Abstract
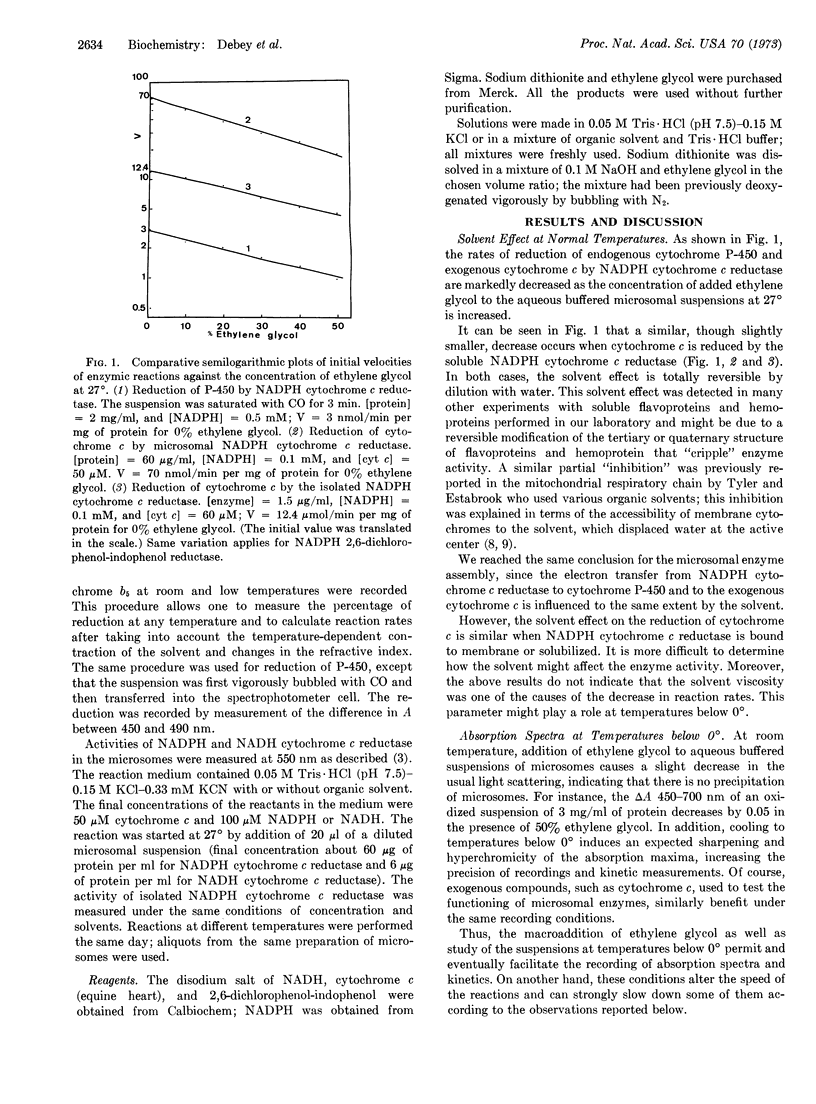
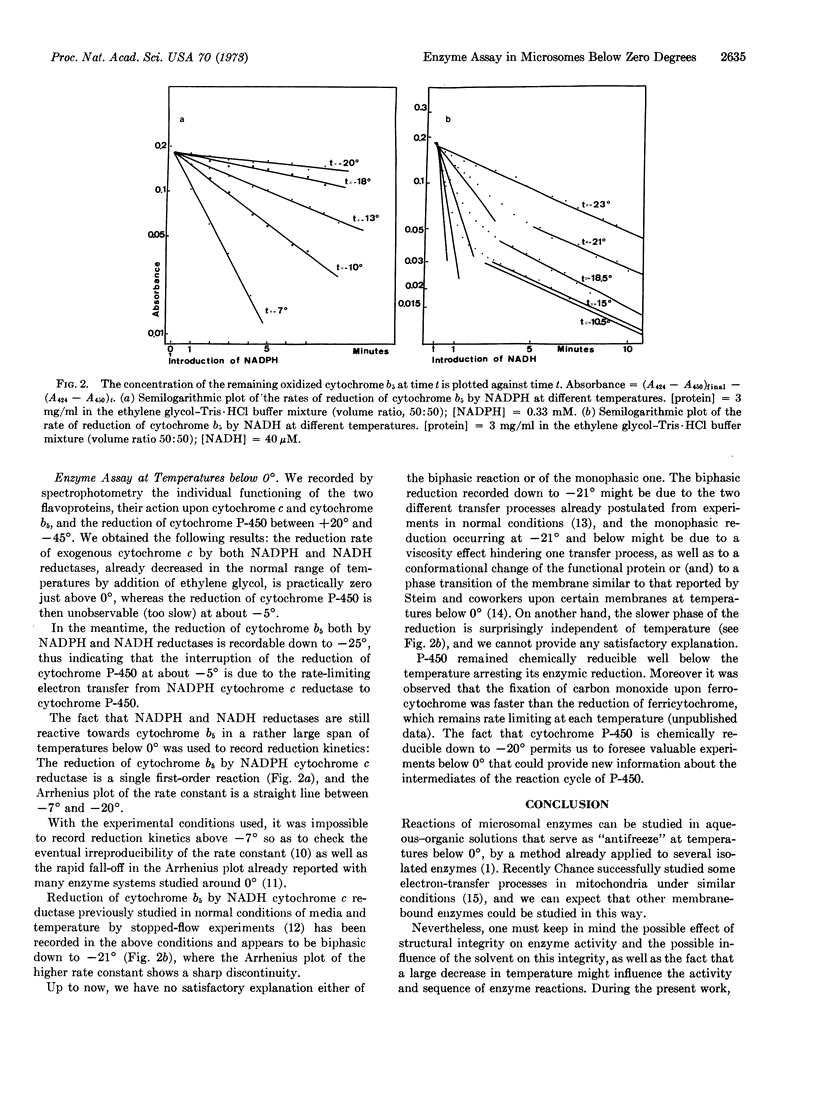
Reactions of a membrane-bound multienzyme complex (electron-transport chain of rat-liver microsomes) suspended in aqueous-organic solvent used as antifreeze at temperatures below 0° were studied. In the presence of a relatively high concentration of ethylene glycol, electron transfer can still be observed and some individual and sequential enzyme assays can be performed over a wide range of temperatures below 0°.
Keywords: ethylene glycol, electron transfer, rats, liver
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B., WILLIAMS G. R. Kinetics of cytochrome b5 in rat liver microsomes. J Biol Chem. 1954 Aug;209(2):945–951. [PubMed] [Google Scholar]
- Douzou P., Sireix R., Travers F. Temporal resolution of individual steps in an enzymic reaction at low temperature. Proc Natl Acad Sci U S A. 1970 Jul;66(3):787–792. doi: 10.1073/pnas.66.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M., Chance B. Studies on the electron transport chain at subzero temperatures: electron transport at site 3. Arch Biochem Biophys. 