). For both DDD85646 and DDD86211, this “activity valley” was a reproducible effect over successive experiments.
Discussion
We have developed a robust assay to screen for inhibitory compounds active against intracellular Leishmania, independent of automated fluorescent microscopy or the time-consuming counting of parasites on Giemsa-stained slides. It is similar to a previously reported assay [30] but uses isolated splenic amastigotes rather than cultured promastigotes for the initial infection of primary macrophages and subsequent reproducible lysis by the mild detergent saponin over a 5 min period, rather than harsher SDS treatment for 30 sec. We have focused on L. donovani as our test organism here, given its importance as the causative agent of the most severe form of leishmaniases, but other Leishmania species and/or host cells could easily be used in this assay format.
Key steps in optimising the assay included determining the best lysis conditions for parasite release while retaining only marginal background signal from any surviving macrophages. For the L. donovani/bone marrow-derived macrophage model used here, lysis with 2 mg·ml−1 saponin in DMEM for 5 min at room temperature gave the best results, with amastigote integrity compromised at higher saponin concentrations (Fig. 2). After macrophage lysis and centrifugation, the amastigote-containing supernatant was supplemented with RPMI medium and incubated at 26°C, triggering amastigote to promastigote transformation and subsequent parasite proliferation. This second stage of the assay was critical for amplification of the signal over background; the number of amastigotes present on the day of lysis was insufficient to achieve this result. By performing this incubation step, the resulting signal is dependent on the initial amastigote number as long as the differentiated promastigotes are in the logarithmic growth phase (Fig. 1). In our hands, this phase continued for at least 4 days, by which time the signal was sufficiently amplified to generate highly reproducible results. While an assay requiring 9 days for completion may be considered non-optimal, this protocol allows a number of compounds to be tested sequentially (possible at medium through-put in culture wells) and has the advantage that counting of parasites is not required rather than the admittedly more exacting counting of Giemsa-stained amastigotes in infected macrophages on slides. Thus results between laboratories should be subject to less variability. A further advantage of this method is its lack of dependence on genetically modified parasites, facilitating compound testing on both resistant and sensitive parasite field isolates.
We want to highlight that both this assay and the Jain assay [30] are apparently good alternatives for screening inhibitory compounds active against intracellular Leishmania parasites, being independent of automated fluorescent microscopy or Giemsa-staining. Our assay is straightforward to establish, highly reproducible and has the potential for semi-automation to facilitate higher through-put screening.
Validation of this assay has been achieved using the anti-leishmanial gold standard drugs, amphotericin B and miltefosine. The resulting EC50 values are in good agreement with those reported against L. donovani elsewhere [42]–[44] and provide confidence that the assay can be used successfully to screen new compounds for activity against Leishmania amastigotes.
To this end, we progressed to testing a number of available NMT inhibitors, including those with advanced lead-like activity in T. brucei blood stream parasites, potent inhibitory activity against L. major NMT [31], [36] and the initial hits from the recent Pfizer HTS screen [35]. These were first tested against purified recombinant LdNMT to confirm their potency and then tested in the extracellular model described above, in which isolated splenic L. donovani amastigotes were incubated in the presence of inhibitor at 26°C. This method has the advantage of using host-derived amastigotes as the starting point as opposed to axenic amastigotes, which differ from intracellular amastigotes [17], [18], especially in their metabolism [18]. Conversely, the model could be problematic for general compound screening, introducing a bias towards compounds targeting parasite differentiation or promastigote replication. The final stage of testing used the new intracellular assay.
Focusing firstly on the TbNMT inhibitors DDD85646, DDD100887 and DDD86211 [31], [36], these compounds gave IC50 values of 4.4 nM, 20 nM and 7.3 nM respectively against L. donovani NMT. However, surprisingly, DDD85646 translated the least effectively from enzyme to cell, with a sharp drop in potency against L. donovani extracellular amastigotes, resulting in an EC50 of 8 µM.
