Abstract
Vesicular trafficking has emerged as an important means by which eukaryotes modulate responses to microbial pathogens, likely by contributing to the correct localization and levels of host components necessary for effective immunity. However, considering the complexity of membrane trafficking in plants, relatively few vesicular trafficking components with functions in plant immunity are known. Here we demonstrate that Arabidopsis thaliana Dynamin-Related Protein 2B (DRP2B), which has been previously implicated in constitutive clathrin-mediated endocytosis (CME), functions in responses to flg22 (the active peptide derivative of bacterial flagellin) and immunity against flagellated bacteria Pseudomonas syringae pv. tomato (Pto) DC3000. Consistent with a role of DRP2B in Pattern-Triggered Immunity (PTI), drp2b null mutant plants also showed increased susceptibility to Pto DC3000 hrcC −, which lacks a functional Type 3 Secretion System, thus is unable to deliver effectors into host cells to suppress PTI. Importantly, analysis of drp2b mutant plants revealed three distinct branches of the flg22-signaling network that differed in their requirement for RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD), the NADPH oxidase responsible for flg22-induced apoplastic reactive oxygen species production. Furthermore, in drp2b, normal MAPK signaling and increased immune responses via the RbohD/Ca2+-branch were not sufficient for promoting robust PR1 mRNA expression nor immunity against Pto DC3000 and Pto DC3000 hrcC−. Based on live-cell imaging studies, flg22-elicited internalization of the plant flagellin-receptor, FLAGELLIN SENSING 2 (FLS2), was found to be partially dependent on DRP2B, but not the closely related protein DRP2A, thus providing genetic evidence for a component, implicated in CME, in ligand-induced endocytosis of FLS2. Reduced trafficking of FLS2 in response to flg22 may contribute in part to the non-canonical combination of immune signaling defects observed in drp2b. In conclusion, this study adds DRP2B to the relatively short list of known vesicular trafficking proteins with roles in flg22-signaling and PTI in plants.
Author Summary
Plants have developed effective mechanisms for protection against pathogens including bacteria, but if a plant is unable to induce defenses, pathogenic bacteria invade and colonize the host, which can lead to reduced yield and nutritional quality of crops. An important aspect of engineering durable crop resistance against bacteria is elucidating and manipulating resistance pathways in the model plant Arabidopsis thaliana. The plant receptor FLAGELLIN SENSING 2 (FLS2) recognizes the bacterial protein flagellin to initiate host defense responses contributing to immunity. Here, we identify Dynamin-Related Protein 2B (DRP2B), previously implicated in membrane trafficking in plants, as a novel component of defense responses against flagellin and bacterial Pseudomonas syringae strains in Arabidopsis thaliana. More specifically, DRP2B functioned in the first line of defense against bacteria, namely in pattern-triggered immunity. We also demonstrated that DRP2B has different roles in three distinct branches of the flg22-signaling network that could be separated by their genetic requirement for the NADPH oxidase RbohD. In drp2b mutant plants, impaired ligand-induced endocytosis of FLS2 may contribute in part to the non-canonical combination of immune defects. Our findings highlight the importance of a functional vesicular trafficking network for plant immune responses and effective immunity against bacteria.
Introduction
Eukaryotes have developed highly effective immune mechanisms for protection against microbial pathogens. As the first line of defense, Pattern-Triggered Immunity (PTI) relies on the perception of conserved microbial features called Pathogen- (or Microbe-) Associated Molecular Patterns (PAMPs or MAMPs) by host receptors referred to as Pattern Recognition Receptors (PRRs) [1]–[3]. The bacterial PAMP flagellin is the main proteinaceous component of flagellum filaments essential for mobility of pathogenic bacteria such as Pseudomonades to infect hosts [4], [5]. In the model plant Arabidopsis thaliana, flagellin or its 22 amino acid active peptide-derivative, flg22, is perceived by the extracellular domain of FLAGELLIN SENSING 2 (FLS2), a receptor kinase localized to the plasma membrane (PM) [6]–[8]. In response to flg22, FLS2 undergoes ligand-induced endocytosis and trafficking through the Trans-Golgi Network/Early Endosomes (TGN/EE) and Multi Vesicular Bodies/Late Endosomes (MVB/LE) for subsequent degradation [9]–[14]. Endocytic degradation of FLS2 results in cellular desensitization to flg22, and subsequent new synthesis of FLS2 leads to flg22-resensitization [13]. Overall, relatively few components functioning in ligand-induced endocytosis of FLS2 have been identified, and potential role(s) of FLS2 endocytosis in flg22-signal initiation and/or attenuation remain mostly undefined.
Flg22-binding to FLS2 also initiates a plethora of flg22-signaling responses that include an increase in cytosolic calcium ([Ca2+]cyt), the production of apoplastic reactive oxygen species (ROS), activation of mitogen-activated protein kinases (MAPKs), transcriptional changes of defense marker genes, production of the plant defense hormone salicylic acid (SA), as well as callose deposition at cell wall sites [7], [15], [16]. Furthermore, flg22 induces rapid changes in the phosphorylation status of many proteins located at the PM as shown by large-scale phosphoproteomics analyses [17], [18]. Increasing evidence indicates that these immune responses form a signaling network consisting of multiple, parallel signaling branches rather than a single linear pathway. However, few mutants and chemical inhibitors have been identified showing separation of these proposed PAMP-signaling branches [13], [19]–[23]. Open questions regarding the network regulation include what components contribute to signal initiation/attentuation through specific branches of the signaling network and whether one signaling branch influences the other(s).
To gain insight into these questions, we used a candidate-based approach focusing on Arabidopsis thaliana Dynamin-Related Protein 2B (DRP2B), a vesicular trafficking protein that is phosphorylated in response to flg22 [17], [18], thus potentially placing DRP2B in the flg22-signaling network. As a member of the superfamily of high molecular weight GTPases with roles in membrane dynamics, DRP2B shares 93% amino acid sequence identity with DRP2A [24], [25]. Consistent with these two closely related DRP2s being functionally redundant in growth and development, drp2a drp2b double mutants arrest early in female and male gametophyte development, resulting in lethality [26], [27]. DRP2A and DRP2B are the only bona fide (or “classical”) dynamins in A. thaliana [25], [26] as they share the same domain structure as mammalian Dynamin 1 and 2. Classical mammalian Dynamins are key components of clathrin-mediated endocytosis (CME) catalyzing the scission and release of clathrin-coated vesicles (CCVs) from the PM [28], [29]. In addition to CME, dynamins contribute to endocytic mechanisms that are independent of clathrin [30], [31]. Consistent with its localization to the PM [17], [18], [32], [33], A. thaliana DRP2B and a related rice Oryza sativa OsDRP2 (Brittle Culm3; BC3) are implicated in CME based on their co-localization with clathrin-light chain [33], [34] as well as a functional study, in which roots of Arabidopsis mutants that express dominant-negative DRP2A/B show defects in the uptake of the endocytic tracer dye, FM4-64 [27]. However, it cannot be excluded that plant DRP2s, like their mammalian counterparts [30], [31], play roles in clathrin-independent endocytosis in plants [35], [36]. In rice, the bc3 allele disrupts cellulose biosynthesis of the secondary cell wall, likely by affecting trafficking of a cellulose synthase catalytic subunit [34], [37]. However, the roles of DRP2s in other cellular responses, including plant innate immunity, remain undefined.
Here, we identified DRP2B to function in flg22-signaling and innate immunity against Pseudomonas syringae pv tomato (Pto) DC3000 and Pto hrcC-. We provide evidence that DRP2B differentially contributes to three distinct branches of the flg22-signaling network. Moreover, we separated the individual flg22-signaling branches based on the genetic requirement for RbohD, which encodes the NADPH oxidase necessary for PAMP-induced apoplastic ROS production. Live-cell imaging studies indicate that DRP2B, but not DRP2A, is required for robust flg22-induced endocytosis of FLS2, and impaired ligand-induced trafficking defects of FLS2 may contribute in part to the non-canonical combination of phenotypic immune defects observed in drp2b.
Materials and Methods
Plant materials and growth conditions
drp2a-1, drp2a-3, drp2b-2, drp2b-5, sid2-2 and rbohD were previously described [26], [38]–[40]. All mutants are in Col-0 ecotype background. The drp2b-2 rbohD double mutant was generated by cross between drp2b-2 and rbohD. drp2b-2 plants expressing apoaequorin (drp2b-2/AEQ) or FLS2-GFP (drp2b/FLS2-GFP) were generated by crossing drp2b-2 with Col-0 plants expressing AEQ [41] or FLS2pro:FLS2-3xMyc-EGFP [9], respectively. Each double mutant was identified by PCR genotyping using primers listed in S1 Table. Arabidopsis seedlings and plant growth was at 22°C as described [38] in a 8-hour light/16-hour dark cycle photoperiod at 82 µmol m−2 s−1. Except when noted, fully expanded rosette leaves from five-to-six week old plants were used for all assays. After cutting leaf tissue or transfer of seedlings, all samples were floated on dH2O overnight at 22°C in continuous light (unless noted otherwise) to reduce wounding response prior to any assays.
PAMPs
PAMP peptides were as described [13], made by GenScript (Scotch Plains, NJ) and used at indicated concentrations.
Apoplastic ROS production
Luminol-based ROS production in leaf tissue was performed as described [38] using indicated PAMP concentrations or using Pto DC3000 or Pto hrcC− [42]. For inhibitor treatments, leaf tissue was pre-treated by floating leaf discs in water-containing 30 µM Wortmannin (Wm; Sigma-Aldrich; St. Louis, MO) for one hour prior to subsequent flg22-elicitation [13]. For ROS assays in six-to-seven-day old plants, cotyledons were cut in half, and the two halves were placed into a 96-well microplate for elicitation. All ROS experiments shown in a same panel were performed in the same 96-well plate at the same time to allow for direct comparison.
Cytosolic calcium measurements
Calcium assays were done as in [43] with minor changes. Leaf discs (1.1 cm2) of five-to-six week old plants or cotyledons of eight-day old seedlings grown on agar plates were cut into halves and placed in individual wells of a 96-well microplate with 150 µL reconstitution buffer [10 µM coelenterazine (Nanolight Technology, Pinetop, AZ), 2 mM MES buffer (pH 5.7), 10 mM CaCl2]. Prior to elicitation, the solution was removed and 0.1 µM flg22 of ddH2O was added to each well. Luminescence was acquired using a Glomax 96 microplate Luminometer (Promega) scanning each row in five-second intervals. Calcium concentration was calculated as described [41].
Bacterial pathogen assays
Pto DC3000lux [44] and Pto DC3000 hrcC [45] were used in leaf tissue at a concentration of OD600 = 0.0005 and OD600 = 0.02, respectively, as previously described [20]. Bacterial infection of two-week old plants were performed as in [46] with the exception that plants were grown on MS agar plates under a 8-hour light/16-hour dark cycle photoperiod at 114 µmol m−2 s−1. Prior to infection, four two-week-old plants were transferred to a microtiter plate containing 2 mL of dH2O, incubated for 15–20 h prior to infection with Pto DC3000lux (2×107 cfu/mL). For quantification of bacterial growth, seedlings were rinsed in dH2O and ground in 500 µL dH20 prior to serial dilution plating.
Quantitative Real-Time PCR (qRT-PCR) analysis
Three leaves of five-to-six week old plants were syringe infiltrated with flg22 or bacterial solution at indicated concentrations or optical densities (OD), respectively, allowed to dry and then placed at 22°C. Tissue was flash-frozen in liquid nitrogen at indicated times. Total RNA was isolated from collected tissue using Trizol Reagent (Sigma) according to the manufacturer's protocol and processed for qRT-PCR as described previously [46], [47] using gene-specific primers (S1 Table) and At2g28390 as a reference gene.
Immunoblot analysis
For immunoblot analyses of elicited samples, three 1.5 cm2 leaf discs (each cut into five strips to maximize exposure of tissue to the elicitation solution) or three seedlings were elicited with indicated flg22-concentrations for specified times and flash frozen in liquid nitrogen following PAMP removal. Sample preparation and immunoblot analysis of total proteins was done as described [38] using antibodies at the dilutions: αDRP2, 1∶2000; αMPK6, 1∶5000, αCalnexin, 1∶3000; αFLS2, 1∶2500; αP-p44/42 MAPK, 1∶3000 (#4370; Cell Signaling Tech, Danvers, MA); αGFP, 1∶2000 (#A-11122; Life Technologies, Grand Island, NY).
Callose deposition and quantification
Leaves were syringe-infiltrated with indicated concentrations of active or inactive flg22. 24 hours post-infiltration, leaf discs (1.5 cm2) were processed using aniline-blue staining as done previously [20], [38]. Callose deposits were visualized by ultraviolent epifluorescence using a Leica M205 FA microscope (Leica Microsystems Inc.; Buffalo Grove, IL, USA) and quantified from digital images as a percentage of each leaf disc covered with bright pixels above a set pixel intensity threshold using Metamorph software (Molecular Devices, Sunnyvale, CA) [48].
Salicylic Acid (SA) measurement and treatment
Plants were grown and treated as done for pathogen infection assay of two-week old plants. SA content in plant tissue was measured using ultra performance liquid chromatography electrospray ionization tandem mass spectrometry (UPLC-ESI-MS/MS) method as described previously [49], [50] with minor modifications. Briefly, around 50 mg of frozen tissue was ground to a fine powder using a tissue homogenizer (TissueLyser II, Qiagen, Venlo, Netherlands) in a tube containing metal beads. 150 µL of extraction buffer (70% methanol/water (v/v) with 0.5% acetic acid) containing known amount of an internal standard, deuterium labeled -SA (d4-SA), was added to the ground tissue and incubated for 30 min at 4°C while mixing. Following a brief centrifugation, the supernatant was transferred to a microcentrifuge tube and centrifuged at 18,000 g for 30 min at 4°C. Five µL of the resulting supernatant was separated on a UPLC BEH C18 column (1.7 µm, 2.1×50 mm; Waters, Milford, MA, USA) attached to an Acquity UPLC H-Class system (Waters) using a 3-min gradient program consisting of 0.15% aqueous formic acid and methanol as mobile phase (0.4 mL/min flow rate at 40°C). Characteristic MS transitions of m/z 137>93 and 141>97 for SA and d4-SA, respectively, were detected using a Xevo TQ-S tandem quadrupole mass spectrometer (Waters) operated at ESI negative ion mode. Peak area was integrated using MassLynx 4.1 software (Waters), and the absolute amount of SA was determined by comparing the relative peak area of SA and d4-SA in the plant extracts to the dose-response curve generated using pure SA and d4-SA standards.
For exogenous application of SA, four two-week-old plants were transferred from MS agar plates to a single well of a 24-well microtiter plate containing 2 mL of dH2O and incubated at 22°C for 15–20 hours. At the time of treatment, dH2O was removed from individual wells of the microtiter plate and replaced with 50 µM SA (Sigma) solution or dH2O (mock-treatment). The microtiter plate was returned to the growth chamber, and tissue was flash frozen in liquid nitrogen 24 hours post treatment for subsequent qRT-PCR analyses of PR1 mRNA levels.
Spinning disc confocal microscopy
Six-day old Col-0 WT FLS2-GFP and drp2b-2 FLS2-GFP seedlings were transferred to 0.6 mL dH2O in a 48-well tissue culture plate (two to three seedlings/well) and incubated at 22°C with continuous light for 16–20 hours. Untreated (0 min flg22) seedlings were mounted in water and immediately imaged. For imaging flg22-induced endocytosis of FLS2, seedlings were submerged in 1 µM flg22 for 30 or 45 minutes and then immediately mounted for imaging over a 10-min period (i.e. 35–45 or 50–60 min, respectively). FLS2-GFP was imaged in the epidermal pavement cell layer of the adaxial (top) cotyledon surface. For each experiment, at least four seedlings were imaged per genotype/treatment, with four to ten fields of view per seedling.
Live-cell imaging experiments were carried out using a custom Olympus IX-71 inverted microscope (Center Valley, PA) equipped with a Yokogawa CSU-X1 5000 rpm spinning disc unit (Tokyo, Japan), Andor iXon Ultra 897 High Speed EMCCD camera (Belfast, United Kingdom), PZ-2000 XYZ series automated stage with Piezo Z-axis top plate (Applied Scientific Instrumentation; Eugene, OR), and 60x-silicon oil objective (Olympus UPlanSApo 60x/1.30 Sil). GFP was excited with a Spectra Physics 488-nm diode laser (Santa Clara, CA), and fluorescence was collected through a series of Semrock Brightline 488-nm single-edge dichroic beamsplitter and 500-550-nm bandpass filters (Rochester, NY). Camera exposure time was set to 150 msec. For each image series, 66 consecutive images at a z-step interval of 0.31 µm (20 µm total depth) were captured using Andor iQ2 software (Belfast, United Kingdom).