1972 Jul;151(1):304–315. doi: 10.1016/0003-9861(72)90501-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MALMSTROM B. G. The temperature dependence of the enolase reaction with different activating metal ions. Biochim Biophys Acta. 1955 Oct;18(2):285–286. doi: 10.1016/0006-3002(55)90070-6. [DOI] [PubMed] [Google Scholar]
- MASTERS B. S., KAMIN H., GIBSON Q. H., WILLIAMS C. H., Jr STUDIES ON THE MECHANISM OF MICROSOMAL TRIPHOSPHOPYRIDINE NUCLEOTIDE-CYTOCHROME C REDUCTASE. J Biol Chem. 1965 Feb;240:921–931. [PubMed] [Google Scholar]
- Omura T., Siekevitz P., Palade G. E. Turnover of constituents of the endoplasmic reticulum membranes of rat hepatocytes. J Biol Chem. 1967 May 25;242(10):2389–2396. [PubMed] [Google Scholar]
- Steim J. M., Tourtellotte M. E., Reinert J. C., McElhaney R. N., Rader R. L. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci U S A. 1969 May;63(1):104–109. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. D., Estabrook R. W. The influence of deuterium oxide and organic solvents on the interaction of respiratory chain components. J Biol Chem. 1966 Apr 25;241(8):1672–1680. [PubMed] [Google Scholar]