Compounds DDD100887 and DDD86211 were more effective, generating EC50 values of 660 nM and 180 nM, respectively. Thus, the chemical modifications introduced into these compounds (moderation of their basicity or reduction in the number of hydrogen bond donors) resulted in an improved translation of enzyme to cell activity, perhaps due to better cellular penetration as compared to DDD85646 [36]. These results were partially reflected in the intracellular model data but the compounds were ∼10-fold (in the case of DDD100887) less active when compared to the purified NMT enzyme, the cellular target. This was the only compound for which a genuine dose-response curve could be plotted and an EC50 value calculated. Both DDD85646 and DDD86211 did not result in an expected dose-response relationship, precluding the EC50 calculation. Initially the compounds displayed cytotoxic effects before an “activity valley” was detected at higher concentrations. Both compounds were cytotoxic, at 45 µM, however, with all amastigotes cleared from the macrophages at this compound concentration. We currently have no rational explanation for the occurrence of this activity valley.
The remarkably good translation between NMT activity and cellular activity of DDD85646 observed in T. brucei blood stream parasites [31] was not observed for L. donovani in either the extra- or the intracellular model. Procyclic (insect) stages of T. brucei parasites were also much less sensitive to DDD85646 (pers. comm. H.P. Price), perhaps due to the different rates of endocytosis between the two parasite stages (with bloodstream trypanosomes having an unusually high rate [47]–[49]. Endocytosis rates are also slower in Leishmania species when compared to the bloodstream form of T brucei, although recent data suggest up-regulated endocytosis in L. mexicana amastigotes [50]. A similar lack of translation from enzyme to cellular activity has been reported for L. major CRK3 kinase inhibitors [51], [52], perhaps reflecting more general problems in targeting amastigote stages of Leishmania. These might include uptake and delivery of compounds into the macrophage, their chemical modification as a result of the acidic environment of the parasitophorous vacuole and/or their export from the phagolysosome via diffusion or active transport.
The second set of compounds tested in this study, derived from the Pfizer screen [35] were no better than the optimised Dundee derivative compounds in the extracellular amastigote assay. However, this analysis aided identification of issues to be addressed in a hit-to-lead development programme. Thus, IMP-0000195 (IC50 76.5 nM, EC50 12 µM) was the best enzyme inhibitor but also showed host cell toxicity and no selectivity. IMP-0000556 did not result in any leishmanicidal activity, probably due to insufficient potency - this is the compound with the least activity in the enzyme assay. More promisingly, IMP-0000083 and IMP-0000197 showed moderate cellular activity in the extracellular assay with no host cell cytotoxicity up to at least 45 µM. However, these two compounds showed no activity in the intracellular assay. Clearly, more work is required to improve both the potency and to optimise the physicochemical properties of these initial hits to improve cellular activity, developments that are currently in progress.
In summary, we report a robust assay for screening compounds against intracellular Leishmania amastigotes that should enable others to screen natural product or other libraries for identification of compounds active against the clinically-relevant life cycle stage. Leishmania NMT inhibitors are in early development but the compounds described here show good potential for further progression with respect to selectivity and cellular potency. In addition, methods that enhance compound delivery into both the parasitophorous vacuole and the intracellular parasites require further investigation. An optimised delivery system might increase the therapeutic “window”, thereby greatly improving the modest selectivity or potency observed so far.
Acknowledgments
We thank Simon Croft and Vanessa Yardley for helpful discussions and members of the Kaye lab (University of York) for advice on working with the in vivo model of VL. We also thank Tom Waugh for synthetic chemistry support.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Wellcome Trust (grant no. 087792). The funders had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript.