Quantitative analysis of FLS2-GFP endocytosis and PM intensity
Z-stack image series were processed and analyzed using Fiji software [51]. Each FLS2-GFP image series was displayed as a maximum-intensity projection (MIP) with brightness and contrast adjusted uniformly for all MIPs. Stomata were removed from the MIP using the Freehand Selection tool, and the total pavement cell surface area (µm2) was measured. FLS2-GFP-containing puncta (endosomes) were detected using the Advanced Weka Segmentation plug-in for Fiji, which uses Weka machine learning capabilities [52] for trainable selection of image features. In brief, a prototype MIP image with clearly defined FLS2-GFP puncta was used to generate a "Vesicle Classifier" that was subsequently applied to all other MIPs. Puncta were counted in the resulting binary images using the Analzye Particles function in Fiji with the following parameters: particle Size = 0.25–2.5 µm2 and Circularity = 0.25–1.00. Every MIP image was visually inspected after puncta detection for accuracy, and a manual adjustment of the puncta count was made if necessary. Puncta density was calculated for each MIP image as the number of FLS2-GFP puncta per 1000 µm2.
For measuring levels of FLS2-GFP at the PM (FLS2-GFP PM intensity), a single optical section of the epidermal pavement cells with juxtaposed PMs was selected from each SDCM Z-series, or captured independently using the same imaging and experimental parameters described. Using Fiji, bright PM regions were highlighted with the Oval Selection tool and subsequently analyzed for mean pixel intensity. The FLS2-GFP PM intensity for each image was calculated as the average value of the pixel intensity measurements from four selected PM regions. For each genotype and treatment, FLS2-GFP PM intensities are reported relative to the FLS2-GFP PM intensity of un-elicited Col-0 FLS2-GFP.
Statistical analysis
Unless stated otherwise, each experiment was done at least three independent times with similar results. Statistical significances based on unpaired Two-tailed student's t-test were determined with Graph Pad Prism4 software (La Jolla, CA).
Accession numbers
FLS2 (At5g46330), DRP2B (At1g59610), DRP2A (At1g10290), RBOHD (At5g47910), PR1 (At2g14610).
Results
DRP2B is a negative regulator of RbohD-dependent ROS production in response to flg22
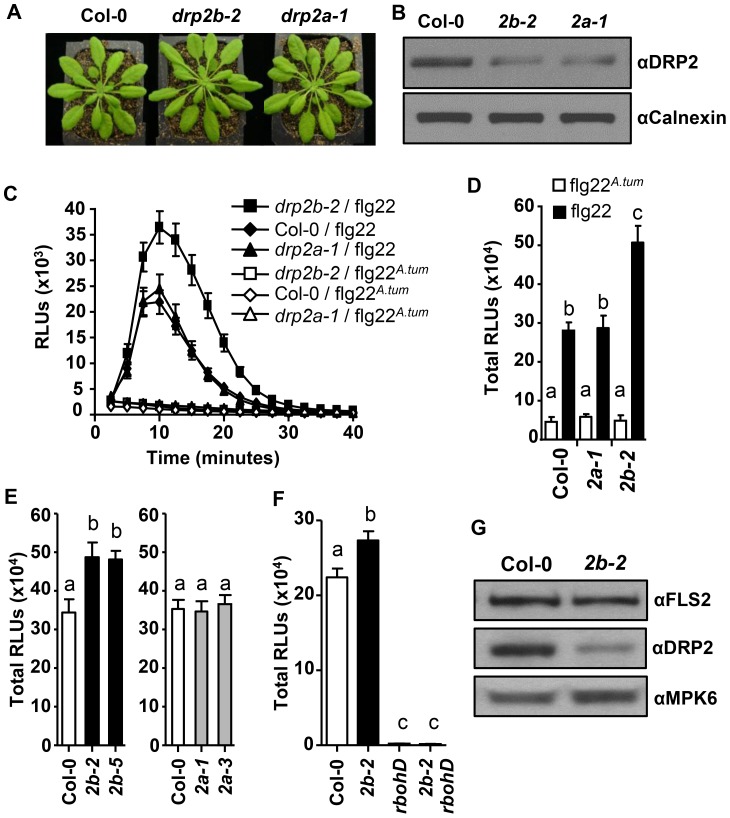
As previous work has shown that drp2a drp2b double null mutants are gametophytic lethal [26], [27], all experiments conducted in this study were performed using our previously published drp2a and drp2b single null mutants [26]. Overall, these single mutants do not show any gross morphological defects (Fig. 1A) [26], [27]. Using an affinity purified polyclonal peptide antibody (αDRP2) that detects both DRP2A and DRP2B proteins due to their high amino acid sequence identity [26], we confirmed by immunoblot analysis that five-to-six week old leaves of drp2a-1 (SALK_071036) or drp2b-2 (SALK_134887) single mutants accumulated significantly reduced levels of DRP2 proteins (Fig. 1B). These results are consistent with reduced DRP2 protein levels seen in drp2 single mutants at the seedling stage [26]. The residual levels of protein detected by the DRP2 antibody in the drp2b-2 mutant likely represented DRP2A, and DRP2B in drp2a-1.
Figure 1. DRP2B is a negative regulator of RbohD-dependent ROS Production in response to flg22.
(A) No gross growth defects were observed in five-to-six week-old drp2b-2 (2b-2) or drp2a-1 (2a-1) plants relative to the wildtype (Col-0). (B) Compared to Col-0, drp2b-2 (2b-2) and drp2a-1 (2a-1) single mutants exhibited reduced DRP2 protein levels as shown in immunoblot analyses of total protein extracts from un-elicited leaf tissue. αDRP2 antibody showed DRP2 protein levels, and αCalnexin was the loading control. (C) In time-course experiments, ROS production was elevated in drp2b-2 compared to Col-0 and drp2a-1 in response to 0.1 µM of active flg22 (filled shapes; n = 24/genotype) but not inactive flg22A.tum (open shapes; n = 8/genotype). (D) Compared to Col-0 and drp2a-1 (2a-1), total ROS production was significantly increased in drp2b-2 (2b-2) after elicitation with 0.1 µM of active flg22 (P<0.0003) but not in response to inactive flg22A.tum (P>0.5). Data were based on time-course experiment shown in Figure (C). (E) Independent drp2b (black bars), but not drp2a (gray bars), null mutant alleles displayed increased flg22-dependent ROS production compared to Col-0 (white bar) (P<0.007) (n = 24/genotype). (F) Similar to rbohD single mutants, drp2b-2 rbohD double mutants (2b-2 rbohD) did not produce any ROS in response to 0.1 µM active flg22 (P>0.125). (n = 24/genotype and treatment). (G) Steady-state protein levels of FLS2 were similar between Col-0 and drp2b-2 (2b-2) as shown by immunoblot analyses of total proteins extracts from un-elicited leaf tissue using αFLS2 and αDRP2 antibodies. αMPK6 served as a loading control. All experiments were done in 5-week old leaf tissue and repeated more than three independent times with similar results. Values are mean ± SE. Different letters indicate significant differences while the same letter indicates no significant differences between samples based on Two-tailed student's t-test. Relative Light Units, RLU.
Analysis of PAMP-elicited ROS has proven to be a valuable tool in identifying and characterizing novel components involved in PAMP-signaling [18], [20], [38], [53], [54]. Therefore, we monitored ROS production in response to flg22 in drp2 single mutants. In drp2b-2 but not drp2a-1 null mutant leaves, flg22-induced ROS production was significantly increased relative to Col-0 when ROS production was plotted over time (Fig. 1C), at its peak at 10–12 minutes post-elicitation (S1A Fig.) and as total ROS produced over 40 minutes (Fig. 1D). In contrast, no significant difference was observed between flg22-elicited ROS in drp2a-1 and Col-0 (Fig. 1C-D; S1A Fig.). In control experiments, little to no ROS was detected in response to inactive flg22 derived from Agrobacterium tumefaciens (flg22A.tum) [55] in either drp2a-1, drp2b-2 or Col-0 (Fig. 1C). Similar results were observed using the independent DRP2 null alleles, drp2b-5 (SALK_041330) and drp2a-3 (SALK_011319) [26] (Fig. 1E; total RLUs) confirming that the increased ROS production in drp2b mutant lines was specific to loss of DRP2B. In drp2b-2, ROS production was also significantly increased after elicitation with elf26, an unrelated bacterial PAMP detected by the PRR Elongation Factor-Tu receptor (EFR) [1]–[3] (S1B Fig.), indicating that DRP2B's function is not restricted to flg22. Taken together, our results show that DRP2B acts as a negative regulator of flg22-induced ROS production, whereas DRP2A has no apparent role in this response.
Next, we investigated whether the increase in amplitude of flg22-induced ROS in drp2b-2 was dependent on RbohD, the plasma membrane-localized NADPH oxidase responsible for rapid apoplastic ROS production after elicitation with PAMPs [18], [39], [56]. In the homozygous drp2b-2 rbohD double mutant, flg22-elicited ROS production was highly reduced and statistically similar to ROS in the rbohD single mutant (Fig. 1F). These results indicate that in drp2b-2, the increase in flg22-induced ROS production is completely RbohD-dependent.
The increased ROS production in drp2b-2 was unlikely due to increased expression of RbohD and FLS2 because in quantitative reverse transcription polymerase chain reaction (qRT-PCR) experiments, no significant difference in steady-state RbohD or FLS2 mRNA levels was observed between drp2b-2 and Col-0 (S1C-D Fig.). Furthermore, FLS2 protein levels were found by immunoblot analysis of total protein extracts from drp2b-2 and Col-0 lines to be similar (Fig. 1G), indicating that changes in the steady-state levels of the FLS2 receptor are not likely to account for the observed increase in flg22-dependent ROS production in drp2b-2.
DRP2B negatively regulates flg22-induced Ca2+-dependent responses but has no apparent role in MAPK-dependent responses
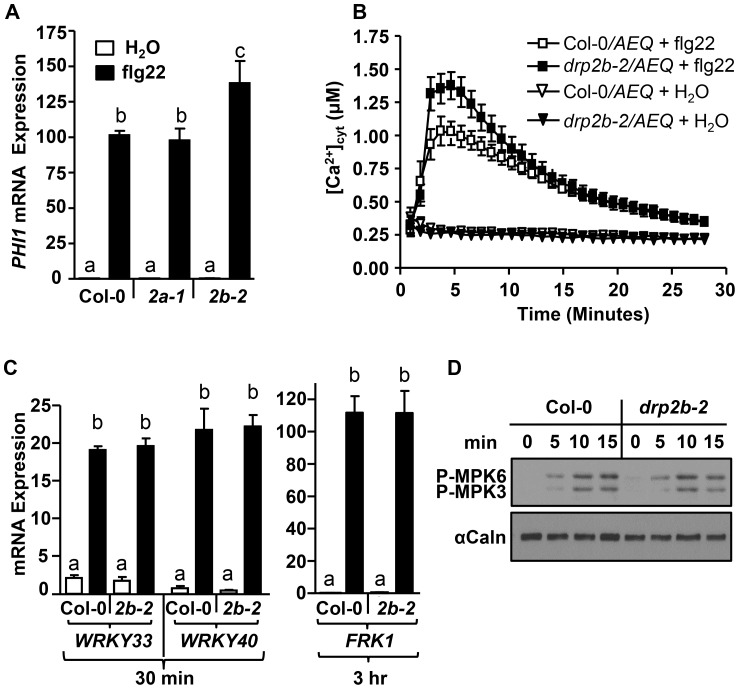
To test whether drp2b-2 displays other flg22-signaling defects, we measured flg22-induced changes in mRNA expression of PHI1, a Ca2+-dependent marker gene that is up-regulated in response to flg22 [19]. As determined by qRT-PCR (Fig. 2A), PHI1 transcript accumulation was significantly increased in drp2b-2 compared to Col-0 and drp2a-1 at 30 minutes post-elicitation with flg22 (Fig. 2A). No statistically significant differences were detected between flg22-treated drp2a-1 and Col-0 or between mock (H20)-treated tissues (Fig. 2A). Because drp2b-2 but not drp2a-1 single mutant plants showed heightened flg22-induced ROS production and PHI1 mRNA levels, we focused the remainder of this study on further characterizing the role of DRP2B in innate immune responses utilizing the drp2b-2 mutant.
Figure 2. DRP2B is a negative regulator of flg22-induced Ca2+-dependent responses but has no apparent role in MAPK-dependent responses.
(A) Using qRT-PCR, PHI1 mRNA levels were increased in drp2b-2 (2b-2; P<0.05), but not in drp2a-1 (2a-1; P>0.5), compared to wild-type Col-0 at 30 min post-elicitation with 1 µM flg22 (black bars). Water treatment (white bars) at 30 min are mock control (P>0.5). (n = 3/genotype and treatment). (B) After 0.1 µM flg22 elicitation, cytosolic Ca2+ levels were significantly elevated in drp2b-2 expressing the Ca2+-reporter Aequorin (drp2b-2/AEQ; closed square) compared to Col-0 expressing Aequorin (Col-0/AEQ; open square). Mock (H2O)-treatment served as control (triangles). (n = 6/genotype and treatment). (C) Using qRT-PCR, mRNA levels of WRKY33, WRKY40 and FRK1 were not significantly different between drp2b-2 (2b-2) and Col-0 at indicated times after elicitation with water (white bars) or 1 µM flg22 (black bars). P-values for WRKY33, WRKY40, FRK1 mRNA levels between drp2b-2 and Col-0 were all P>0.5. (n = 3/genotype and treatment). (D) No apparent difference in flg22-induced phosphorylation of MPK3 and MPK6 was observed between Col-0 and drp2b-2 (2b-2) after elicitation over 15 minutes (min) following elicitation with 0.1 µM flg22. Immunoblot analysis was done on total protein extracts probed with an antibody for phosphorylated MAPKs (P-MPK3 and P-MPK6). αCalnexin (αCaln) served as loading control. Experiments in (A,C and D) utilized 5–6 week old leave tissue, and experiments in (B) used cotyledons from 8-day old seedlings. All experiments were repeated at least 3 times with similar results. Values are mean ± SE. Statistical analysis was done as in Fig. 1.
One of the earliest signaling events occurring within 1–2 minutes after PAMP perception is a rapid increase in [Ca2+]cyt which in turn contributes to PAMP-induced ROS production and PHI1 mRNA levels [19], [57]. To examine whether DRP2B also functioned in flg22-induced changes of [Ca2+]cyt, drp2b-2 was crossed with a Col-0 plant line expressing the cytosolic calcium reporter aequorin (AEQ), a well-established photoreporter protein to assess changes in cellular calcium levels [41], [57]. When elicited with flg22, 8-day as well as five-to six-week old homozygous drp2b-2/AEQ plants displayed a highly significant increase in [Ca2+]cyt compared to Col-0/AEQ (Fig. 2B and S2A Fig., respectively). Specificity of this response was demonstrated by the lack of increased [Ca2+]cyt in mock (H2O)-treated samples (Fig. 2B). Pretreatment with LaCl3, a Ca2+ -channel blocker [57], [58], abolished flg22-induced [Ca2+]cyt in both drp2b-2/AEQ and Col-0/AEQ to statistically similar levels (S2B Fig.). Similarly, no difference in flg22-induced ROS production was detected between drp2b-2 mutant and wild-type plants after LaCl3 pretreatment (S2C Fig.). These LaCl3 results indicate that in drp2b, the flg22-elicited increase in [Ca2+]cyt and ROS production was dependent on the activity of Ca2+-channel(s).
To further investigate the transcriptional reprogramming that occurs downstream of DRP2B, we measured the flg22-induced changes in mRNA expression of PER62, PER4, and NHL10, which are marker genes reported to be synergistically affected by CDPKs and MAPKs [19], as well as the MAPK-dependent marker genes WRKY33, WRKY40 or FRK1 [19], [59]. As determined by qRT-PCR comparing drp2b-2 to Col-0, no significant differences were observed for transcript accumulation for any of these genes before or after flg22-treatment (S3 Fig., PER62, PER4, NHL10; Fig. 2C, WRKY33, WRKY40, FRK1). Furthermore, we probed total protein samples with an antibody that detects phosphorylated MAPKs [38] to examine activation of the MAPK-pathway in response to flg22. No statistically significant differences in flg22-induced phosphorylation of MPK3 and MPK6 were evident in drp2b-2 compared to Col-0 between 0 to 45 min (Fig. 2D and S4A Fig. for 0 to 15 min; S4B and S4C Figs. for 0 to 45 min).
