Abstract
Key Clinical Message
Umbilical vein thrombosis is a rare anomaly with high mortality that frequently occurs in association with fetomaternal conditions. The unfavorable outcome of our case highlights the need for consensus on severity criteria, including the percentage of vascular occlusion determined by power Doppler, in order to improve outcome.
Keywords: Fetal loss, prenatal diagnosis, thrombosis, umbilical vein
What's already known about this topic?
Umbilical vein thrombosis is a rare anomaly of uncertain clinical significance, with high mortality and morbidity.
It frequently occurs in association with fetomaternal conditions.
What does this study add?
We provide a clinical-pathological description of a case of antenatally diagnosed umbilical cord thrombosis with an unfavorable outcome.
It highlights the need for consensus on severity criteria, including the percentage of vascular occlusion determined by power Doppler, in order to improve outcome.
The umbilical vein thrombosis is a rare event and its management at the time of diagnosis remains undefined.
We present the unfavorable course of umbilical cord thrombosis revealed by ultrasound scan at 32 weeks of gestation (WG). Despite the close follow-up, the fetus died in utero.
The 27-year-old mother, gravida 1, had a previously unremarkable medical and obstetrical history. Her first (12 WG) and second (22 WG) ultrasound scans were normal. The oral glucose tolerance test, performed because of familial diabetes, was recorded normal at 28 weeks. The third 32-week scan revealed a large insertion of the umbilical cord on the placental side, suggestive of a cyst, and the patient was referred to our tertiary care prenatal center. The repeat scan indicated ectasia of the umbilical vein at the level of placental cord insertion, with turbulent and pulsatile flow, extended by partial thrombosis of the umbilical vein (Fig.1). The cord displayed 3 vessels and the coiling index was not increased (0.2/cm). The rest of the cord was normal, including the intra-abdominal part of the umbilical vein. Doppler ultrasound of the umbilical artery indicated a slightly increased resistance index of 0.76 (93rd percentile). Doppler ultrasound indicated a normal ductus venosus. Placental location and appearance was normal. The estimated fetal weight (2091 g) was concordant with the gestational age (48th percentile), as was the amount of amniotic fluid. Fetal movements were appropriate. Fetal heart rate patterns were normal. We decided to follow up the patient with daily fetal heart monitoring at home, and weekly ultrasound scans at our institution. Nevertheless, 3 days later, the mother presented to the emergency room because of perceived reduced fetal movements over the previous 12 h. Examination revealed intrauterine fetal demise. Induction of labor was followed by a normal vaginal delivery of a stillborn girl weighing 2200 g.
Figure 1.
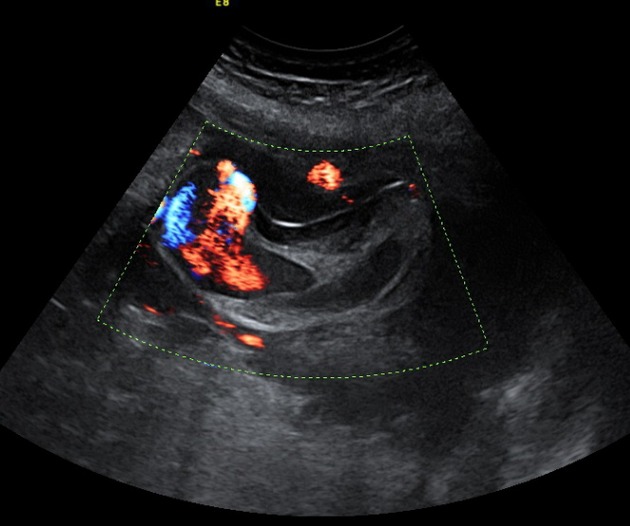
US scan: ectasia of the umbilical vein at the level of placental cord insertion with turbulent and pulsatile flow extended by partial thrombosis of the umbilical vein.
Histopathological examination revealed a macerated, eutrophic fetus with no malformations. The placental examination revealed a massively dilated chorionic vein with a 5 cm vascular ectasia and intimal dysplasia with thrombosis (Fig.2). The cord displayed 3 vessels and was highly twisted (coiling index: 1/1 cm), counter-clockwise. Histological examination of placental parenchyma showed, in addition to venous thrombosis, generally avascular chorionic villi.
Figure 2.
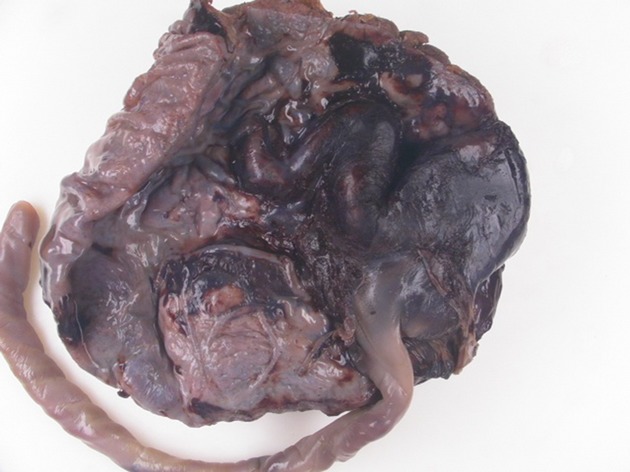
Pathology: vascular wall intimal dysplasia with thrombosis.