References
- 1. El-Sayed NM, Myler PJ, Blandin G, Berriman M, Crabtree J, et al. (2005) Comparative genomics of trypanosomatid parasitic protozoa. Science 309: 404–409. [DOI] [PubMed] [Google Scholar]
- 2. Aslett M, Aurrecoechea C, Berriman M, Brestelli J, Brunk BP, et al. (2010) TriTrypDB: a functional genomic resource for the Trypanosomatidae. Nucleic Acids Res 38: D457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stuart K, Brun R, Croft S, Fairlamb A, Gurtler RE, et al. (2008) Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest 118: 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barrett MP, Croft SL (2012) Management of trypanosomiasis and leishmaniasis. Br Med Bull 104: 175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bahia MT, de Andrade IM, Martins TA, do Nascimento AF, Diniz Lde F, et al. (2012) Fexinidazole: a potential new drug candidate for Chagas disease. PLoS Negl Trop Dis 6: e1870 doi: 1810.1371/journal.pntd.0001870. Epub 0002012 Nov 0001871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wyllie S, Patterson S, Stojanovski L, Simeons FR, Norval S, et al. (2012) The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis. Sci Transl Med 4: 119re111 doi: 110.1126/scitranslmed.3003326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pinazo MJ, Espinosa G, Gallego M, Lopez-Chejade PL, Urbina JA, et al. (2010) Successful treatment with posaconazole of a patient with chronic Chagas disease and systemic lupus erythematosus. Am J Trop Med Hyg 82: 583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diniz Lde F, Caldas IS, Guedes PM, Crepalde G, de Lana M, et al. (2010) Effects of ravuconazole treatment on parasite load and immune response in dogs experimentally infected with Trypanosoma cruzi. Antimicrob Agents Chemother 54: 2979–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Urbina JA, Payares G, Sanoja C, Lira R, Romanha AJ (2003) In vitro and in vivo activities of ravuconazole on Trypanosoma cruzi, the causative agent of Chagas disease. Int J Antimicrob Agents 21: 27–38. [DOI] [PubMed] [Google Scholar]
- 10. Vila-Nova NS, de Morais SM, Falcao MJ, Alcantara TT, Ferreira PA, et al. (2013) Different susceptibilities of Leishmania spp. promastigotes to the Annona muricata acetogenins annonacinone and corossolone, and the Platymiscium floribundum coumarin scoparone. Exp Parasitol 133: 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benitez J, Cavalcanti de Queiroz A, Correia I, Alves MA, Alexandre-Moreira MS, et al. (2013) New oxidovanadium(IV) N-acylhydrazone complexes: Promising antileishmanial and antitrypanosomal agents. Eur J Med Chem 62C: 20–27. [DOI] [PubMed] [Google Scholar]
- 12. Chan MM, Bulinski JC, Chang KP, Fong D (2003) A microplate assay for Leishmania amazonensis promastigotes expressing multimeric green fluorescent protein. Parasitol Res 89: 266–271. [DOI] [PubMed] [Google Scholar]
- 13. Ahmed SB, Sghaier RM, Guesmi F, Kaabi B, Mejri M, et al. (2011) Evaluation of antileishmanial, cytotoxic and antioxidant activities of essential oils extracted from plants issued from the leishmaniasis-endemic region of Sned (Tunisia). Nat Prod Res 25: 1195–1201. [DOI] [PubMed] [Google Scholar]
- 14. Sereno D, Lemesre JL (1997) Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob Agents Chemother 41: 972–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Callahan HL, Portal AC, Devereaux R, Grogl M (1997) An axenic amastigote system for drug screening. Antimicrob Agents Chemother 41: 818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antoine JC, Prina E, Lang T, Courret N (1998) The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol 6: 392–401. [DOI] [PubMed] [Google Scholar]
- 17. Holzer TR, McMaster WR, Forney JD (2006) Expression profiling by whole-genome interspecies microarray hybridization reveals differential gene expression in procyclic promastigotes, lesion-derived amastigotes, and axenic amastigotes in Leishmania mexicana. Mol Biochem Parasitol 146: 198–218. [DOI] [PubMed] [Google Scholar]