Taken together, our results reveal that loss of DRP2B differentially affected distinct branches of the flg22-signaling network. Specifically, we observed that DRP2B functioned as a negative regulator of early Ca2+-dependent responses while having no apparent role in MAPK-dependent signaling.
Elevated flg22-induced ROS production in drp2b mutants is Wortmannin- but not Tyrphostin A23-sensitive
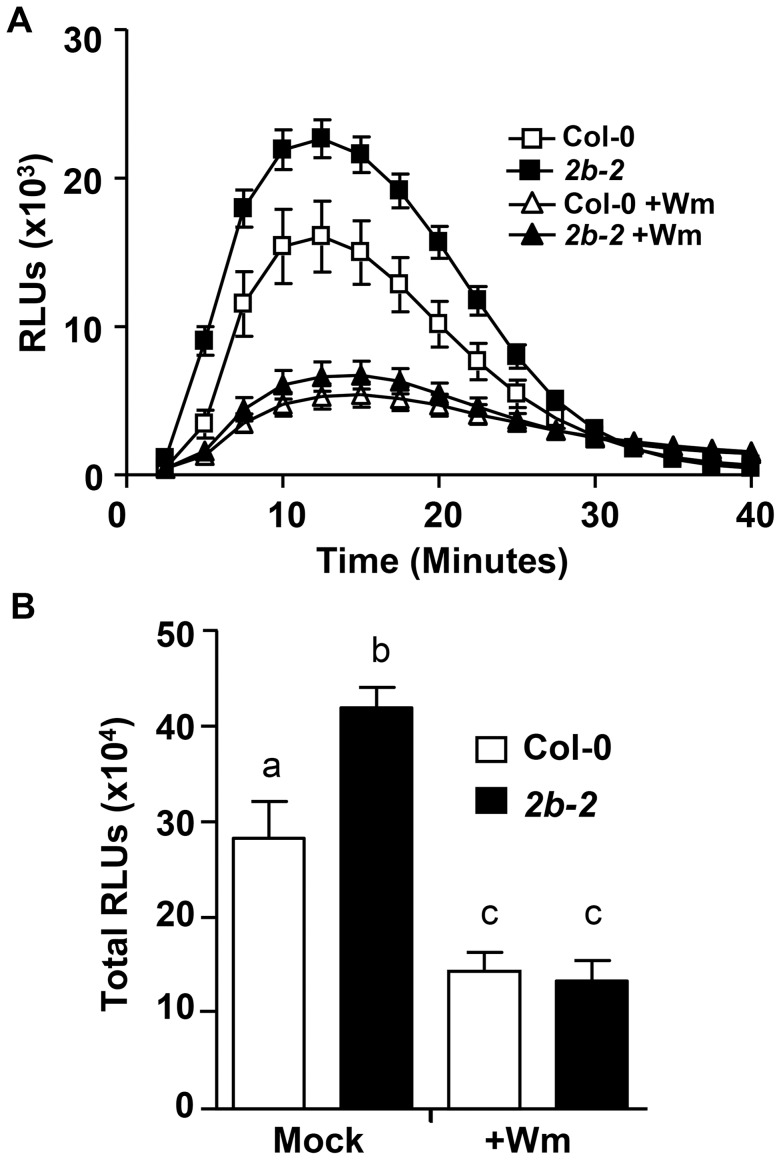
Recently, we have shown that flg22-elicited ROS production but not MAPK phosphorylation is sensitive to chemical interference with the vesicular trafficking inhibitor Wortmannin (Wm) [13]. Wm is a well-established phosphatidylinositol (PI)-3- and PI-4-kinase inhibitor [13], which interferes with the maturation of late endosomes and multivesicular bodies [60], [61]. In plant cells, Wm also inhibits the formation of endocytic vesicles at the PM [62], thereby impeding ligand-induced endocytosis and degradation of FLS2 [9], [12], [13]. Given that both loss of DRP2B (Figs. 1 and 2) and Wm-treatment [13] affect flg22-elicited ROS production but not MAPK phosphorylation, we examined whether the increase in flg22-induced ROS production in drp2b-2 was Wm-sensitive. Prior to flg22-elicitation, leaf tissue was pre-incubated for one hour with 30 µM Wm, a concentration previously shown to interfere with flg22-induced endocytic degradation of FLS2 [9], [13] and ROS production [13]. In agreement with our previous study [13], pretreating Col-0 leaf discs with Wm resulted in a significant decrease in flg22-induced ROS production compared to mock-treated wild-type tissue (Fig. 3A-B; compare mock and +Wm for Col-0). For drp2b-2, the inhibitor pretreatment also significantly decreased flg22-elicited ROS production (Fig. 3A-B; compare mock and +Wm for drp2b-2), and importantly, we consistently observed no statistically significant difference in flg22-elicited ROS levels between drp2b-2 and Col-0 lines following Wm treatment (Fig. 3A-B; compare +Wm for drp2b-2 and Col-0).
Figure 3. For drp2b, increased flg22-induced ROS production is sensitive to Wortmannin.
(A) After a one-hour pretreatment in the absence (square) or presence (triangle) of 30 µM Wortmannin (Wm), ROS production was measured over 40 minutes in drp2b-2 (2b-2, closed symbols) and Col-0 (open symbols) in response to 1 µM flg22 (n = 24/treatment and genotype). (B) Total ROS production based on time-course experiments shown in (A) indicates that in drp2b-2 (2b-2, black bars), the increase in flg22-induced ROS production is Wm-sensitive (compare 2b-2+Wm vs Col-0+Wm, P>0.5). Experiments were done using five-to-six week old leaf tissue and repeated at least three independent times with similar results. Values are mean ± SE. Statistical analysis was done as in Fig. 1. Relative Light Units, RLU.
Next, we tested whether in drp2b-2, the increase in flg22-induced ROS is sensitive to chemical interference with Tyrphostin A23 (TyrA23). TyrA23 was initially identified as a tyrosine kinase inhibitor [63]; however, it also functions as a vesicular trafficking inhibitor by interfering with the internalization of endocytic vesicles from the PM [63]–[68]. We have recently shown that similar to Wm, pretreatment with TyrA23 results in reduced flg22-elicited ROS production in wild-type plants [13]; but importantly, reduced ROS levels can be attributed to its function as a Tyr kinase inhibitor rather than its role as a vesicular trafficking inhibitor [13]. When pretreating leaf tissue with 100 µM TyrA23, flg22-induced ROS production was significantly decreased in both drp2b-2 and Col-0 leaf tissue (S5A and S5B Figs.; compare –T23 vs +T23 for either Col-0 or drp2b-2; P<0.0001) indicating that this inhibitor was functional. However, when comparing ROS levels between mutant and wild-type in the presence of TyrA23, we consistently observed that flg22-induced ROS production was still significantly increased in drp2b-2 compared to Col-0 (S5A and S5B Figs.; compare +T23 between drp2b-2 and Col-0; P≤0.025). Taking these chemical interference results together, DRP2B's role as a negative regulator in flg22-induced ROS production appears to be part of a signaling pathway that is Wm- but not fully TyrA23-sensitive.
Loss of DRP2B differentially affects late flg22-responses in a non-canonical manner
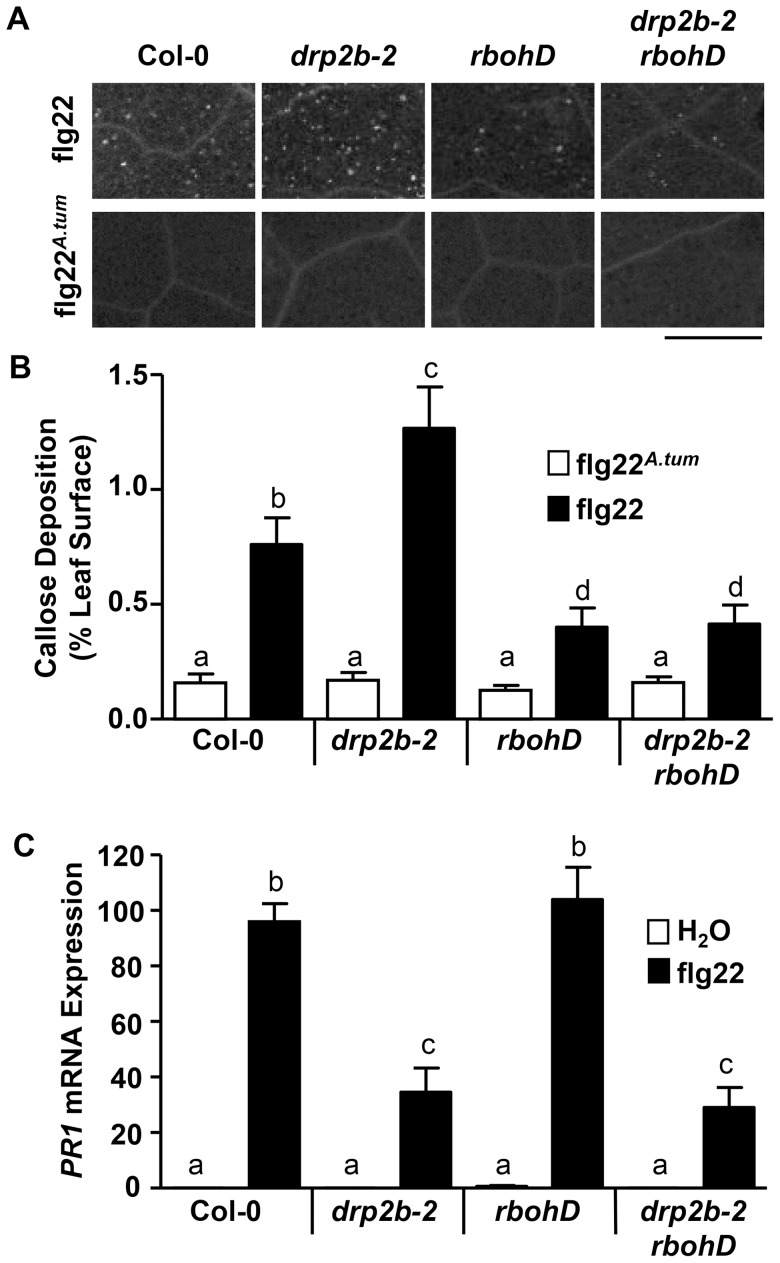
To investigate whether loss of DRP2B affects late flg22-responses, callose, a β-1,3-glucan polymer deposited at the cell wall in response to flg22, was visualized by aniline blue staining. When leaves of mature plants were infiltrated with active flg22, drp2b-2 showed a significant increase in flg22-induced callose deposits compared to Col-0 at 24 hours post-infiltration (Figs. 4A-B). In control experiments, no difference was detected in response to inactive flg22A .tum (Fig. 4A-B) indicating that in drp2b-2, the increased number of callose deposits was not due to a wound-effect or pre-existing callose deposits. Because flg22-induced callose deposition requires functional RbohD [48], [56], we utilized the drp2b-2 rbohD double mutant to examine whether in drp2b-2, the increase in flg22-elicited callose deposition was RbohD-dependent. A significant decrease in flg22-induced callose deposition was observed in both rbohD single and drp2b-2 rbohD double mutants compared to Col-0 and drp2b-2 (Figs. 4A-B). Importantly, no statistical difference was observed between rbohD and drp2b-2 rbohD (Fig. 4B) indicating that DRP2B functions as a negative regulator of flg22-induced callose deposition in a RbohD-dependent manner, and that in drp2b-2, increased callose deposition may be a downstream result of enhanced RbohD-dependent ROS production.
Figure 4. Loss of DRP2B affects late flg22-responses in a non-canonical manner.
(A) Callose deposition in five-to-six week old leaf tissue of Col-0, drp2b-2, rbohD, and drp2b-2 rbohD at 24 hr after infiltration of 10 nM flg22 or 10 nM flg22A.tum. A representative image is shown depicting differences in callose depositions between genotypes and treatments. Scale bar = 0.5 mm. (B) Percentage of total leaf surface area covered by aniline blue-stained fluorescent callose at 24 hr after infiltration of 10 nM flg22 (black bars) or 10 nM flg22A.tum (white bars). (n>20/genotype and treatment). (C) Using qRT-PCR, PR1 mRNA levels were significantly reduced in drp2b-2 and drp2b-2 rbohD compared to Col-0 (P<0.0001) or rbohD (P<0.0001) at 24 hrs after infiltration with 1 µM flg22 (black bars). Treatment with water (white bars) served as mock control (P>0.5). (n = 4/genotype and treatment). All experiments were done using five-to-six week old leaf tissue and repeated at least three independent times with similar results. Values are mean ± SE. Statistical analysis was done as in Fig. 1.
Expression of Pathogen-Related 1 (PR1), a commonly used late marker gene typically associated with SA-regulated signaling responses, is up-regulated after flg22-treatment or pathogen infection [45]. After twenty-four hours elicitation with flg22, but not with mock (H2O) treatment, an up-regulation of PR1 mRNA levels was observed for both drp2b-2 and Col-0. However, the increase in PR1 mRNA expression level was significantly reduced in drp2b-2 compared to Col-0 as determined by qRT-PCR (Fig. 4C). Because RbohD has been proposed to function as a negative regulator of SA-responses [21], [69], we examined whether the decreased flg22-induced PR1 expression in the drp2b-2 mutant was due to an increase in RbohD-dependent responses. As shown in Fig. 4C, flg22-elicited PR1 mRNA expression levels in rbohD were similar to Col-0. PR1 mRNA levels in the drp2b-2 rbohD double mutant, however, were not restored to those measured in rbohD but were similar to those in drp2b (Fig. 4C). Thus, we conclude that in drp2b-2, the increased RbohD-dependent responses are unlikely to be responsible for diminished levels of flg22-induced PR1 mRNA expression.
To test whether decreased PR1 mRNA levels in drp2b were due to altered Ca2+-channel activity, we co-treated plants with 1 µM flg22 and 10 mM LaCl3 for 24 hours prior to qRT-PCR analysis. Compared to flg22 elicitation alone, LaCl3 co-treatment resulted in a significant decrease in flg22-induced PR1 mRNA levels in both Col-0 and drp2b-2 (S6 Fig.) indicating that robust transcriptional activation of PR1 required activity of Ca2+ channel(s). But importantly, PR1 mRNA levels were still significantly decreased in drp2b-2 compared to Col-0 under these co-treatment conditions (S6 Fig.). Based on these results, we conclude that DRP2B functions as a positive regulator of flg22-induced PR1 mRNA expression that was partially independent of Ca2+-channel activity. Thus, DRP2B's role in modulating PR1 mRNA levels appears to be different from its role as a negative regulator in ROS production and callose deposition, which were dependent on both RbohD and Ca2+-channel activities.
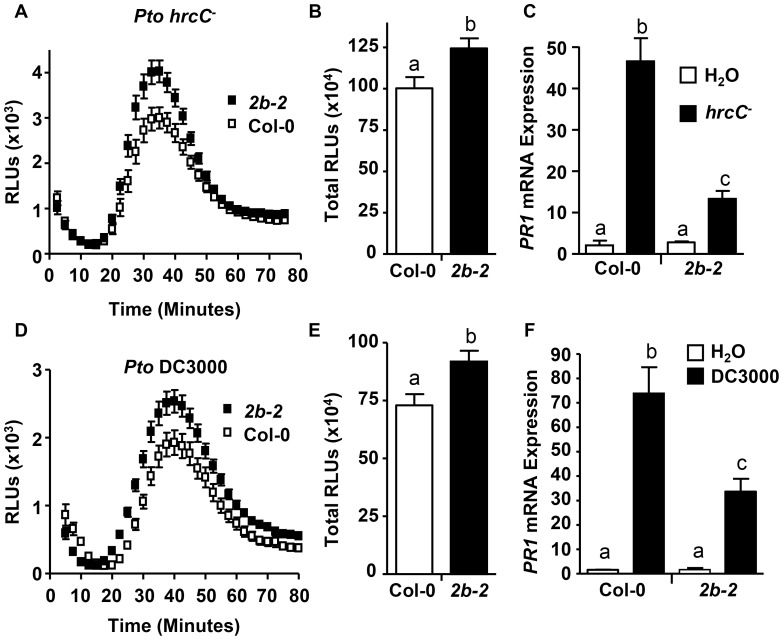
drp2b shows increased ROS production and decreased PR1 mRNA in response to living Pto strains in a Type 3 Secretion System-independent manner
Next, we investigated whether DRP2B's role as a negative regulator for ROS production but as positive a regulator for PR1 mRNA levels was specific to flg22 or whether its regulatory roles may be also observed in response to flagellated bacterial Pto strains. Live Pto cells elicit an early and transient ROS production that is independent of the Type 3 Secretion System (T3SS) and is fully dependent on RbohD and FLS2 [42]. Utilizing this ROS bioassay, ROS production was measured in response to the live Pto DC3000 hrcC− (Pto hrcC−) that elicits PAMP-dependent responses; but Pto hrcC− lacks a functional T3SS, and thus in contrast to the virulent bacterial strain Pto DC3000, Pto hrcC− is defective in effector delivery and cannot suppress Pattern-Triggered Immunity (PTI) [70]. For drp2b-2, treatment with Pto hrcC− resulted in increased ROS production including increased peak ROS (Fig. 5A; around 40 minutes post-treatment) as well as total ROS production (Fig. 5B) compared to Col-0. Similar results were obtained when treating drp2b-2 leaf discs with Pto DC3000 (Fig. 5D-E).
