Abstract
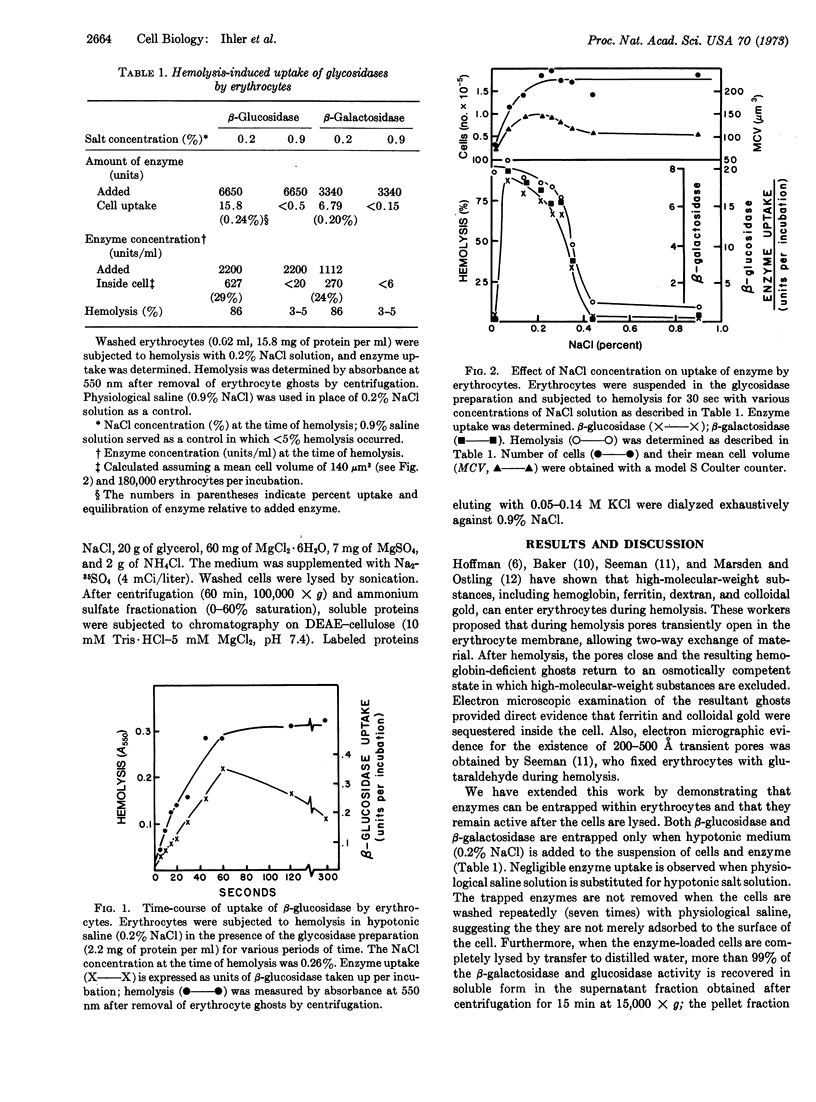
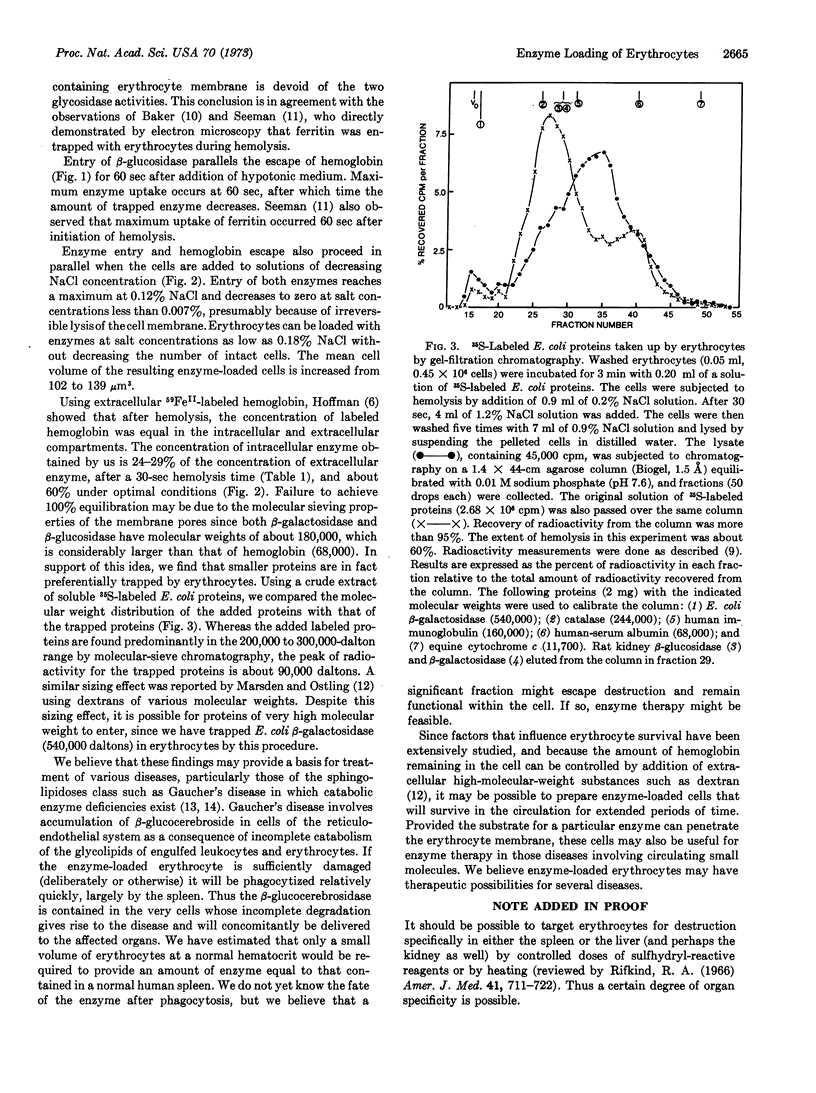
We demonstrated that β-glucosidase and β-galactosidase can be trapped inside erythrocytes by rapid hemolysis of the cell in the presence of these enzymes. Enzyme enters only during hemolysis, and optimum uptake occurs within 60 sec. There is no loss in cell number after hemolysis-induced enzyme uptake, and the ghosts have only a slightly increased mean cell volume. Smaller proteins enter more readily than larger proteins, although enzymes with a molecular weight of at least 180,000 can be readily entrapped by erythrocytes. This finding may provide a useful approach to the problem of enzyme replacement in certain diseases, including Gaucher's disease.
Keywords: sphingolipidoses, Gaucher's disease, β-glucocerebroside, β-glucocerebrosidase, spherocytes
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADY R. O., KANFER J., SHAPIRO D. THE METABOLISM OF GLUCOCEREBROSIDES. I. PURIFICATION AND PROPERTIES OF A GLUCOCEREBROSIDE-CLEAVING ENZYME FROM SPLEEN TISSUE. J Biol Chem. 1965 Jan;240:39–43. [PubMed] [Google Scholar]
- Baker R. F. Entry of ferritin into human red cells during hypotonic haemolysis. Nature. 1967 Jul 22;215(5099):424–425. doi: 10.1038/215424a0. [DOI] [PubMed] [Google Scholar]
- Beutler E., Kuhl W. The diagnosis of the adult type of Gaucher's disease and its carrier state by demonstration of deficiency of beta-glucosidase activity in peripheral blood leukocytes. J Lab Clin Med. 1970 Nov;76(5):747–755. [PubMed] [Google Scholar]