- 18. Rochette A, Raymond F, Corbeil J, Ouellette M, Papadopoulou B (2009) Whole-genome comparative RNA expression profiling of axenic and intracellular amastigote forms of Leishmania infantum. Mol Biochem Parasitol 165: 32–47. [DOI] [PubMed] [Google Scholar]
- 19. Paape D, Lippuner C, Schmid M, Ackermann R, Barrios-Llerena ME, et al. (2008) Transgenic, fluorescent Leishmania mexicana allow direct analysis of the proteome of intracellular amastigotes. Mol Cell Proteomics 7: 1688–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hart DT, Coombs GH (1982) Leishmania mexicana: energy metabolism of amastigotes and promastigotes. Exp Parasitol 54: 397–409. [DOI] [PubMed] [Google Scholar]
- 21. Siqueira-Neto JL, Song OR, Oh H, Sohn JH, Yang G, et al. (2010) Antileishmanial high-throughput drug screening reveals drug candidates with new scaffolds. PLoS Negl Trop Dis 4: e675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Muylder G, Ang KK, Chen S, Arkin MR, Engel JC, et al. (2011) A screen against Leishmania intracellular amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl Trop Dis 5: e1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rochette A, Raymond F, Ubeda JM, Smith M, Messier N, et al. (2008) Genome-wide gene expression profiling analysis of Leishmania major and Leishmania infantum developmental stages reveals substantial differences between the two species. BMC Genomics 9: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scheltema RA, Decuypere S, T'Kindt R, Dujardin JC, Coombs GH, et al. (2010) The potential of metabolomics for Leishmania research in the post-genomics era. Parasitology 137: 1291–1302. [DOI] [PubMed] [Google Scholar]
- 25. Saunders EC, Ng WW, Kloehn J, Chambers JM, Ng M, et al. (2014) Induction of a stringent metabolic response in intracellular stages of Leishmania mexicana leads to increased dependence on mitochondrial metabolism. PLoS Pathog 10: e1003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kutzleb R, Schweyen RJ, Kaudewitz F (1973) Extrachromosomal inheritance of paromomycin resistance in Saccharomyces cerevisiae. Genetic and biochemical characterization of mutants. Mol Gen Genet 125: 91–98. [DOI] [PubMed] [Google Scholar]
- 27. Denise H, Coombs GH, Mottram JC (2004) Generation of Leishmania mutants lacking antibiotic resistance genes using a versatile hit-and-run targeting strategy. FEMS Microbiol Lett 235: 89–94. [DOI] [PubMed] [Google Scholar]
- 28. Misslitz A, Mottram JC, Overath P, Aebischer T (2000) Targeted integration into a rRNA locus results in uniform and high level expression of transgenes in Leishmania amastigotes. Mol Biochem Parasitol 107: 251–261. [DOI] [PubMed] [Google Scholar]
- 29. Sereno D, Cordeiro da Silva A, Mathieu-Daude F, Ouaissi A (2007) Advances and perspectives in Leishmania cell based drug-screening procedures. Parasitol Int 56: 3–7. [DOI] [PubMed] [Google Scholar]
- 30. Jain SK, Sahu R, Walker LA, Tekwani BL (2012) A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J Vis Exp 70 4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frearson JA, Brand S, McElroy SP, Cleghorn LA, Smid O, et al. (2010) N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nature 464: 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Price HP, Menon MR, Panethymitaki C, Goulding D, McKean PG, et al. (2003) Myristoyl-CoA:Protein N-Myristoyltransferase, an Essential Enzyme and Potential Drug Target in Kinetoplastid Parasites. J Biol Chem 278: 7206–7214. [DOI] [PubMed] [Google Scholar]
- 33. Gelb MH, Van Voorhis WC, Buckner FS, Yokoyama K, Eastman R, et al. (2003) Protein farnesyl and N-myristoyl transferases: piggy-back medicinal chemistry targets for the development of antitrypanosomatid and antimalarial therapeutics. Molecular and Biochemical Parasitology 126: 155–163. [DOI] [PubMed] [Google Scholar]