Figure 5. drp2b shows increased ROS and decreased PR1 mRNA to living Pto in a T3SS-independent manner.
(A) and (D) Time-course of ROS production in Col-0 (open symbol) and drp2b-2 (2b-2; filled symbol) in response to living (A) Pto hrcC- or (D) Pto DC3000 (OD600 = 0.1). (n = 32/genotype). (B) and (E) Compared to Col-0, total ROS production was significantly increased in drp2b-2 (2b-2) after elicitation with (B) Pto hrcC− (P<0.01) or (E) Pto DC3000 (P<0.007) based on values shown in (A) or (D), respectively. Relative Light Units, RLU. (C) and (F) Using qRT-PCR, PR1 mRNA levels were significantly reduced in drp2b-2 (black bars) compared to Col-0 (white bars) at 24 hr after infiltration with (C) Pto hrcC− (OD600 = 0.02) (P<0.045) or (F) Pto DC3000 (DC; OD600 = 0.02) (P<0.005). Infiltration with water (white bars) served as mock control. (n = 6/genotype and treatment). All experiments were done using five-to-six week old leaf tissue and repeated at least three independent times with similar results. Values are mean ± SE. Statistical analysis was done as in Fig. 1.
For measuring PR1 mRNA levels, drp2b-2 mutant and wildtype leaves were syringe-infiltrated with Pto hrcC− or Pto DC3000. As determined by qRT-PCR, PR1 mRNA levels were significantly induced by either bacterial strain for both drp2b-2 and Col-0 compared to mock-treated tissue at 24 hours after infiltration; but the Pto-induced expression of PR1 was significantly lower in drp2b-2 than in Col-0 in response to Pto hrcC− or Pto DC3000 (Fig. 5C or 5F, respectively). Thus consistent with the flg22 results, DRP2B is a negative regulator of ROS production but a positive regulator of PR1 mRNA levels in response to live bacterial Pto strains in a T3SS-independent manner.
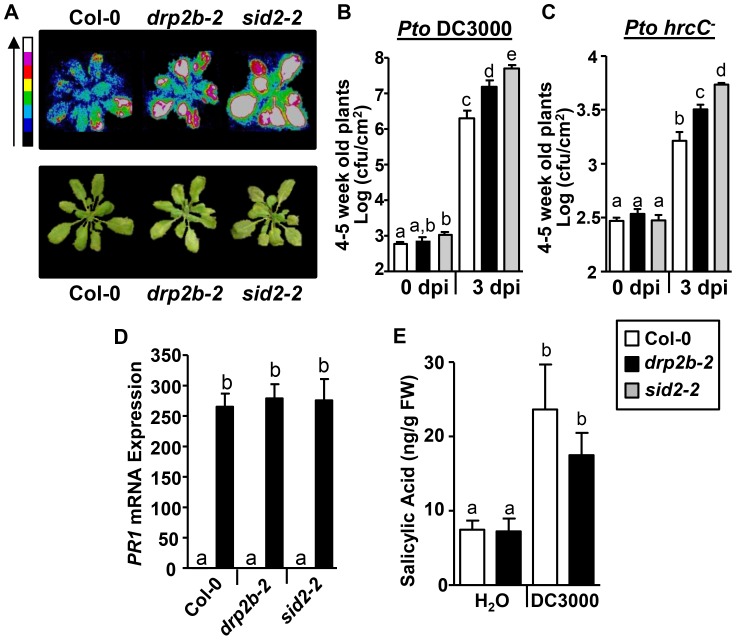
Consistent with decreased PR1 mRNA levels, drp2b plants are more susceptible to Pto DC3000 infection
To examine whether the observed defects in flg22 and Pto-induced signaling responses in drp2b-2 correlated with changes in resistance to these bacterial pathogens, we monitored the growth of Pto DC3000 strains after infiltration into mature five-to-six week old leaves. First, we utilized Pto DC3000 that stably expresses the luxCDABE operon (Pto DC3000lux) [44] allowing for the visualization of growth of this bioluminescent bacterial strain in planta using a Photek luminescent camera [20], [46]. Three days post-infection (3 dpi) with syringe-infiltrated Pto DC3000lux, drp2b-2 exhibited increased bacterial growth compared to Col-0 (Fig. 6A); but drp2b-2 appeared consistently less susceptible than sid2-2 plants known to be highly susceptible to Pto DC3000lux infection because they do not express functional SID2 (SALICYLIC ACID INDUCTION-DEFICIENT 2), a key enzyme in defense-related SA biosynthesis [40]. These results were confirmed by bacterial serial dilution plating assays in that bacterial growth was significantly higher in drp2b-2 than in Col-0 but lower than in sid2-2 at 3 dpi (Fig. 6B). No statistical difference in bacterial growth was observed between drp2b-2 and Col-0 or sid2-2 leaves at 0 dpi (Fig. 6B). Similar results were obtained when drp2b-2 leaves were infiltrated with Pto hrcC− (Fig. 6C), and this increased susceptibility of drp2b-2 to Pto hrcC− was consistent with a function of DRP2B in PTI.
Figure 6. drp2b single mutants are more susceptible to Pseudomonas syringae pv. tomato (Pto) DC3000 and Pto hrcC− infection.
(A) In planta growth of Pto DC3000 LuxCDABE (DC3000lux) in 4–5 week old Col-0, drp2b-2 (2b-2), and sid2-2 plants. Plants were imaged at 3 days post infiltration (dpi) after syringe-infiltration with DC3000lux (OD600 = 0.0005) by bright field or with a Photek camera. A representative plant is shown for each genotype and treatment. Color scale bar indicates increasing photon intensity. (n = 4 plants/genotype and treatment). (B) Compared to Col-0 (white bars), bacterial growth was significantly increased in drp2b-2 plants (black bar) (P<0.0065) at 3 dpi with DC3000lux (OD600 = 0.0005) as measured by serial dilution plating. sid2-2 served as control (gray bars; sid2-2 to Col-0: P<0.0001; to drp2b-2: P<0.02). (n = 7-12/genotype and treatment). (C) Compared to Col-0 (white bars), bacterial growth was significantly increased in drp2b-2 plants (black bar) (P<0.01) at 3 dpi with Pto hrcC- (OD600 = 0.02) as measured by serial dilution planting. sid2-2 served as control (gray bars; sid2-2 to Col-0: P<0.0001; to drp2b-2: P = 0.0005). (n = 6/genotype and treatment). (D) Using qRT-PCR, no difference in PR1 mRNA levels was observed between drp2b-2, sid2-2 and Col-0 24 hrs after treatment with 50 µM exogenous SA (black bars). Treatment with water (H2O; white bars) served as mock control. (n = 4/genotype an treatment). (E) Levels of SA in drp2b-2 (black bars) compared to Col-0 (white bars) at 24 hr post infection (hpi) with Pto DC3000lux (OD600 = 0.02) (P = 0.375). Treatment with water (H2O; white bars) served as mock control (P = 0.91). (n = 4/genotype and treatment; with each n containing 6 seedlings). Experiments in (A-C) and in (D-E) utilized 4–5 week or 2 week old plants, respectively. All experiments were repeated three times with similar results. Values are mean ± SE. Statistical analysis was done as in Fig. 1.
In complementary approaches, plants were grown for two weeks on MS plates and then were fully immersed in a solution of Pto DC3000lux [46] for pathogen infection assays. At this developmental stage, no significant difference in weight was observed between the drp2b-2, Col-0 and sid2-2 plant lines (S7A Fig.). Similar to phenotypic defects observed in five-to-six week old mature drp2b-2 plants (see Figs. 1C-F and 4C), two week old drp2b-2 plants exhibited increased ROS production and reduced PR1 transcript accumulation in response to flg22 (S7B-D Fig.) as well as increased Pto DC3000lux susceptibility and decreased PR1 transcript accumulation in response to Pto DC3000lux compared to Col-0 (S7E-F Fig.).
To gain insight into whether decreased PR1 levels and increased susceptibility in drp2b-2 may be due to defects in SA perception or accumulation, we first measured PR1 mRNA levels after exogenous application of 50 µM SA to 2-week-old plants. As determined by qRT-PCR, drp2b-2 accumulated similar levels of PR1 mRNA levels compared to Col-0 and sid2-2 (Fig. 6D) indicating that SA perception remained intact in drp2b-2 plants. Next, we measured SA levels (ng/fresh weight) in 2-week-old drp2b-2 and Col-0 plants 24 hpi with Pto DC3000lux. No significant difference in SA levels was observed between drp2b-2 and Col-0 after treatment with Pto DC3000lux (Fig. 6E). Likewise, no significant difference in SA levels was observed between drp2b-2 and Col-0 after treatment with flg22 (S7G Fig.). Because SA accumulation was measured under the same experimental conditions as PR1 gene induction and resistance to Pto (S7D-F Fig.), we conclude that the increased susceptibility to Pto DC3000 in drp2b-2 appears to occur independently of SA accumulation.
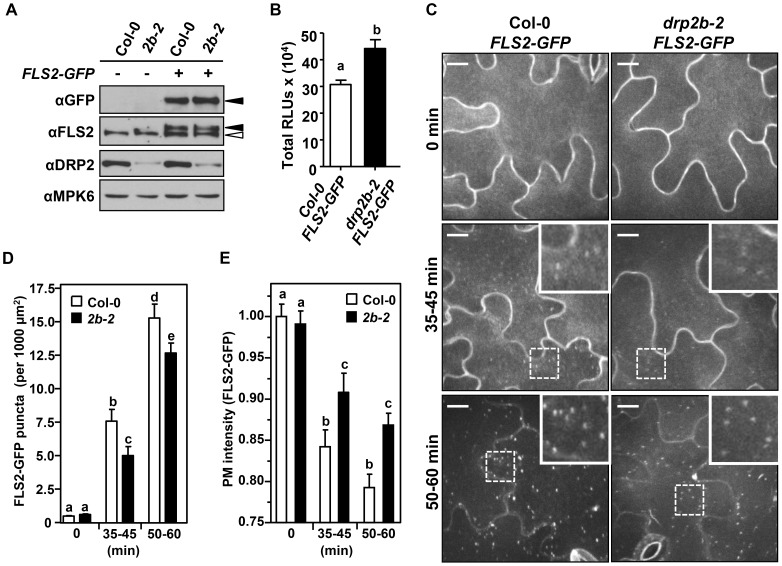
Robust ligand-induced endocytosis of FLS2 requires DRP2B but not DRP2A
Based on the subcellular localization of DRP2B to sites of CME from the PM and its role in constitutive endocytosis of yet unknown cargo [27], [33], DRP2B may modulate the trafficking of PM-resident proteins involved in flg22-signaling. FLS2 is a likely candidate for DRP2B-dependent trafficking because FLS2 undergoes ligand-induced endocytosis and subsequent degradation at 60 minutes post-elicitation [9], [12], [13].
To detect potential spatial and temporal defects in FLS2 localization, we crossed Col-0 plants expressing FLS2pro:FLS2-3xMyc-EGFP [9] with drp2b-2 plants to generate homozygous Col-0 FLS2-GFP and drp2b-2 FLS2-GFP sibling plants for live cell imaging studies. To ensure equal expression of FLS2-GFP in both Col-0 and drp2b-2, we performed immunoblot analyses of un-elicited seedlings under conditions grown and prepared for microscopy. Similar to drp2b-2 mutants, drp2b-2 FLS2-GFP seedlings displayed reduced levels of DRP2 protein (Fig. 7A, αDRP2). Consistent with Fig. 1G, similar levels of endogenous FLS2 were detected between Col-0 FLS2-GFP and drp2b-2 FLS2-GFP plants (Fig. 7A; αFLS2). Importantly, equivalent expression was also observed for GFP-tagged FLS2 in Col-0 and drp2b seedlings (Fig. 7A; αGFP). As observed in drp2b-2 which expressed endogenous FLS2 but not FLS2-GFP (Fig. 1D), drp2b-2 FLS2-GFP cotyledons displayed increased flg22-induced ROS production compared to Col-0 FLS2-GFP (Fig. 7B). This result indicates that ectopic FLS2-GFP expression did not abrogate the difference in flg22-induced ROS production between Col-0 and drp2b-2. Therefore, FLS2-GFP cotyledons can be used as a biologically relevant tissue for microscopic analysis of the role of DRP2B in FLS2 trafficking.
Figure 7. DRP2B is partially required for flg22-induced endocytosis of FLS2.
(A) Col-0 FLS2-GFP and drp2b-2 FLS2-GFP (2b-2) homozygous F4 seedlings expressed similar levels of both endogenous FLS2 and FLS2-GFP as shown by immunoblot analyses of total protein extracts. αFLS2 detected both native FLS2 (open arrow) and FLS2-GFP (closed arrow) while αGFP detected FLS2-GFP only (closed arrow). αDRP2 was used to confirm drp2b-2 mutants, and αMPK6 was used as a loading control. (B) In response to 1 µM flg22, total ROS production was elevated in drp2b-2 FLS2-GFP (black bar) compared to Col-0 FLS2-GFP (white bars) cotyledons (P<0.001). (n = 30 cotyledons/genotype). Relative Light Units, RLU. (C) Flg22-induced endocytosis of FLS2-GFP was not blocked in drp2b-2 cotyledons. Col-0 FLS2-GFP (Col-0) and drp2b-2 FLS2-GFP whole seedlings were treated with 1 µM flg22 to observe un-elicited (constitutive; 0 min) and ligand-induced (35–45, 50–60 min) endocytosis of FLS2-GFP by spinning disc confocal microscopy. Representative maximum-intensity projection images and zoomed insets of FLS2-GFP fluorescence are shown, with bright pixels corresponding to increased abundance of FLS2-GFP at a given location. Scale bars = 10 µm. (D) Quantification of FLS2-GFP in puncta at 0, 35–45 or 50–60 min after elicitation with 1 µM flg22 indicates that loss of DRP2B resulted in ∼20% decrease in flg22-stimulated endocytosis of FLS2-GFP (35–45 min, P = 0.0204; 50–60 min, P = 0.0396). No change in unstimulated accumulation of FLS2-GFP in puncta (P>0.05, 0 min) was observed. Images from two independent experiments were included in the analysis. (n = 44 to 67 images analyzed per genotype/treatment). (E) In drp2b-2 cotyledons, decreased accumulation of FLS2-GFP in puncta correlates with increased PM intensity of FLS2-GFP after elicitation with 1 µM flg22 relative to Col-0 (35–45 min, P = 0.0302; 50–60 min, P = 0.0001). No significant differences are observed in the PM intensity of FLS2-GFP in unstimulated Col-0 and drp2b-2 cotyledons (P>0.05, 0 min). Images from 2–3 independent experiments were included in the analysis. (n = 76–151 images analyzed per genotype/treatment). All experiments were repeated at least three independent times with similar results. Values are mean ± SE. Statistical analysis was done as in Fig. 1.
