Abstract
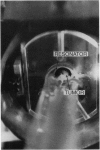
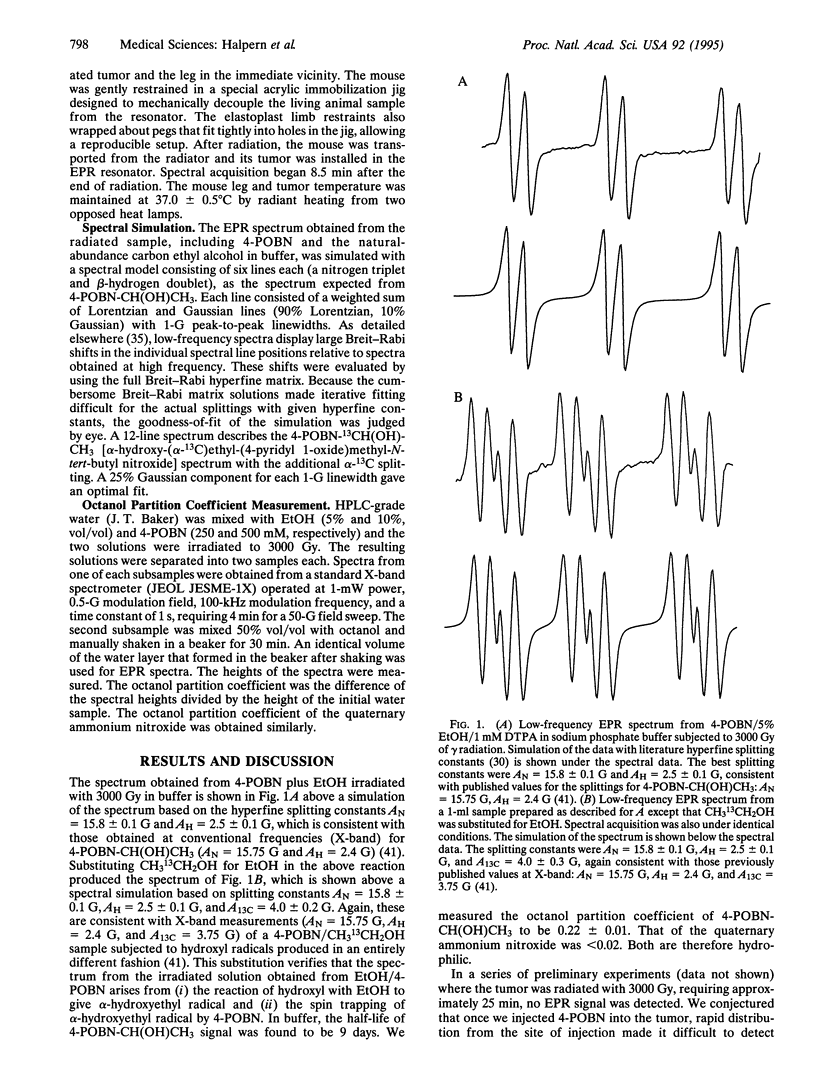
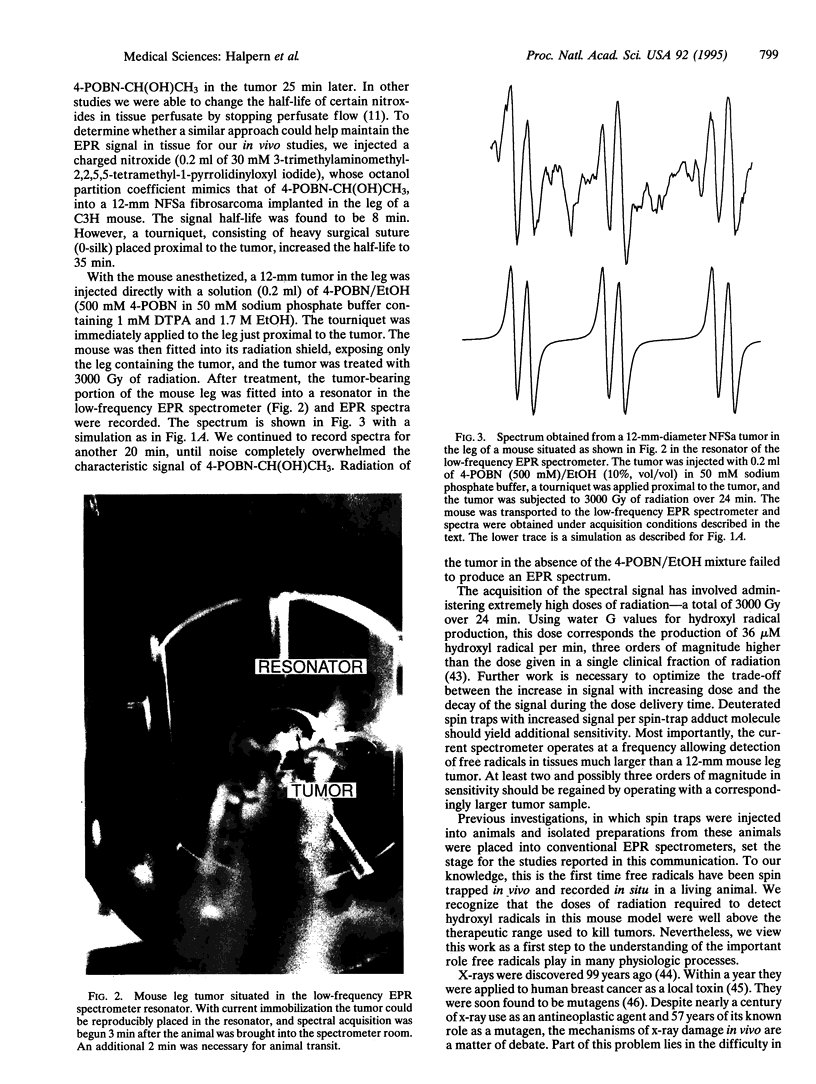
Hydroxyl radicals are thought to be responsible for the toxicity associated with ionizing radiation in tissues. Measurements of hydroxyl radicals generated by ionizing radiation in cellular systems have failed thus far to elucidate higher-level homeostatic responses to this and other reactive oxygen species. Careful assessment of prior indirect hydroxyl radical assays in living tissues indicates that they are prone to a variety of artifacts, making all but the most qualitative relationships difficult to establish. This paper describes the detection of hydroxyl radicals produced during radiation in the leg tumor of a living mouse, where the free radicals evolve; detection uses low-frequency electron paramagnetic resonance in combination with in vivo spin trapping. To our knowledge, this is the first report of such a direct measurement of free radical production in the tissues of a living animals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Oxygen radicals and 8-hydroxyguanine in DNA. Jpn J Cancer Res. 1991 Dec;82(12):1460–1461. [PubMed] [Google Scholar]
- Baker J. E., Felix C. C., Olinger G. N., Kalyanaraman B. Myocardial ischemia and reperfusion: direct evidence for free radical generation by electron spin resonance spectroscopy. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2786–2789. doi: 10.1073/pnas.85.8.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley P. A., Andrew E. R. RF magnetic field penetration, phase shift and power dissipation in biological tissue: implications for NMR imaging. Phys Med Biol. 1978 Jul;23(4):630–643. doi: 10.1088/0031-9155/23/4/006. [DOI] [PubMed] [Google Scholar]
- Burkitt M. J., Mason R. P. Direct evidence for in vivo hydroxyl-radical generation in experimental iron overload: an ESR spin-trapping investigation. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8440–8444. doi: 10.1073/pnas.88.19.8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Production of hydroxyl radical by decomposition of superoxide spin-trapped adducts. Mol Pharmacol. 1982 Mar;21(2):262–265. [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980 Mar;200(1):1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990 Sep;11(9):1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- Hoult D. I., Chen C. N., Sank V. J. The field dependence of NMR imaging. II. Arguments concerning an optimal field strength. Magn Reson Med. 1986 Oct;3(5):730–746. doi: 10.1002/mrm.1910030509. [DOI] [PubMed] [Google Scholar]