- Glew R. H., Heath E. C. Studies on the extracellular alkaline phosphatase of Micrococcus sodonensis. I. Isolation and characterization. J Biol Chem. 1971 Mar 25;246(6):1556–1565. [PubMed] [Google Scholar]
- Glew R. H., Lee R. E. Composition of the membranous deposits occurring in Gaucher's disease. Arch Biochem Biophys. 1973 Jun;156(2):626–639. doi: 10.1016/0003-9861(73)90314-7. [DOI] [PubMed] [Google Scholar]
- HOFFMAN J. F. Physiological characteristics of human red blood cell ghosts. J Gen Physiol. 1958 Sep 20;42(1):9–28. doi: 10.1085/jgp.42.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell C. M., Canellos G. P., Leventhal B. G., Carbone P. P., Block J. B., Serpick A. A., Selawry O. S. L-asparaginase: therapeutic and toxic effects in patients with neoplastic disease. N Engl J Med. 1969 Nov 6;281(19):1028–1034. doi: 10.1056/NEJM196911062811902. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee R. E. The fine structure of the cerebroside occurring in Gaucher's disease. Proc Natl Acad Sci U S A. 1968 Oct;61(2):484–489. doi: 10.1073/pnas.61.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSDEN N. V., OSTLING S. G. Accumulation of dextran in human red cells after haemolysis. Nature. 1959 Aug 29;184(Suppl 10):723–724. doi: 10.1038/184723a0. [DOI] [PubMed] [Google Scholar]
- Rifkind R. A. Destruction of injured red cells in vivo. Am J Med. 1966 Nov;41(5):711–723. doi: 10.1016/0002-9343(66)90032-5. [DOI] [PubMed] [Google Scholar]
- Seeman P. Transient holes in the erythrocyte membrane during hypotonic hemolysis and stable holes in the membrane after lysis by saponin and lysolecithin. J Cell Biol. 1967 Jan;32(1):55–70. doi: 10.1083/jcb.32.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]