Next, Col-0 FLS2-GFP and drp2b-2 FLS2-GFP seedlings were used for quantitative live cell imaging using Spinning Disc Confocal Microscopy (SCDM), a technique previously utilized to quantify ligand-induced endocytosis of FLS2-GFP in A. thaliana [9], [14]. First, we confirmed that under un-elicited conditions, FLS2-GFP resided primarily at the PM of pavement cells on the adaxial surface of both Col-0 and drp2b-2 cotyledons (Fig. 7C, 0 min) with detection of some FLS2-GFP in intracellular compartments (Fig. 7D, 0 min) as determined by quantification of the number of FLS2-GFP-positive puncta (per 1000 µm2 area) within cells. In addition, we detected equivalent levels of FLS2-GFP at the PM in un-elicited tissues based on PM intensity measurements (Fig. 7E, 0 min), indicating that altered PM abundance of FLS2 was not a likely cause of early flg22-reponse defects in drp2b. Consistent with previous reports showing ligand-induced internalization of FLS2-GFP and subsequent movement through early and late endosomal compartments that appear as FLS2-GFP-containing puncta [9], [10], [12], [14], elicitation of Col-0 with 1 µM flg22 led to significant internalization of FLS2-GFP into endosomal compartments at 35–45 min and 50–60 min (Fig. 7C-D; Col-0). Endosomal accumulation of FLS2-GFP coincided with a significant decrease in FLS2-GFP detected at the PM (Fig. 7E; Col-0). Similar timing of FLS2-GFP accumulation in endosomes was observed for drp2b-2 (Fig. 7C-D; 35–45 min and 50–60 min, drp2b-2); but importantly, quantification of the number of FLS2-GFP-positive endosomes (puncta per 1000 µm2 area) after flg22-elicitation showed a significant and consistent 20% decrease in drp2b-2 compared to Col-0 (Fig. 7D). This reduction correlated with a significant decrease in removal of FLS2-GFP from the PM in drp2b-2 as determined by PM intensity measurements (Fig. 7E; compare drp2b-2 with Col-0 at 35–45 and 50–60 min). Combined, these results implicate DRP2B in ligand-induced endocytosis of FLS2.
To test for specificity in flg22-induced endocytosis of FLS2 within the DRP2 protein family, we generated drp2a-3 FLS2-GFP and sibling Col-0 FLS2-GFP plants for live-cell imaging that express equivalent levels of endogenous FLS2 and FLS2-GFP (S8A Fig.). First, we confirmed that as for drp2a mutant plants not expressing FLS2-GFP (Figs. 1D-E), drp2a-3 FLS2-GFP cotyledons did not show any significant difference in flg22-induced ROS production compared to Col-0 FLS2-GFP (S8B Fig.). Importantly, as determined by quantitative live cell imaging, no significant differences in the number of FLS2-GFP endosomes were observed in drp2a-3 compared to Col-0 in un-elicted (S8C-D Fig., 0 min) or flg22-elicted cotyledons (S8C-D Fig., 50–60 min), indicating that DRP2A plays no apparent role in ligand-induced internalization of FLS2-GFP.
In conclusion, these live-cell imaging results (Fig. 7 and S8 Fig.) indicate that FLS2 undergoes flg22-induced endocytosis that is partially dependent upon DRP2B, but not the close homolog DRP2A.
Discussion
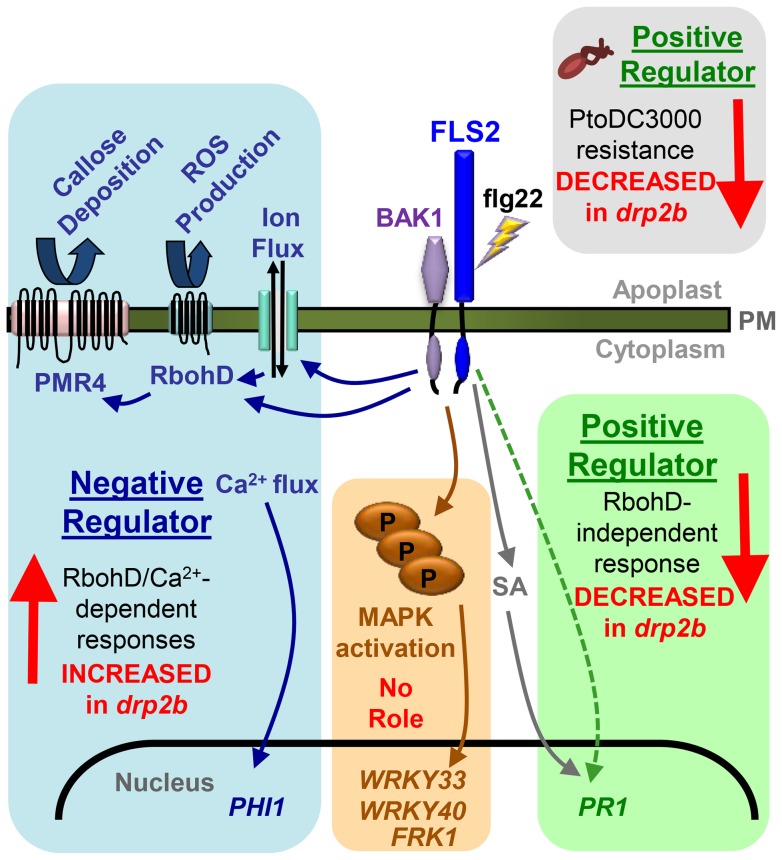
Here, we identified DRP2B, previously implicated as a CCV component with roles in CME in plants [33], [34], as a novel factor functioning in flg22-signaling and innate immunity against Pto DC3000 and Pto hrcC-. We also provide evidence for a role of DRP2B in ligand-induced endocytosis of the plant flagellin receptor FLS2. As summarized in Fig. 8, loss of DRP2B revealed separation of innate immune signaling responses into at least three distinct branches of the flg22-signaling network that differ in their requirement for the NADPH oxidase RbohD.
Figure 8. Summary of non-canonical response defects in drp2b within the different branches of the flg22-signaling network.
Flg22-binding to FLS2 and its co-receptor BAK1 initiates at least three distinct branches of flg22-signaling networks that are differentially affected by loss of DRP2B compared to wildtype plants. All tested RbohD/Ca2+-dependent responses are increased in drp2b plants (blue box) consistent with DRP2's function as a negative regulator of these responses. Loss of DRP2B has no effect upon flg22-induced MAPK pathway activation (brown box) implying that DRP2B has no apparent role in the MAPK pathway. However, drp2b displays decreased PR1 mRNA expression in response to flg22, which occurs independently of RbohD (green box) and may be at least in part, independent of SA. Correlating with decreased transcription of PR1, drp2b plants show decreased resistance to bacterial pathogen infection (Pto DC3000) (gray box) indicating that DRP2B is a positive regulator of resistance to this bacterial strain. The non-canonical combination of phenotypic defects observed in drp2b may be in part due to altered vesicular trafficking of FLS2 and potentially other yet unknown PM-resident cargo proteins. For simplicity, not all known components of the flg22-signaling network are included in this model.
Role of DRP2B in distinct immune signaling branches
Our results strongly support the emerging conceptual change in the field of plant immunity that flg22-signaling events occur through a signaling network rather than a single linear pathway. More specifically, our results discovered that DRP2B has roles as a negative regulator of flg22-induced cytosolic Ca2+ levels, ROS production, PHI1 mRNA levels and callose deposition (Figs. 1C-E, 2A-B and 4A-B). In light of our results and other studies [19], [48], [56], these signaling responses can be placed into the same branch of the flg22-signaling network, which we refer to as the RbohD/Ca2+-dependent branch (Fig. 8). Activation of RbohD has been reported to occur via Ca2+-dependent and independent mechanisms [19], [58], [71]–[73]; but our LaCl3 study indicate that for drp2b, the flg22-elicited increase in cytosolic Ca2+ was fully responsible for the increase in ROS production (S2 Fig.). The identification of a DRP2B peptide as an in vitro substrate for Ca2+-dependent protein kinases (CDPKs) provides further evidence of a link between DRP2B and the Ca2+-dependent response pathway(s) [74].
In contrast to its role as a negative regulator in the RbohD/Ca2+-signaling branch, DRP2B functions as a positive regulator of PR1 mRNA levels in response to flg22 and to Pto in a T3SS-independent manner (Figs. 4C, 5C and 5F). Further support for the proposed separation of these signaling pathways into two distinct flg22-signaling branches (Fig. 8) was provided by assessing the genetic requirement of RbohD for specific flg22-responses. Increases in flg22-induced ROS production and callose deposition observed in drp2b were RbohD-dependent (Figs. 1F and 4B). In contrast, the decrease in flg22-induced PR1 mRNA accumulation, a commonly used marker gene for SA responses, was RbohD-independent (Fig. 4C). If RbohD were to function as a negative regulator of SA-pathway responses within PAMP-triggered immunity as previously proposed [21], [69], then a rescue to wildtype PR1 mRNA levels in drp2b rbohD plants would have been expected after flg22-treatment. Instead, both drp2b and drp2b rbohD plants showed a similarly reduced induction of PR1, indicating that component(s) other than RbohD may function as a negative regulator(s) of PR1/SA-responses. While the inability of drp2b plants to induce wildtype levels of PR1 mRNA correlates well with its increased susceptibility to Pto DC3000 and Pto hrcC- (Figs. 5C, 5F and 6A-C), these phenotypic defects appear to be mostly independent or downstream of SA perception and accumulation (Fig. 6D-E). Consistent with the latter, a recent study dissecting signaling networks triggered by pathogenic Pto strains provides evidence for a separation of PR1 induction and SA accumulation [75]. Furthermore, in response to a pathogenic Pseudomonas strain, the ARF-guanine nucleotide exchange factor (ARF-GEF) AtMIN7 has been recently reported to positively regulate PR1 expression via a SA-independent pathway as a atmin7 mutant displays reduced PR1 expression without a significant reduction in SA accumulation [76]. However in response to flg22, loss of AtMIN7 results in decreased callose deposition but has no effect on PR1 levels [77], indicating that AtMIN7 and DRP2B likely have distinct roles in flg22-signaling.
DRP2B did not appear to play any significant role in modulating the MAPK pathway (MPK6 and MPK3 phosphorylation, upregulation of WRKY33, WRKY40 and FRK1 mRNA levels; Fig. 2C-D and S4 Fig.), which comprises the third branch of the flg22-signaling network in our model (Fig. 8). Thus, our findings provide independent support of recent studies showing separation between flg22-dependent ROS production and MAPK activation using plants defective in gene expression of specific signaling components such as MAPK, CDPKs and RbohD [13], [19], [22], [78]. In agreement, we have recently shown that pretreatment with the chemical inhibitors Wm and TyrA23 impair flg22-induced ROS production but not MAPK phosphorylation [13]. Based upon the results of these chemical interference and genetic studies, it appears that MAPK pathway activation is not dependent upon flg22-stimulated changes in vesicular trafficking. Here, we have expanded on these studies showing that pre-treatment with Wm (Fig. 3), but not TyrA23 (S5 Fig.), disrupts DRP2B function to the extent that genetic loss of DRP2B no longer has a significant effect upon flg22-induced ROS production. As an inhibitor of PI-3 and PI-4 kinase activity, Wm impedes phosphorylation of phosphoinositol lipids within biological membranes, thus potentially disrupting the correct targeting of lipid-binding proteins to specific cellular membranes. For flg22-induced ROS production, Wm may affect the localization of one or more yet unidentified lipid-binding protein(s) in direct or indirect manners. DRP2B is a potential candidate as it contains a Pleckstrin-Homology (PH) domain, which is a lipid-binding domain that enables protein recruitment to phosphorylated phosphoinositides involved in vesicular trafficking or signal transduction; but the lipid binding properties of the DRP2B PH-domain have not been characterized. However, it is also likely that Wm affects (directly or indirectly) other components involved in flg22-induced ROS and/or endocytosis of FLS2. Wm-pretreatment results in strongly impaired ligand-induced endocytic degradation of FLS2 and a greater than 70% reduction of FLS2 accumulation in endosomes [9], [13], compared with the 20% reduction of FLS2 accumulation in endosomes in the drpb2 mutant. Furthermore, flg22-elicitation resulted in increased ROS in drp2b but in decreased ROS after Wm-pretreatment (Fig. 3). Given the fact that Wm reduced ROS production in drp2b to the same level as in WT (Col-0), one explanation for this differential effect could be that besides impeding FLS2 endocytosis, Wm may have additional inhibitory effects on components required for ROS production that function upstream of DRP2B. We cannot exclude the possibility, however, that Wm affects components in separate pathway(s) than DRP2B.
Analysis of the drp2b mutant provided additional insights into how the diverse signaling branches may contribute to effective flg22-signaling and immunity against pathogenic bacteria. Our data supports a previously proposed model [20], [79] in which an important element of robust immunity is transcriptional reprogramming including PR gene expression at a relatively late phase of the immune response. Increased immune responses in the RbohD/Ca2+ branch (at least to the extent observed in drp2b) or normal induction of the MAPK branch were not sufficient to overcome decreased PR1 gene induction or promote wildtype resistance to Pto DC3000 or Pto hrcC-. It is noteworthy that similar to drp2b, mutations in SCD1, a gene encoding a vesicular trafficking protein with putative Rab-GEF activity [80], [81], also result in a non-canonical combination of flg22-signaling responses [20]. These immune response defects are at least in part opposite to those described in this study for drp2b mutant plants. Reduced SCD1 protein levels lead to decreased flg22-induced ROS-production but increased PR1 mRNA levels, as well as increased resistance to Pto DC3000. Thus, SCD1 is a negative regulator [20] while DRP2B is a positive regulator (this study) of immunity against Pto DC3000. Similar to DRP2s, SCD1 is associated with clathrin-coated vesicles (CCVs) [80], [81]; but the exact role(s) of SCD1 in CCV formation or ligand-induced endocytosis of FLS2, or its functional relationship to DRP2B, remain unknown.
Integration of DRP2B's role in immune signaling and trafficking
Overall, relatively little is known about a potential role(s) of flg22-induced endocytosis and subsequent degradation of FLS2 in modulating immune responses [13], [82]. A possible explanation for how loss of DRP2B may result in the non-canonical combination of immune defects may stem, at least in part, from DRP2B's function in ligand-induced endocytosis of FLS2. Drawing from mammalian studies, ligand-induced endocytosis of cell surface receptors may contribute to termination of signaling responses initiated at the PM [83], [84]. Internalization may also ensure contact between receptors and endosomal signaling components to initiate distinct signaling events from endosomes [85], [86].
As shown for mammalian Toll-like receptor 4 (TLR4), the PRR for the bacterial PAMP lipopolysaccharide (LPS), these functions are not mutually exclusive [87]. When disrupting cellular dynamin functions with Dynasore, a chemical inhibitor that generally blocks large GTPase activity including that of dynamins [88], or when expressing a dominant-negative dynamin protein (Dyn K44A), internalization of TLR4 and LPS are disrupted [89], [90]. Analogous to the non-canonical combination of immune defects in drp2b plants, inhibition of dynamin-dependent internalization results in increased signaling through the MyD88/NFκB-branch but decreased signaling through the TRAM-TRIFF-branch [89], [90]. Although much less is known about how trafficking of TLR5, the mammalian cell surface receptor for flagellin, affects immune signaling, flagellin-induced gene expression of pro-inflammatory cytokine and chemokines was significantly reduced after treatment Dynasore [91].
For the Arabidopsis FLS2-flg22 system, it is possible that the very early and transient signaling responses may be in part regulated through FLS2 receptor dynamics, such that delayed endocytosis may lead to heightened signaling from activated FLS2 at the PM. In support of this, drp2b null mutant plants display enhanced flg22-induced cytosolic Ca2+ levels and ROS production, which could be due to the 20% reduction of flg22-induced internalization of FLS2 (Fig. 7D). Endocytic internalization of FLS2 may contribute to a dampening of these early signaling responses. In drp2b, an increase in PHI1 mRNA and callose deposition are likely a consequence of enhanced Ca2+ and ROS production, which are known to function upstream of these later responses. While FLS2 internalization into endosomes may affect some responses, others such as initiation of the MAPK-signaling branch may be independent of ligand-induced endocytosis of FLS2. A delay in FLS2 trafficking through the endosomal compartments could explain the reduced PR1 mRNA levels in drp2b, and such correlation between flg22-induced FLS2 endocytosis and PR1 mRNA expression may likely occur via indirect mechanisms due to the disconnect in timing (within 1 hour for FLS2 internalization and endocytic degradation versus 24 hours for induced PR1 expression). Interestingly, the Lycopersium esculentum (tomato) receptor-like protein LeEix2 has been previously reported to require trafficking from the PM to endosomal compartments to initiate downstream signaling responses upon recognition of the fungal protein ethylene-inducing xylanase (EIX) [92]. Similar to our drp2b results, this study implicates dynamins in ligand-induced endocytosis based on the observation that treatment with the chemical inhibitor Dynasore leads to reduced EIX-induced endocytosis of transiently expressed LeEIX2-GFP in Nicotiana benthamiana [92]. In contrast to our study, however, Dynasore treatment results in reduction of all of the reported EIX-induced responses including impaired ROS production [92]. This difference in signaling response defects may reflect the use of Dynasore, which is known to affect activities of multiple DRPs and large GTPases [88], whereas our study has focused in the analysis of a loss-of-function mutant in a single gene (DRP2B). The number of DRPs in N. benthamiana has not been defined; but A. thaliana contains 16 different DRPs that fall into six subgroups and have diverse cellular functions and subcellular localizations [25].