- Kasai H., Chung M. H., Jones D. S., Inoue H., Ishikawa H., Kamiya H., Ohtsuka E., Nishimura S. 8-Hydroxyguanine, a DNA adduct formed by oxygen radicals: its implication on oxygen radical-involved mutagenesis/carcinogenesis. J Toxicol Sci. 1991 Feb;16 (Suppl 1):95–105. doi: 10.2131/jts.16.supplementi_95. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Rosen H. Ethylene formation by polymorphonuclear leukocytes. Role of myeloperoxidase. J Exp Med. 1978 Aug 1;148(2):490–506. doi: 10.1084/jem.148.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht K. T., Mason R. P. In vivo spin trapping of xenobiotic free radical metabolites. Arch Biochem Biophys. 1993 Jun;303(2):185–194. doi: 10.1006/abbi.1993.1272. [DOI] [PubMed] [Google Scholar]
- Lai E. K., Crossley C., Sridhar R., Misra H. P., Janzen E. G., McCay P. B. In vivo spin trapping of free radicals generated in brain, spleen, and liver during gamma radiation of mice. Arch Biochem Biophys. 1986 Jan;244(1):156–160. doi: 10.1016/0003-9861(86)90104-9. [DOI] [PubMed] [Google Scholar]
- Milas L., Wike J., Hunter N., Volpe J., Basic I. Macrophage content of murine sarcomas and carcinomas: associations with tumor growth parameters and tumor radiocurability. Cancer Res. 1987 Feb 15;47(4):1069–1075. [PubMed] [Google Scholar]
- Mizumoto Y., Nakae D., Yoshiji H., Andoh N., Horiguchi K., Endoh T., Kobayashi E., Tsujiuchi T., Shimoji N., Denda A. Inhibitory effects of 2-O-octadecylascorbic acid and other vitamin C and E derivatives on the induction of enzyme-altered putative preneoplastic lesions in the livers of rats fed a choline-deficient, L-amino acid-defined diet. Carcinogenesis. 1994 Feb;15(2):241–246. doi: 10.1093/carcin/15.2.241. [DOI] [PubMed] [Google Scholar]
- Muller H. J. ARTIFICIAL TRANSMUTATION OF THE GENE. Science. 1927 Jul 22;66(1699):84–87. doi: 10.1126/science.66.1699.84. [DOI] [PubMed] [Google Scholar]
- Nakazawa H., Ichimori K., Shinozaki Y., Okino H., Hori S. Is superoxide demonstration by electron-spin resonance spectroscopy really superoxide? Am J Physiol. 1988 Jul;255(1 Pt 2):H213–H215. doi: 10.1152/ajpheart.1988.255.1.H213. [DOI] [PubMed] [Google Scholar]