Thrombosis of the umbilical cord vessels is a rare but life-threatening event. Its incidence varies from 1/1300 to 1/1500 deliveries and 1/1000 in perinatal autopsies [1,2]. The male/female ratio is 1.6:1. Umbilical vein thrombosis appears to occur more often than umbilical artery thrombosis (71–85% vs. 11–15%), but poor fetal outcome is more frequently reported in the literature with umbilical artery thrombosis [1].
Heifetz [1] has reported fifty-two cases of umbilical cord thrombosis, in over 90% of which thrombosis was associated with additional umbilical cord abnormalities (i.e., knots, vessel stretching…), obstetrical complications (infection, preeclampsia, phlebitis), or systemic fetal conditions (diabetes, fetomaternal hemorrhage) that the author considered the likely cause of both the thrombosis and the poor fetal outcome. Cord compression may cause stasis of the blood and lead to thrombosis in the umbilical vessels. This mechanism may occur in true umbilical cord knots formed in a long cord. Inversely, short cords are more susceptible to vessel stretching during labor, which may also lead to vessel damage and eventually thrombosis [3,4].
Maternal diabetes mellitus is another known risk factor for fetal thrombus formation [5]: infants of diabetic mothers have an increased level of α 2-antiplasmin and decreased fibrinolysin activity with a higher risk of developing thrombosis. They also have an imbalance between vasodilatation and vasoconstriction factors, with enhanced susceptibility to vasoconstriction and platelet aggregation.
The other fetal conditions reported to play a role in the development of thrombi are hemolytic diseases, fetal hydrops, and fetomaternal transfusion, with anemia as a common factor, resulting in fetal heart failure, stasis of blood and thrombosis [1,2].
Redline et al. [6] reported extensive avascular villi (obliterative fetal vasculopathy) as a distinct process with substantial perinatal morbidity. The mother tested negative for thrombophilia.
Kanenishi et al. [7] presented a case of umbilical cord thrombosis with a favorable outcome. An elective cesarean section was performed because of bidirectional turbulent blood flow inside the varix on power Doppler.
It might be argued that in our case we should have decided on elective cesarean section at the time of diagnosis of umbilical vein anomaly in light of the usual complications linked to the late preterm delivery. However, in the absence of the established severity criteria, particularly the percentage of vascular occlusion, we were unable to predict fetal well-being, and so favored (on the basis of risk-benefit balance) ongoing fetal development with close follow-up.
Doppler sonography, particularly power Doppler, is considered as an essential ancillary technique for the documentation of thrombus [8,9], but the criteria used to define the frequency of monitoring should be evaluated. We think that cord thrombosis should be considered as indicating high severity and should prompt an increased frequency of monitoring to improve outcome.
Conflict of Interest
None declared.
References
- 1.Heinfetz SA. Thrombosis of the umbilical cord: analysis of 52 cases and literature review. Pediatr. Pathol. 1988;8:37–54. doi: 10.3109/15513818809022278. [DOI] [PubMed] [Google Scholar]
- 2.Schröcksnadel H, Holböck E, Mitterschiffthaler G, Tötsch M, Dapunt O. Thrombotic occlusion of an umbilical vein varix causing fetal death. Arch. Gynecol. Obstet. 1991;248:213–215. doi: 10.1007/BF02390361. [DOI] [PubMed] [Google Scholar]
- 3.Hasaart TH, Delarue MW, de Bruine AP. Intra-partum fetal death due to thrombosis of the ductus venosus: a clinicopathological case report. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994;56:201–203. doi: 10.1016/0028-2243(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 4.Devlieger H, Moerman P, Lauweryns J, et al. Thrombosis of the right umbilical artery, presumely related to shortness of the umbilical cord: an unusual case of fetal distress. Eur. J. Obstet. Gynecol. Reprod. Biol. 1983;16:123–127. doi: 10.1016/0028-2243(83)90109-0. [DOI] [PubMed] [Google Scholar]
- 5.Fritz MA, Christopher CR. Umbilical vein thrombosis and maternal diabetes mellitus. J. Reprod. Med. 1981;26:320–324. [PubMed] [Google Scholar]
- 6.Redline RW, Ariel I, Baergen RN, Desa DJ, Kraus FT, Roberts DJ, et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatr. Dev. Pathol. 2004;7:443–452. doi: 10.1007/s10024-004-2020-x. [DOI] [PubMed] [Google Scholar]
- 7.Kanenishi K, Nitta E, Mashima M, Hanaoka U, Koyano K, Tanaka H, et al. HDlive imaging of intra-amniotic umbilical vein varix with thrombosis. Placenta. 2013;34:1110–1112. doi: 10.1016/j.placenta.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Viora E, Sciarrone A, Bastonero S, Errante G, Campogrande M. Thrombosis of umbilical vein varix. Ultrasound Obstet. Gynecol. 2002;19:212–213. doi: 10.1046/j.0960-7692.2001.00617.x. [DOI] [PubMed] [Google Scholar]
- 9.Allen SL, Bagnall C, Roberts AB, Teele RL. Thrombosing umbilical vein varix. J. Ultrasound Med. 1998;17:189–192. doi: 10.7863/jum.1998.17.3.189. [DOI] [PubMed] [Google Scholar]