In addition to FLS2, the activities of other PM-localized factors that function in flg22-responses may be regulated directly or indirectly by DRP2B. One potential candidate may be RbohD, as the diffusion and clustering of RbohD-GFP within the PM has been shown to be impaired in mutant plants lacking Clathrin Heavy Chain 2 (CHC2) [93], which similar to DRP2B, encodes a CCV component functioning in CME [94]. However, it remains to be determined whether mutant plants lacking CHC2 display defects in RbohD-dependent ROS production [93]. Interestingly, immunoprecipitation experiments recently identified DRP2B and RbohD as two of 62 proteins that may form complex(es) with Botrytis-induced kinase 1 (BIK1) [58], a dual specificity receptor-like cytoplasmic kinase with roles in flg22-signaling and plant innate immunity via interactions with FLS2 and BAK1 [95]–[97]. BIK1 directly interacts with and phosphorylates RbohD on multiple serine residues in a calcium-independent manner [58], [72] that are critical for flg22-induced ROS production and contribute to immunity against Pto [18], [58], [72]. The functional significance of the potential (direct or indirect) interaction between DRP2B and BIK1, however, remains unknown. In the longer term, it will be interesting to determine whether the increased flg22-induced ROS production in drp2b may be due to defects in RbohD trafficking and whether these defects may be dependent on BIK1.
To date, very few vesicular trafficking proteins are implicated in affecting FLS2 endocytosis. As for DRP2B, consistent with being a CCV component with roles in CME [33], [34], DRP2B's role in ligand-induced endocytosis of FLS2 may occur via clathrin-dependent mechanisms; but we cannot exclude the possibility that DRP2B, like the mammalian dynamins [30], [31], may function in clathrin-independent endocytosis [35], [36]. Similar to DRP2B, the Arabidopsis ESCRT-1 subunit VPS37-1 was recently shown to be partially required for flg22-stimulated endocytosis of FLS2, in that vps37-1 mutant plants exhibited an approximately 20% reduction in FLS2-GFP accumulation in endosomal compartments [14]. However unlike drp2b, vsp37-1 plants do not display increased ROS production or callose deposition in response to flg22. One possible explanation is that VPS37-1 and DRP2B may regulate flg22-stimulated endocytosis of FLS2 at different trafficking steps, which could have differential effects on flg22-signaling. Consistent with this idea, VPS37-1 is predicted to function at the MVB/LE [14] while DRP2B is reported to localize and function at the PM [17], [18], [32], [33].
Flg22-induced polyubiquitination and subsequent degradation of FLS2 is dependent on two closely-related E3 ligases PUB12 and PUB13 [11]. Loss of PUB12/13 results in elevated flg22-induced ROS and callose deposition [11]. While both drp2b and pub12/13 mutants display increased RbohD-dependent responses, we did not observe a block in flg22-stimulated degradation of FLS2 in drp2b as reported for pub12/13 [11]; but so far, the subcellular compartment(s) at which FLS2 accumulates in the pub12/13 mutants remains to be determined. Furthermore in contrast to drp2b, mutations in pub13 have been reported to result in increased accumulation of SA and PR1 mRNA levels in the absence of any stimulus [98], which may contribute to the increased resistance to infection by various Pto strains in these pub mutants under certain growth conditions [11], [98]. Thus, it would seem that the increase in RbohD-dependent responses and flg22-dependent trafficking defects of FLS2 in drp2b and pub12/13 plants may occur through different mechanisms.
In conclusion, we provide evidence that DRP2B, but not DRP2A, is required for robust ligand-induced endocytosis of FLS2. The identification of DRP2B as a novel component functioning in flg22-signaling and immunity against Pto hrcC− adds DRP2B to the relatively short list of vesicular trafficking proteins involved in PAMP-signaling and PTI. Moreover, our findings underline the importance of a functional vesicular trafficking network for plant innate immune responses for effective immunity against invading pathogenic microbes. In view of the described diversity in immune defects for only a limited number of mutants that affect FLS2 endocytic trafficking, a more refined set of genetic tools is needed to more distinctly dissect to what extent ligand-induced endocytosis of FLS2 contributes to the regulation of individual signaling pathways. As such, the drp2b mutant provides an intriguing tool to gain further insights into how vesicular trafficking and diverse flg22-signaling branches contribute to effective immunity against pathogenic bacteria.
Supporting Information
drp2b mutants display increased ROS in response to multiple PAMPs independent of RbohD and FLS2 mRNA levels. (A) Compared to Col-0 and drp2a-1 (2a-1), peak ROS production (at 10–15 minutes post-elicitation) was significantly increased in drp2b-2 (2b-2) after elicitation with 0.1 µM of active flg22 (black bars) (P<0.0001). Responses to inactive flg22A.tum (white bars) were not different between genotypes (P>0.5). Data were based on time-course experiment from Fig. 1C. (n = 24/genotype and treatment). (B) Compared to Col-0 (white bar) and drp2a-1 (2a-1; gray bar), peak ROS production (10–15 minutes post elicitation) was significantly increased in drp2b-2 (2b-2; black bar) after elicitation with 0.1 µM elf26 (P<0.005). (n = 32/genotype). (C) Using quantitative Real-Time PCR (qRT-PCR) with At2g28390 as the reference gene, mRNA levels of RbohD were not significantly different between drp2b-2 (2b-2; black bar) and Col-0 (white bar). Tissues were cut and prepared exactly as those for ROS experiments in 96-well plates and collected immediately prior to flg22-elicitation (n = 3/genotype; P = 0.9). (D) Based on experimental design and qRT-PCR as described in (C), mRNA levels of FLS2 were not significantly different between drp2b-2 (2b-2; black bar) and Col-0 (white bar) (P = 0.33). (n = 3/genotype). For (A - B), luminol-based ROS production is shown as Relative Light Units (RLU). For (A - D), all experiments were done in 4-5 week old leaf tissue and repeated more than three independent times with similar results. Values are mean ± SE. Different letters indicate significant differences while the same letter indicates no significant differences between samples based on Two tailed student's t-test. ROS experiments shown in the same panel were performed in the same 96-well plate at the same time to allow for direct comparison.
(PDF)
Treatment with the calcium channel blocker LaCl3 abolishes flg22-induced increases in cytosolic Ca2+ levels and ROS production. (A) After 0.1 µM flg22 elicitation, cytosolic Ca2+ levels were significantly elevated in leaf discs from 4–5 week old drp2b-2 expressing the Ca2+-reporter Aequorin (drp2b-2/AEQ; closed symbols) compared to Col-0 expressing AEQ (Col-0/AEQ; open symbols). (n = 6/genotype and treatment). (B) LaCl3 abolished flg22-induced elevations of cytosolic Ca2+ levels to similar levels in drp2b-2/AEQ (closed symbols) compared to Col-0/AEQ (open symbols). 8-day old plants were pretreated with 10 mM LaCl3 (triangles) or water (squares) for 30 minutes, washed with water and then elicited with 0.1 µM flg22. (n = 6/genotype and treatment). (C) LaCl3 abolished flg22-induced ROS production to similar levels in drp2b-2 (square) compared to Col-0 (diamond). Leaf discs from 4–5 week old plants were pretreated with 1 mM LaCl3 (open symbols) or water (closed symbols) for 30 minutes, washed with water and then elicited with 0.1 µM flg22. (n = 24/genotype and treatment). For all experiments, values are mean ± SE. Each experiment was repeated more than three independent times with similar results. Statistical analysis was done as in S1 Fig.
(PDF)
DRP2B has no apparent role in the flg22-induced expression of PER62, PER4 and NHL10 . (A) and (B) Using qRT-PCR with At2g28390 as the reference gene, mRNA levels of PER62 and PER4 were not significantly different between drp2b-2 and Col-0 after elicitation for 30 minutes with water (white bars) or 1 µM flg22 (black bars). P-values for PER62 or PER4 mRNA levels between drp2b-2 and Col-0 were all P>0.5. (n = 3/genotype and treatment). (C) Using qRT-PCR with At2g28390 as the reference gene, mRNA levels of NHL10 were not significantly different between drp2b-2 and Col-0 after elicitation for 60 minutes with water (white bars) or 1 µM flg22 (black bars). P-values for NHL10 mRNA levels between drp2b-2 and Col-0 were all P>0.5. (n = 3/genotype and treatment). All experiments were done in leaf tissue of 4–5 week old plants and repeated at least 3 times with similar results. Values are mean ± SE. Statistical analysis was done as in S1 Fig.
(PDF)
DRP2B has no apparent role in flg22-induced MAPK phosphorylation over 45 minutes post-elicitation. (A) Quantification of protein bands from Fig. 2C using Bio-Rad Quantity One software. Data is presented as the ratio of either phosphorylated MPK6 (P-MPK6) or MPK3 (P-MPK3) relative to Calnexin protein levels. For quantification of P-MPK6 and P-MPK3, all data were normalized to the respective phosphorylated MAPK levels of the Col-0/10 minute timepoint. Quantified data represent the means ± SE from four independent biological repeats. (B) No apparent difference in flg22-induced phosphorylation of MPK3 and MPK6 was observed between Col-0 and drp2b-2 over 45 minutes (min) after elicitation with 0.1 µM active flg22. Immunoblot analysis was done on total protein extracts probed with an antibody for phosphorylated MAPKs (P-MPK3 and P-MPK6). αCalnexin served as loading control. The depicted blot is representative of 3 individual experiments showing similar results. (C) Quantification of protein bands from S4B Fig. using Bio-Rad Quantity One software. Data is presented as the ratio of phosphorylated MPK6 (P-MPK6), MPK3 (P-MPK3), and an unknown MAPK (P-MPK?, potentially P-MPK4 or P-MPK11) relative to Calnexin protein expression. For quantification of P-MPK6, P-MPK3, and P-MPK?, all data was normalized to the respective phosphorylated MAPK levels of the Col-0/15 minute timepoint. Quantified data represent the means ± SE from four independent biological repeats. Statistical analysis was done as in S1 Fig.
(PDF)
For drp2b , the increase in flg22-induced ROS production is not fully sensitive to Tyrphostin A23. (A) After a one-hour pretreatment in the absence (square) or presence (triangle) of 100 µM Tyrphostin A23 (T23), ROS production was measured over 40 minutes in drp2b-2 (closed symbols) and Col-0 (open symbols) in response to 1 µM flg22 (n = 24/treatment and genotype). (B) Total ROS production based on time-course experiments shown in (A) indicates that in drp2b-2 (black bars), the increase in flg22-induced ROS production was not completely TyrA23-sensitive (drp2b-2+T23 vs Col-0+T23, P<0.025). Experiments were done using 4–5 week old leaf tissue and repeated at least 3 independent times with similar results. Values are mean ± SE. Statistical analysis was done as in S1 Fig. Relative Light Units, RLU.
(PDF)
The Ca2+ channel blocker LaCl3 further reduces flg22-induced PR1 mRNA levels in drp2b-2 . Using qRT-PCR with At2g28390 as the reference gene, flg22-induced PR1 mRNA levels were significantly reduced in drp2b-2 (black bars) compared to Col-0 (white bars) 24 hr after co-treatment with flg22 and LaCl3 (P<0.05). Eight-day-old seedlings were treated with water, 10 mM LaCl3, 1 µM flg22 or co-treatment with 10 mM LaCl3 and 1 µM flg22 (flg22+ LaCl3). Water and LaCl3 treatment served as a controls. Data represent the mean ± SE of four or more independent experiments (n≥12). Statistical analysis was done as in S1 Fig.
(PDF)
14-day old drp2b-2 plants display similar phenotypic defects in response to flg22 or Pto DC3000 as described for 5-6 week old drp2b-2 plants. (A) No significant difference in the fresh weight of 14-day old plants was observed between drp2b-2 (black bar) and sid2-2 (gray bar) compared to Col-0 (white bar) (P>0.5). (n = 21/genotype). (B) In time-course experiments, ROS production was elevated in 14-day old drp2b-2 plants (filled shapes) compared to Col-0 (open shapes) in response to 0.1 µM flg22. (n = 32/genotype). (C) In 14-day old plants, total ROS production was significantly increased in drp2b-2 plants (black bar) compared to Col-0 (white bar) after elicitation with 0.1 µM flg22 (P<0.01). Data were based on time-course experiment shown in Figure (B). (D) Using qRT-PCR with At2g28390 as the reference gene, PR1 mRNA levels were significantly reduced in drp2b-2 (black bars) compared to Col-0 (white bars) at 24 hr after treatment with 1 µM flg22 (P<0.05). Treatment with water served as mock control. (n = 4/genotype and treatment). (E) Compared to Col-0 (white bars), bacterial growth was significantly increased in 14-day old drp2b-2 plants (black bar) at 3 dpi with Pto DC3000lux (OD600 = 0.0005) as measured by serial dilution plating (P<0.05). sid2-2 (gray bar) served as positive control (P<0.0001). (n = 8/genotype). (F) Using qRT-PCR, PR1 mRNA levels were significantly reduced in drp2b-2 (black bars) compared to Col-0 (white bars) at 24 hr after treatment with Pto DC3000lux (OD600 = 0.0005) (P<0.009). Treatment with water (white bars) served as mock control. (n = 4/genotype). (G) Levels of SA in Col-0 (white bars) and drp2b-2 (black bars) 12-day old plants treated for 24 hr with 1 µM flg22 +0.1% DMSO in water (P = 0.2; n = 3/genotype; each n contained 25 seedlings). Treatment with 0.1% DMSO in water served as mock control (P = 0.3; n = 2/genotype; each n contained 25 seedlings). Experiment was repeated twice with similar results. Unless stated differently, all experiments were done using 14-day old plants and repeated at least 3 independent times with similar results. Values are mean ± SE, and statistical analysis was done as in S1 Fig. ROS experiments shown in the same panel were performed in the same 96-well plate at the same time to allow for direct comparison. Relative Light Units, RLU.
(PDF)
DRP2A is not required for robust flg22-induced endocytosis of FLS2. (A) Col-0 FLS2-GFP and drp2a-3 FLS2-GFP homozygous F4 seedlings expressed similar levels of both endogenous FLS2 and FLS2-GFP as shown by immunoblot analyses of total protein extracts. αFLS2 detected both native FLS2 (open arrow) and FLS2-GFP (closed arrow), while αGFP detected FLS2-GFP only (closed arrow). αDRP2 was used to confirm drp2a-3 mutants, and αMPK6 was used as a loading control. (B) In response to 1 µM flg22, no significant differences are observed in total ROS between Col-0 FLS2-GFP and drp2a-3 FLS2-GFP (P = 0.4) (n = 30 cotyledons/genotype). Relative Light Units, RLU. (C) Flg22-induced endocytosis of FLS2-GFP in Col-0 FLS2-GFP (Col-0) and drp2a-3 FLS2-GFP was determined by spinning disc confocal microscopy. Whole seedlings were treated with 1 µM flg22 to observe un-elicited (constitutive; 0 min) and ligand-induced (50–60 min) endocytosis of FLS2-GFP. Representative maximum-intensity projection images and zoomed insets of FLS2-GFP fluorescence are shown. Scale bars = 10 µm. (D) Quantification of FLS2-GFP in puncta at 0 min and 50–60 min after elicitation with 1 µM flg22 showed no significant differences between drp2a-3 and Col-0 (0 min, P = 0.268; 50–60 min, P = 0.702). (n = 28–55 images analyzed per genotype/treatment). All experiments were done using 7-day old seedlings and were repeated at least three independent times with similar results. Values are mean ± SE, and statistical analysis was done as in S1 Fig.
(PDF)
List of oligonucleotide sequences used as primers.
(PDF)
Supplemental Materials and Methods.