- Pou S., Hassett D. J., Britigan B. E., Cohen M. S., Rosen G. M. Problems associated with spin trapping oxygen-centered free radicals in biological systems. Anal Biochem. 1989 Feb 15;177(1):1–6. doi: 10.1016/0003-2697(89)90002-x. [DOI] [PubMed] [Google Scholar]
- Pou S., Ramos C. L., Gladwell T., Renks E., Centra M., Young D., Cohen M. S., Rosen G. M. A kinetic approach to the selection of a sensitive spin trapping system for the detection of hydroxyl radical. Anal Biochem. 1994 Feb 15;217(1):76–83. doi: 10.1006/abio.1994.1085. [DOI] [PubMed] [Google Scholar]
- Pou S., Rosen G. M. Spin-trapping of superoxide by 5,5-dimethyl-1-pyrroline N-oxide: application to isolated perfused organs. Anal Biochem. 1990 Nov 1;190(2):321–325. doi: 10.1016/0003-2697(90)90202-k. [DOI] [PubMed] [Google Scholar]
- Ramos C. L., Pou S., Britigan B. E., Cohen M. S., Rosen G. M. Spin trapping evidence for myeloperoxidase-dependent hydroxyl radical formation by human neutrophils and monocytes. J Biol Chem. 1992 Apr 25;267(12):8307–8312. [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen G. M., Halpern H. J., Brunsting L. A., Spencer D. P., Strauss K. E., Bowman M. K., Wechsler A. S. Direct measurement of nitroxide pharmacokinetics in isolated hearts situated in a low-frequency electron spin resonance spectrometer: implications for spin trapping and in vivo oxymetry. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7772–7776. doi: 10.1073/pnas.85.20.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röschmann P. Radiofrequency penetration and absorption in the human body: limitations to high-field whole-body nuclear magnetic resonance imaging. Med Phys. 1987 Nov-Dec;14(6):922–931. doi: 10.1118/1.595995. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Decker M. A., Wells R. M., Democko C. A new method for the detection of hydroxyl radical production by phagocytic cells. Biochim Biophys Acta. 1980 Feb 21;628(1):90–97. doi: 10.1016/0304-4165(80)90354-2. [DOI] [PubMed] [Google Scholar]
- Samuni A., Krishna C. M., Riesz P., Finkelstein E., Russo A. Superoxide reaction with nitroxide spin-adducts. Free Radic Biol Med. 1989;6(2):141–148. doi: 10.1016/0891-5849(89)90111-1. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]