- 34. Tate EW, Bell AS, Rackham MD, Wright MH (2013) N-Myristoyltransferase as a potential drug target in malaria and leishmaniasis. Parasitology 24: 1–13. [DOI] [PubMed] [Google Scholar]
- 35. Bell AS, Mills JE, Williams GP, Brannigan JA, Wilkinson AJ, et al. (2012) Selective inhibitors of protozoan protein N-myristoyltransferases as starting points for tropical disease medicinal chemistry programs. PLoS Negl Trop Dis 6: e1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brand S, Cleghorn LA, McElroy SP, Robinson DA, Smith VC, et al. (2011) Discovery of a novel class of orally active trypanocidal N-myristoyltransferase inhibitors. J Med Chem 55: 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. WO2010/026365 (2010) N-myristoyl transferase inhibitors. International patent [Google Scholar]
- 38. Brannigan JA, Roberts SM, Bell AS, Hutton JA, Hodgkinson MR, et al. (2014) Diverse modes of binding in structures of Leishmania major N-myristoyltransferase with selective inhibitors. IUCrJ 1: 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hutton JA, Goncalves V, Brannigan JA, Paape D, Wright MH, et al. (2014) Structure-based design of potent and selective leishmania N-myristoyltransferase inhibitors. J Med Chem 57: 8664–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smelt SC, Engwerda CR, McCrossen M, Kaye PM (1997) Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J Immunol 158: 3813–3821. [PubMed] [Google Scholar]
- 41. Weinheber N, Wolfram M, Harbecke D, Aebischer T (1998) Phagocytosis of Leishmania mexicana amastigotes by macrophages leads to a sustained suppression of IL-12 production. Eur J Immunol 28: 2467–2477. [DOI] [PubMed] [Google Scholar]
- 42. Seifert K, Escobar P, Croft SL (2010) In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J Antimicrob Chemother 65: 508–511. [DOI] [PubMed] [Google Scholar]
- 43. Cojean S, Houze S, Haouchine D, Huteau F, Lariven S, et al. (2012) Leishmania resistance to miltefosine associated with genetic marker. Emerg Infect Dis 18: 704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seifert K, Croft SL (2006) In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob Agents Chemother 50: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seifert K, Perez-Victoria FJ, Stettler M, Sanchez-Canete MP, Castanys S, et al. (2007) Inactivation of the miltefosine transporter, LdMT, causes miltefosine resistance that is conferred to the amastigote stage of Leishmania donovani and persists in vivo. Int J Antimicrob Agents 30: 229–235. [DOI] [PubMed] [Google Scholar]
- 46. Goncalves V, Brannigan JA, Thinon E, Olaleye TO, Serwa R, et al. A fluorescence-based assay for N-myristoyltransferase activity. Anal Biochem 421: 342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Overath P, Engstler M (2004) Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol Microbiol 53: 735–744. [DOI] [PubMed] [Google Scholar]
- 48. Engstler M, Thilo L, Weise F, Grunfelder CG, Schwarz H, et al. (2004) Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei. J Cell Sci 117: 1105–1115. [DOI] [PubMed] [Google Scholar]
- 49. Liu J, Qiao X, Du D, Lee MG (2000) Receptor-mediated endocytosis in the procyclic form of Trypanosoma brucei. J Biol Chem 275: 12032–12040. [DOI] [PubMed] [Google Scholar]
- 50. Ali HZ, Harding CR, Denny PW (2012) Endocytosis and Sphingolipid Scavenging in Leishmania mexicana Amastigotes. Biochem Res Int 2012: 691363 10.1155/2012/691363. Epub 692011 Sep 691321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walker RG, Thomson G, Malone K, Nowicki MW, Brown E, et al. (2011) High throughput screens yield small molecule inhibitors of Leishmania CRK3:CYC6 cyclin-dependent kinase. PLoS Negl Trop Dis 5: e1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cleghorn LA, Woodland A, Collie IT, Torrie LS, Norcross N, et al. (2011) Identification of inhibitors of the Leishmania cdc2-related protein kinase CRK3. ChemMedChem 6: 2214–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.