(PDF)
Acknowledgments
The authors thank Sean Rogers, Kelsey Mescher, Dan Salamango (all Univ. of Missouri) and Dr. Kiwamu Tanaka (Washington State Univ.) for technical assistance and help; Carina Collins, Drs. Scott Peck, Jeff Anderson and Walter Gassmann (all Univ. of Missouri) for helpful discussions and comments; Dr. Jeanne Mihail (Univ. of Missouri) for discussion on statistical analyses; the 2012 Fiji Workshop organized by the Midwest Plant Cell Dynamics Meeting and LOCI group (Univ. of Wisconsin-Madison) for introduction to using Fiji for image analysis. Thanks to Dr. Scott Peck (Univ. of Missouri) for rbohD and MPK6 antibody, Dr. Marc Knight (Durham University, England) for the AEQ reporter line, Dr. Silke Robatzek (Sainsbury Laboratory, England) for the FLS2-GFP line and Dr. Neil Hoffman (USDA, Maryland) for Calnexin antibody.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the National Science Foundation (IOS; No. 1147032) (AH), University of Missouri-Research Board (AH), Daniel F. Millikan Graduate Fellowship (University of Missouri-Division of Plant Sciences) (JMS), University of Missouri CAFNR-Undergraduate Research Internship (Dudley & Virgie Alexander Scholarship) (SJR), and National Science Foundation (No. 0446157) (SYB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Macho Alberto P, Zipfel C (2014) Plant PRRs and the Activation of Innate Immune Signaling. Molecular Cell 54: 263–272. [DOI] [PubMed] [Google Scholar]
- 2. Boller T, Felix G (2009) A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annual Review of Plant Biology 60: 379–406. [DOI] [PubMed] [Google Scholar]
- 3. Kumar H, Kawai T, Akira S (2011) Pathogen Recognition by the Innate Immune System. International Reviews of Immunology 30: 16–34. [DOI] [PubMed] [Google Scholar]
- 4. Bardy SL, Ng SYM, Jarrell KF (2003) Prokaryotic motility structures. Microbiology 149: 295–304. [DOI] [PubMed] [Google Scholar]
- 5. Clarke CR, Chinchilla D, Hind SR, Taguchi F, Miki R, et al. (2013) Allelic variation in two distinct Pseudomonas syringae flagellin epitopes modulates the strength of plant immune responses but not bacterial motility. New Phytologist 200: 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robatzek S, Wirthmueller L (2013) Mapping FLS2 function to structure: LRRs, kinase and its working bits. Protoplasma 250: 671–681. [DOI] [PubMed] [Google Scholar]
- 7. Monaghan J, Zipfel C (2012) Plant pattern recognition receptor complexes at the plasma membrane. Current Opinion in Plant Biology 15: 349–357. [DOI] [PubMed] [Google Scholar]
- 8. Sun Y, Li L, Macho AP, Han Z, Hu Z, et al. (2013) Structural Basis for flg22-Induced Activation of the Arabidopsis FLS2-BAK1 Immune Complex. Science 342: 624–628. [DOI] [PubMed] [Google Scholar]
- 9. Beck M, Zhou J, Faulkner C, MacLean D, Robatzek S (2012) Spatio-Temporal Cellular Dynamics of the Arabidopsis Flagellin Receptor Reveal Activation Status-Dependent Endosomal Sorting. Plant Cell 24: 4205–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi S-w, Tamaki T, Ebine K, Uemura T, Ueda T, et al. (2013) RABA Members Act in Distinct Steps of Subcellular Trafficking of the FLAGELLIN SENSING2 Receptor. Plant Cell 25: 1174–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu D, Lin W, Gao X, Wu S, Cheng C, et al. (2011) Direct Ubiquitination of Pattern Recognition Receptor FLS2 Attenuates Plant Innate Immunity. Science 332: 1439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robatzek S, Chinchilla D, Boller T (2006) Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes & Development 20: 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith JM, Salamango DJ, Leslie ME, Collins CA, Heese A (2014) Sensitivity to Flg22 Is Modulated by Ligand-Induced Degradation and de Novo Synthesis of the Endogenous Flagellin-Receptor FLAGELLIN-SENSING2. Plant Physiology 164: 440–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spallek T, Beck M, Ben Khaled S, Salomon S, Bourdais G, et al. (2013) ESCRT-I Mediates FLS2 Endosomal Sorting and Plant Immunity. PLoS Genet 9: e1004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicaise V, Roux M, Zipfel C (2009) Recent Advances in PAMP-Triggered Immunity against Bacteria: Pattern Recognition Receptors Watch over and Raise the Alarm. Plant Physiol 150: 1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tena G, Boudsocq M, Sheen J (2011) Protein kinase signaling networks in plant innate immunity. Current Opinion in Plant Biology 14: 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benschop JJ, Mohammed S, O'Flaherty M, Heck AJR, Slijper M, et al. (2007) Quantitative Phosphoproteomics of Early Elicitor Signaling in Arabidopsis. Molecular & Cellular Proteomics 6: 1198–1214. [DOI] [PubMed] [Google Scholar]
- 18. Nühse TS, Bottrill AR, Jones AME, Peck SC (2007) Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant Journal 51: 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, et al. (2010) Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464: 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Korasick DA, McMichael C, Walker KA, Anderson JC, Bednarek SY, et al. (2010) Novel Functions of Stomatal Cytokinesis-Defective 1 (SCD1) in Innate Immune Responses against Bacteria. Journal of Biological Chemistry 285: 23342–23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F (2009) Network Properties of Robust Immunity in Plants. PLoS Genet 5: e1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu J, Xie J, Yan C, Zou X, Ren D, et al. (2014) A chemical genetic approach demonstrates that MPK3/MPK6 activation and NADPH oxidase-mediated oxidative burst are two independent signaling events in plant immunity. Plant Journal 77: 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henty-Ridilla JL, Li J, Day B, Staiger CJ (2014) ACTIN DEPOLYMERIZING FACTOR4 Regulates Actin Dynamics during Innate Immune Signaling in Arabidopsis. Plant Cell 26: 340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bednarek SY, Backues SK (2010) Plant dynamin-related protein families DRP1 and DRP2 in plant development. Biochemical Society Transactions 038: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hong Z, Bednarek SY, Blumwald E, Hwang I, Jurgens G, et al. (2003) A unified nomenclature for Arabidopsis dynamin-related large GTPases based on homology and possible functions. Plant Molecular Biology 53: 261–265. [DOI] [PubMed] [Google Scholar]
- 26. Backues SK, Korasick DA, Heese A, Bednarek SY (2010) The Arabidopsis Dynamin-Related Protein2 Family Is Essential for Gametophyte Development. Plant Cell 22: 3218–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor N (2011) A role for Arabidopsis dynamin related proteins DRP2A/B in endocytosis; DRP2 function is essential for plant growth. Plant Molecular Biology 76: 117–129. [DOI] [PubMed] [Google Scholar]
- 28. Ferguson SM, De Camilli P (2012) Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 13: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pucadyil TJ, Schmid SL (2009) Conserved Functions of Membrane Active GTPases in Coated Vesicle Formation. Science 325: 1217–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Howes MT, Mayor S, Parton RG (2010) Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Current Opinion in Cell Biology 22: 519–527. [DOI] [PubMed] [Google Scholar]
- 31. Sandvig K, Pust S, Skotland T, van Deurs B (2011) Clathrin-independent endocytosis: mechanisms and function. Current Opinion in Cell Biology 23: 413–420. [DOI] [PubMed] [Google Scholar]
- 32. Fujimoto M, Arimura S-i, Nakazono M, Tsutsumi N (2008) Arabidopsis dynamin-related protein DRP2B is co-localized with DRP1A on the leading edge of the forming cell plate. Plant Cell Rep 27: 1581–1586. [DOI] [PubMed] [Google Scholar]
- 33. Fujimoto M, Arimura S-i, Ueda T, Takanashi H, Hayashi Y, et al. (2010) Arabidopsis dynamin-related proteins DRP2B and DRP1A participate together in clathrin-coated vesicle formation during endocytosis. Proc Natl Acad Sci U S A 107: 6094–6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xiong G, Li R, Qian Q, Song X, Liu X, et al. (2010) The rice dynamin-related protein DRP2B mediates membrane trafficking, and thereby plays a critical role in secondary cell wall cellulose biosynthesis. Plant Journal 64: 56–70. [DOI] [PubMed] [Google Scholar]
- 35. Bandmann V, Homann U (2012) Clathrin-independent endocytosis contributes to uptake of glucose into BY-2 protoplasts. Plant J 70: 578–584. [DOI] [PubMed] [Google Scholar]
- 36. Li R, Liu P, Wan Y, Chen T, Wang Q, et al. (2012) A membrane microdomain-associated protein, Arabidopsis Flot1, is involved in a clathrin-independent endocytic pathway and is required for seedling development. Plant Cell 24: 2105–2122 doi: 2110.1105/tpc.2112.095695. Epub 092012 May 095615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hirano K, Kotake T, Kamihara K, Tsuna K, Aohara T, et al. (2010) Rice BRITTLE CULM 3 (BC3) encodes a classical dynamin OsDRP2B essential for proper secondary cell wall synthesis. Planta 232: 95–108. [DOI] [PubMed] [Google Scholar]
- 38. Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, et al. (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci U S A 104: 12217–12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci U S A 99: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565. [DOI] [PubMed] [Google Scholar]
- 41. Knight H, Trewavas AJ, Knight MR (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith J, Heese A (2014) Rapid bioassay to measure early reactive oxygen species production in Arabidopsis leave tissue in response to living Pseudomonas syringae. Plant Methods 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanaka K, Swanson SJ, Gilroy S, Stacey G (2010) Extracellular Nucleotides Elicit Cytosolic Free Calcium Oscillations in Arabidopsis. Plant Physiology 154: 705–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fan J, Crooks C, Lamb C (2008) High-throughput quantitative luminescence assay of the growth in planta of Pseudomonas syringae chromosomally tagged with Photorhabdus luminescens luxCDABE . Plant Journal 53: 393–399. [DOI] [PubMed] [Google Scholar]
- 45. Tsuda K, Sato M, Glazebrook J, Cohen JD, Katagiri F (2008) Interplay between MAMP-triggered and SA-mediated defense responses. Plant Journal 53: 763–775. [DOI] [PubMed] [Google Scholar]
- 46. Anderson JC, Bartels S, Besteiro MAG, Shahollari B, Ulm R, et al. (2011) Arabidopsis MAP Kinase Phosphatase 1 (AtMKP1) negatively regulates MPK6-mediated PAMP responses and resistance against bacteria. Plant Journal 67: 258–268. [DOI] [PubMed] [Google Scholar]
- 47. Libault M, Wan J, Czechowski T, Udvardi M, Stacey G (2007) Identification of 118 Arabidopsis Transcription Factor and 30 Ubiquitin-Ligase Genes Responding to Chitin, a Plant-Defense Elicitor. Molecular Plant-Microbe Interactions 20: 900–911. [DOI] [PubMed] [Google Scholar]
- 48. Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, et al. (2011) Callose Deposition: A Multifaceted Plant Defense Response. Molecular Plant-Microbe Interactions 24: 183–193. [DOI] [PubMed] [Google Scholar]
- 49. Koo AJK, Cooke TF, Howe GA (2011) Cytochrome P450 CYP94B3 mediates catabolism and inactivation of the plant hormone jasmonoyl-L-isoleucine. Proc Natl Acad Sci U S A 108: 9298–9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koo AJK, Gao X, Daniel Jones A, Howe GA (2009) A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant Journal 59: 974–986. [DOI] [PubMed] [Google Scholar]
- 51. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Meth 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hall M, Frank E, Holmes G, Pfahringer B, Reutemann P, et al. (2009) The WEKA data mining software: an update. SIGKDD Explor Newsl 11: 10–18. [Google Scholar]
- 53. Boutrot F, Segonzac C, Chang KN, Qiao H, Ecker JR, et al. (2010) Direct transcriptional control of the Arabidopsis immune receptor FLS2 by the ethylene-dependent transcription factors EIN3 and EIL1. Proc Natl Acad Sci U S A 107: 14502–14507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, et al. (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500. [DOI] [PubMed] [Google Scholar]
- 55. Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant Journal 18: 265–276. [DOI] [PubMed] [Google Scholar]
- 56. Zhang J, Shao F, Li Y, Cui H, Chen L, et al. (2007) A Pseudomonas syringae Effector Inactivates MAPKs to Suppress PAMP-Induced Immunity in Plants. Cell Host & Microbe 1: 175–185. [DOI] [PubMed] [Google Scholar]
- 57. Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D (2011) Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant Journal 68: 100–113. [DOI] [PubMed] [Google Scholar]
- 58. Li L, Li M, Yu L, Zhou Z, Liang X, et al. (2014) The FLS2-Associated Kinase BIK1 Directly Phosphorylates the NADPH Oxidase RbohD to Control Plant Immunity. Cell Host & Microbe 15: 329–338. [DOI] [PubMed] [Google Scholar]
- 59. Mao G, Meng X, Liu Y, Zheng Z, Chen Z, et al. (2011) Phosphorylation of a WRKY Transcription Factor by Two Pathogen-Responsive MAPKs Drives Phytoalexin Biosynthesis in Arabidopsis. Plant Cell 23: 1639–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Munnik T, Nielsen E (2011) Green light for polyphosphoinositide signals in plants. Current Opinion in Plant Biology 14: 489–497. [DOI] [PubMed] [Google Scholar]
- 61. Wang J, Cai Y, Miao Y, Lam SK, Jiang L (2009) Wortmannin induces homotypic fusion of plant prevacuolar compartments. Journal of Experimental Botany 60: 3075–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ito E, Fujimoto M, Ebine K, Uemura T, Ueda T, et al. (2012) Dynamic behavior of clathrin in Arabidopsis thaliana unveiled by live imaging. Plant Journal 69: 204–216. [DOI] [PubMed] [Google Scholar]
- 63. Banbury DN, Oakley JD, Sessions RB, Banting G (2003) Tyrphostin A23 Inhibits Internalization of the Transferrin Receptor by Perturbing the Interaction between Tyrosine Motifs and the Medium Chain Subunit of the AP-2 Adaptor Complex. Journal of Biological Chemistry 278: 12022–12028. [DOI] [PubMed] [Google Scholar]
- 64. Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, et al. (2007) Clathrin-Mediated Constitutive Endocytosis of PIN Auxin Efflux Carriers in Arabidopsis. Current Biology 17: 520–527. [DOI] [PubMed] [Google Scholar]
- 65. Irani NG, Di Rubbo S, Mylle E, Van den Begin J, Schneider-Pizoń J, et al. (2012) Fluorescent castasterone reveals BRI1 signaling from the plasma membrane. Nat Chem Biol 8: 583–589. [DOI] [PubMed] [Google Scholar]
- 66. Konopka CA, Bednarek SY (2008) Comparison of the Dynamics and Functional Redundancy of the Arabidopsis Dynamin-Related Isoforms DRP1A and DRP1C during Plant Development. Plant Physiol 147: 1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leborgne-Castel N, Lherminier J, Der C, Fromentin J, Houot V, et al. (2008) The Plant Defense Elicitor Cryptogein Stimulates Clathrin-Mediated Endocytosis Correlated with Reactive Oxygen Species Production in Bright Yellow-2 Tobacco Cells. Plant Physiology 146: 1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ortiz-Zapater E, Soriano-Ortega E, Marcote MJ, Ortiz-Masiá D, Aniento F (2006) Trafficking of the human transferrin receptor in plant cells: effects of tyrphostin A23 and brefeldin A. Plant Journal 48: 757–770. [DOI] [PubMed] [Google Scholar]
- 69. Torres MA, Jones JDG, Dangl JL (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37: 1130–1134. [DOI] [PubMed] [Google Scholar]
- 70. Mansfield JW (2009) From bacterial avirulence genes to effector functions via the hrp delivery system: an overview of 25 years of progress in our understanding of plant innate immunity. Molecular Plant Pathology 10: 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dubiella U, Seybold H, Durian G, Komander E, Lassig R, et al. (2013) Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci U S A 110: 8744–8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, et al. (2014) Direct Regulation of the NADPH Oxidase RBOHD by the PRR-Associated Kinase BIK1 during Plant Immunity. Molecular Cell 54: 43–55. [DOI] [PubMed] [Google Scholar]
- 73. Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, et al. (2007) Calcium-Dependent Protein Kinases Regulate the Production of Reactive Oxygen Species by Potato NADPH Oxidase. Plant Cell 19: 1065–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Curran A, Chang I-F, Chang C-L, Garg S, Miguel RM, et al. (2011) Calcium-dependent protein kinases from Arabidopsis show substrate specificity differences in an analysis of 103 substrates. Front Plant Sci 2: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tsuda K, Mine A, Bethke G, Igarashi D, Botanga CJ, et al. (2013) Dual Regulation of Gene Expression Mediated by Extended MAPK Activation and Salicylic Acid Contributes to Robust Innate Immunity in Arabidopsis thaliana. PLoS Genet 9: e1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gangadharan A, Sreerekha M-V, Whitehill J, Ham JH, Mackey D (2013) The Pseudomonas syringae pv. tomato Type III Effector HopM1 Suppresses Arabidopsis Defenses Independent of Suppressing Salicylic Acid Signaling and of Targeting AtMIN7. PLoS ONE 8: e82032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nomura K, Mecey C, Lee Y-N, Imboden LA, Chang JH, et al. (2011) Effector-triggered immunity blocks pathogen degradation of an immunity-associated vesicle traffic regulator in Arabidopsis. Proc Natl Acad Sci U S A 108: 10774–10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Segonzac C, Feike D, Gimenez-Ibanez S, Hann DR, Zipfel C, et al. (2011) Hierarchy and Roles of Pathogen-Associated Molecular Pattern-Induced Responses in Nicotiana benthamiana. Plant Physiology 156: 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lu X, Tintor N, Mentzel T, Kombrink E, Boller T, et al. (2009) Uncoupling of sustained MAMP receptor signaling from early outputs in an Arabidopsis endoplasmic reticulum glucosidase II allele. Proc Natl Acad Sci U S A 106: 22522–22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Falbel TG, Koch LM, Nadeau JA, Segui-Simarro JM, Sack FD, et al. (2003) SCD1 is required for cytokinesis and polarized cell expansion in Arabidopsis thaliana. Development 130: 4011–4024. [DOI] [PubMed] [Google Scholar]
- 81. McMichael CM, Reynolds GD, Koch LM, Wang C, Jiang N, et al. (2013) Mediation of Clathrin-Dependent Trafficking during Cytokinesis and Cell Expansion by Arabidopsis STOMATAL CYTOKINESIS DEFECTIVE Proteins. Plant Cell 25: 3910–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Beck M, Heard W, Mbengue M, Robatzek S (2012) The INs and OUTs of pattern recognition receptors at the cell surface. Current Opinion in Plant Biology 15: 367–374. [DOI] [PubMed] [Google Scholar]
- 83. Scita G, Di Fiore PP (2010) The endocytic matrix. Nature 463: 464–473. [DOI] [PubMed] [Google Scholar]
- 84. Sorkin A, von Zastrow M (2009) Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol 10: 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Irani NG, Russinova E (2009) Receptor endocytosis and signaling in plants. Current Opinion in Plant Biology 12: 653–659. [DOI] [PubMed] [Google Scholar]
- 86. Kagan Jonathan C (2012) Signaling Organelles of the Innate Immune System. Cell 151: 1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. McGettrick AF, O'Neill LAJ (2010) Localisation and trafficking of Toll-like receptors: an important mode of regulation. Current Opinion in Immunology 22: 20–27. [DOI] [PubMed] [Google Scholar]
- 88. Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, et al. (2006) Dynasore, a Cell-Permeable Inhibitor of Dynamin. Developmental Cell 10: 839–850. [DOI] [PubMed] [Google Scholar]
- 89. Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, et al. (2006) Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J 25: 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang Y, Yang Y, Liu X, Wang N, Cao H, et al. (2012) Inhibition of clathrin/dynamin-dependent internalization interferes with LPS-mediated TRAM–TRIF-dependent signaling pathway. Cellular Immunology 274: 121–129. [DOI] [PubMed] [Google Scholar]
- 91. Parker D, Prince A (2013) Epithelial Uptake of Flagella Initiates Proinflammatory Signaling. PLoS ONE 8: e59932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sharfman M, Bar M, Ehrlich M, Schuster S, Melech-Bonfil S, et al. (2011) Endosomal signaling of the tomato leucine-rich repeat receptor-like protein LeEix2. Plant Journal 68: 413–423. [DOI] [PubMed] [Google Scholar]
- 93. Hao H, Fan L, Chen T, Li R, Li X, et al. (2014) Clathrin and Membrane Microdomains Cooperatively Regulate RbohD Dynamics and Activity in Arabidopsis. The Plant Cell Online 26: 1729–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kitakura S, Vanneste S, Robert S, Löfke C, Teichmann T, et al. (2011) Clathrin Mediates Endocytosis and Polar Distribution of PIN Auxin Transporters in Arabidopsis. Plant Cell 23: 1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lu D, Wu S, Gao X, Zhang Y, Shan L, et al. (2010) A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci U S A 107: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang J, Li W, Xiang T, Liu Z, Laluk K, et al. (2010) Receptor-like Cytoplasmic Kinases Integrate Signaling from Multiple Plant Immune Receptors and Are Targeted by a Pseudomonas syringae Effector. Cell Host & Microbe 7: 290–301. [DOI] [PubMed] [Google Scholar]
- 97. Lin W, Li B, Lu D, Chen S, Zhu N, et al. (2014) Tyrosine phosphorylation of protein kinase complex BAK1/BIK1 mediates Arabidopsis innate immunity. Proc Natl Acad Sci U S A 111: 3632–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li W, Ahn I-P, Ning Y, Park C-H, Zeng L, et al. (2012) The U-Box/ARM E3 Ligase PUB13 Regulates Cell Death, Defense, and Flowering Time in Arabidopsis. Plant Physiology 159: 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
drp2b mutants display increased ROS in response to multiple PAMPs independent of RbohD and FLS2 mRNA levels. (A) Compared to Col-0 and drp2a-1 (2a-1), peak ROS production (at 10–15 minutes post-elicitation) was significantly increased in drp2b-2 (2b-2) after elicitation with 0.1 µM of active flg22 (black bars) (P<0.0001). Responses to inactive flg22A.tum (white bars) were not different between genotypes (P>0.5). Data were based on time-course experiment from Fig. 1C. (n = 24/genotype and treatment). (B) Compared to Col-0 (white bar) and drp2a-1 (2a-1; gray bar), peak ROS production (10–15 minutes post elicitation) was significantly increased in drp2b-2 (2b-2; black bar) after elicitation with 0.1 µM elf26 (P<0.005). (n = 32/genotype). (C) Using quantitative Real-Time PCR (qRT-PCR) with At2g28390 as the reference gene, mRNA levels of RbohD were not significantly different between drp2b-2 (2b-2; black bar) and Col-0 (white bar). Tissues were cut and prepared exactly as those for ROS experiments in 96-well plates and collected immediately prior to flg22-elicitation (n = 3/genotype; P = 0.9). (D) Based on experimental design and qRT-PCR as described in (C), mRNA levels of FLS2 were not significantly different between drp2b-2 (2b-2; black bar) and Col-0 (white bar) (P = 0.33). (n = 3/genotype). For (A - B), luminol-based ROS production is shown as Relative Light Units (RLU). For (A - D), all experiments were done in 4-5 week old leaf tissue and repeated more than three independent times with similar results. Values are mean ± SE. Different letters indicate significant differences while the same letter indicates no significant differences between samples based on Two tailed student's t-test. ROS experiments shown in the same panel were performed in the same 96-well plate at the same time to allow for direct comparison.
(PDF)
Treatment with the calcium channel blocker LaCl3 abolishes flg22-induced increases in cytosolic Ca2+ levels and ROS production. (A) After 0.1 µM flg22 elicitation, cytosolic Ca2+ levels were significantly elevated in leaf discs from 4–5 week old drp2b-2 expressing the Ca2+-reporter Aequorin (drp2b-2/AEQ; closed symbols) compared to Col-0 expressing AEQ (Col-0/AEQ; open symbols). (n = 6/genotype and treatment). (B) LaCl3 abolished flg22-induced elevations of cytosolic Ca2+ levels to similar levels in drp2b-2/AEQ (closed symbols) compared to Col-0/AEQ (open symbols). 8-day old plants were pretreated with 10 mM LaCl3 (triangles) or water (squares) for 30 minutes, washed with water and then elicited with 0.1 µM flg22. (n = 6/genotype and treatment). (C) LaCl3 abolished flg22-induced ROS production to similar levels in drp2b-2 (square) compared to Col-0 (diamond). Leaf discs from 4–5 week old plants were pretreated with 1 mM LaCl3 (open symbols) or water (closed symbols) for 30 minutes, washed with water and then elicited with 0.1 µM flg22. (n = 24/genotype and treatment). For all experiments, values are mean ± SE. Each experiment was repeated more than three independent times with similar results. Statistical analysis was done as in S1 Fig.
(PDF)
DRP2B has no apparent role in the flg22-induced expression of PER62, PER4 and NHL10 . (A) and (B) Using qRT-PCR with At2g28390 as the reference gene, mRNA levels of PER62 and PER4 were not significantly different between drp2b-2 and Col-0 after elicitation for 30 minutes with water (white bars) or 1 µM flg22 (black bars). P-values for PER62 or PER4 mRNA levels between drp2b-2 and Col-0 were all P>0.5. (n = 3/genotype and treatment). (C) Using qRT-PCR with At2g28390 as the reference gene, mRNA levels of NHL10 were not significantly different between drp2b-2 and Col-0 after elicitation for 60 minutes with water (white bars) or 1 µM flg22 (black bars). P-values for NHL10 mRNA levels between drp2b-2 and Col-0 were all P>0.5. (n = 3/genotype and treatment). All experiments were done in leaf tissue of 4–5 week old plants and repeated at least 3 times with similar results. Values are mean ± SE. Statistical analysis was done as in S1 Fig.
(PDF)
DRP2B has no apparent role in flg22-induced MAPK phosphorylation over 45 minutes post-elicitation. (A) Quantification of protein bands from Fig. 2C using Bio-Rad Quantity One software. Data is presented as the ratio of either phosphorylated MPK6 (P-MPK6) or MPK3 (P-MPK3) relative to Calnexin protein levels. For quantification of P-MPK6 and P-MPK3, all data were normalized to the respective phosphorylated MAPK levels of the Col-0/10 minute timepoint. Quantified data represent the means ± SE from four independent biological repeats. (B) No apparent difference in flg22-induced phosphorylation of MPK3 and MPK6 was observed between Col-0 and drp2b-2 over 45 minutes (min) after elicitation with 0.1 µM active flg22. Immunoblot analysis was done on total protein extracts probed with an antibody for phosphorylated MAPKs (P-MPK3 and P-MPK6). αCalnexin served as loading control. The depicted blot is representative of 3 individual experiments showing similar results. (C) Quantification of protein bands from S4B Fig. using Bio-Rad Quantity One software. Data is presented as the ratio of phosphorylated MPK6 (P-MPK6), MPK3 (P-MPK3), and an unknown MAPK (P-MPK?, potentially P-MPK4 or P-MPK11) relative to Calnexin protein expression. For quantification of P-MPK6, P-MPK3, and P-MPK?, all data was normalized to the respective phosphorylated MAPK levels of the Col-0/15 minute timepoint. Quantified data represent the means ± SE from four independent biological repeats. Statistical analysis was done as in S1 Fig.
(PDF)
For drp2b , the increase in flg22-induced ROS production is not fully sensitive to Tyrphostin A23. (A) After a one-hour pretreatment in the absence (square) or presence (triangle) of 100 µM Tyrphostin A23 (T23), ROS production was measured over 40 minutes in drp2b-2 (closed symbols) and Col-0 (open symbols) in response to 1 µM flg22 (n = 24/treatment and genotype). (B) Total ROS production based on time-course experiments shown in (A) indicates that in drp2b-2 (black bars), the increase in flg22-induced ROS production was not completely TyrA23-sensitive (drp2b-2+T23 vs Col-0+T23, P<0.025). Experiments were done using 4–5 week old leaf tissue and repeated at least 3 independent times with similar results. Values are mean ± SE. Statistical analysis was done as in S1 Fig. Relative Light Units, RLU.
(PDF)
The Ca2+ channel blocker LaCl3 further reduces flg22-induced PR1 mRNA levels in drp2b-2 . Using qRT-PCR with At2g28390 as the reference gene, flg22-induced PR1 mRNA levels were significantly reduced in drp2b-2 (black bars) compared to Col-0 (white bars) 24 hr after co-treatment with flg22 and LaCl3 (P<0.05). Eight-day-old seedlings were treated with water, 10 mM LaCl3, 1 µM flg22 or co-treatment with 10 mM LaCl3 and 1 µM flg22 (flg22+ LaCl3). Water and LaCl3 treatment served as a controls. Data represent the mean ± SE of four or more independent experiments (n≥12). Statistical analysis was done as in S1 Fig.
(PDF)
14-day old drp2b-2 plants display similar phenotypic defects in response to flg22 or Pto DC3000 as described for 5-6 week old drp2b-2 plants. (A) No significant difference in the fresh weight of 14-day old plants was observed between drp2b-2 (black bar) and sid2-2 (gray bar) compared to Col-0 (white bar) (P>0.5). (n = 21/genotype). (B) In time-course experiments, ROS production was elevated in 14-day old drp2b-2 plants (filled shapes) compared to Col-0 (open shapes) in response to 0.1 µM flg22. (n = 32/genotype). (C) In 14-day old plants, total ROS production was significantly increased in drp2b-2 plants (black bar) compared to Col-0 (white bar) after elicitation with 0.1 µM flg22 (P<0.01). Data were based on time-course experiment shown in Figure (B). (D) Using qRT-PCR with At2g28390 as the reference gene, PR1 mRNA levels were significantly reduced in drp2b-2 (black bars) compared to Col-0 (white bars) at 24 hr after treatment with 1 µM flg22 (P<0.05). Treatment with water served as mock control. (n = 4/genotype and treatment). (E) Compared to Col-0 (white bars), bacterial growth was significantly increased in 14-day old drp2b-2 plants (black bar) at 3 dpi with Pto DC3000lux (OD600 = 0.0005) as measured by serial dilution plating (P<0.05). sid2-2 (gray bar) served as positive control (P<0.0001). (n = 8/genotype). (F) Using qRT-PCR, PR1 mRNA levels were significantly reduced in drp2b-2 (black bars) compared to Col-0 (white bars) at 24 hr after treatment with Pto DC3000lux (OD600 = 0.0005) (P<0.009). Treatment with water (white bars) served as mock control. (n = 4/genotype). (G) Levels of SA in Col-0 (white bars) and drp2b-2 (black bars) 12-day old plants treated for 24 hr with 1 µM flg22 +0.1% DMSO in water (P = 0.2; n = 3/genotype; each n contained 25 seedlings). Treatment with 0.1% DMSO in water served as mock control (P = 0.3; n = 2/genotype; each n contained 25 seedlings). Experiment was repeated twice with similar results. Unless stated differently, all experiments were done using 14-day old plants and repeated at least 3 independent times with similar results. Values are mean ± SE, and statistical analysis was done as in S1 Fig. ROS experiments shown in the same panel were performed in the same 96-well plate at the same time to allow for direct comparison. Relative Light Units, RLU.
(PDF)
DRP2A is not required for robust flg22-induced endocytosis of FLS2. (A) Col-0 FLS2-GFP and drp2a-3 FLS2-GFP homozygous F4 seedlings expressed similar levels of both endogenous FLS2 and FLS2-GFP as shown by immunoblot analyses of total protein extracts. αFLS2 detected both native FLS2 (open arrow) and FLS2-GFP (closed arrow), while αGFP detected FLS2-GFP only (closed arrow). αDRP2 was used to confirm drp2a-3 mutants, and αMPK6 was used as a loading control. (B) In response to 1 µM flg22, no significant differences are observed in total ROS between Col-0 FLS2-GFP and drp2a-3 FLS2-GFP (P = 0.4) (n = 30 cotyledons/genotype). Relative Light Units, RLU. (C) Flg22-induced endocytosis of FLS2-GFP in Col-0 FLS2-GFP (Col-0) and drp2a-3 FLS2-GFP was determined by spinning disc confocal microscopy. Whole seedlings were treated with 1 µM flg22 to observe un-elicited (constitutive; 0 min) and ligand-induced (50–60 min) endocytosis of FLS2-GFP. Representative maximum-intensity projection images and zoomed insets of FLS2-GFP fluorescence are shown. Scale bars = 10 µm. (D) Quantification of FLS2-GFP in puncta at 0 min and 50–60 min after elicitation with 1 µM flg22 showed no significant differences between drp2a-3 and Col-0 (0 min, P = 0.268; 50–60 min, P = 0.702). (n = 28–55 images analyzed per genotype/treatment). All experiments were done using 7-day old seedlings and were repeated at least three independent times with similar results. Values are mean ± SE, and statistical analysis was done as in S1 Fig.
(PDF)
List of oligonucleotide sequences used as primers.
(PDF)
Supplemental Materials and Methods.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.