Abstract
Volumetric MRI scans from 26 women with repeated episodes of childhood sexual abuse (CSA), and 17 healthy women (18–22 years) were analyzed for sensitive periods effects on hippocampal and amydgala volume, frontal cortex gray matter volume and corpus callosum area. Hipppocampal volume was reduced in association with CSA at 3–5 years (β=−0.69, p<0.0001) and 11–13 years (β=−0.25, p<0.05). Corpus callosum was reduced with CSA at 9–10 years (β=−0.44, p<0.005), and frontal cortex was attenuated in subjects with CSA at ages 14–16 (β=−0.48, p<0.005). Brain regions have unique windows of vulnerability to the effects of traumatic stress.
Keywords: hippocampus, frontal cortex, corpus callosum childhood sexual abuse, stress, maltreatment, adolescents
Introduction
Childhood abuse is a major risk factor in the development of psychopathology (see {1–4}), and is also associated with a host of neuropsychological and neurocognitive consequences {5}. Recent studies suggest that clinical sequelae may stem, at least in part, from enduring adverse effects on brain development {6}. The nature and severity of the effects will likely depend on genetic predisposition {7, 8}, frequency, severity and multiplicity of the stressors {1, 8–10}, gender {9, 11}, and timing of the insult {9}.
Generally, early onset and longer duration of abuse have been associated with greater morphological change {9}, but this may be an oversimplification. An alternative hypothesis is that stress-susceptible brain regions have their own unique sensitive periods (or windows of vulnerability) to the effects of early stress {12}. In practice these two hypotheses may not appear that different, as longer periods of abuse may be more likely to intersect a sensitive period, and many brain regions probably have a relatively early (prepubertal) window of vulnerability. Nevertheless, this is a critical distinction, which could substantially enhance our understanding of the neuropsychiatric effects of abuse, and shed new light on the underlying temporal aspects of gene x environment interactions that lay at the heart of most psychiatric vulnerabilities.
The concept that brain regions go though stages when they are maximally sensitive to experience emerged from the landmark studies of Hubel and Weisel {13}. They found that binocular deprivation affected development of the visual cortex in cats if it occurred early in postnatal life, but had no impact after puberty. Little evidence exists for sensitive periods in human brain development. Postnatal sensitive periods have been delineated for development of speech, language {14–16} and binocular vision {17}. However, a vast array of stimuli and experiences are likely to affect brain development throughout a host of sensitive periods that await discovery.
Stress has been identified as a key experiential factor that programs and modifies brain development {12, 18}. Exposure to physical or sexual abuse resulting in psychopathology have been associated with attenuated left hemisphere maturation {19, 20}, diminished size of the corpus callosum {9, 11, 20, 21}, reduced hippocampal volume in adults {22–25} (but not children {9, 21, 26, 27}), and alterations in gray matter volume (GMV), symmetry and neuronal integrity of frontal cortex {21, 26, 28}.
The aim of this study was to test the hypothesis that stress-sensitive brain regions have their own developmental time windows when they are maximally vulnerable to the effects of early stress. This is a relatively easy hypothesis to test in a preclinical study, where stress can be administered during specific developmental stages. It is much harder to test this hypothesis in humans, as there is a great deal of heterogeneity in onset and duration of abuse, and the frequent co-occurrence of other forms of stress. A secondary aim of this study was to propose an analytical strategy to facilitate the exploration of sensitive period effects for complex phenomenon such as childhood sexual abuse (CSA).
In order to test this hypothesis measures of hippocampal, corpus callosum, frontal cortex and amygdala size were obtained from MRI scans of female college students with self-reported histories of CSA that occurred at different ages and healthy sociodemographically comparable controls. The study was predicated on the following hypotheses. The hippocampus would have an early period of vulnerability based on observation that hippocampal synaptogenesis is strongly influenced by variations in maternal care and availability {29, 30}. The slowly maturing frontal cortex (e.g., {31}) would have a late period of vulnerability, and may be resistant to the effects of early stress given our observation that exposure to early isolation stress in rats affected synaptic density in hippocampus but had no sustained effect on synaptic density in prefrontal cortex {30}. The corpus callosum would likely have an intermediate period of vulnerability in females based on our observation that sexual abuse was associated with reduced corpus callosum area in females while neglect (generally an earlier problem) was associated with reduced corpus callosum area in males {11}. Finally, we predicted that the amygdala would not have a prominent period of vulnerability, based on reports of normal amygdaloid volumes in abused subjects {9, 22, 25, 26}.
We now provide evidence for discrete regional sensitive periods to the effects of CSA, with the hippocampus having the earliest period of vulnerability and the frontal cortex having the latest.
Methods
Subjects
Physically healthy, unmedicated, right-handed individuals aged 18–22 years were recruited via advertisements looking for individuals interested in participating in “psychiatric research.” {5}. Primary entry criterion was a history of three or more episodes of forced contact CSA that ended at least two years prior to enrollment. CSA was defined as forced involuntary contact with the sexual part of the victim’s or the perpetrator’s body. Contact had to be accompanied by threats of harm to self or others, or feelings of fear or terror. History of CSA was supported by written response and by consistent result of a lengthy structured interview conducted by a certified clinician using the Traumatic Antecedents Questionnaire {32}.
Rigorous exclusion criteria were applied to select subjects in which differences in brain morphology could be most clearly attributable to CSA. They included neurological disorders; medical disorder affecting growth or development; treatment with corticosteroids; pregnancy; past or present alcohol/substance abuse; premature birth or complications during mother’s pregnancy or delivery; in utero exposure to alcohol or drugs; or a history of physical abuse. Exclusion criteria also included exposure to any other forms of preceding or subsequent trauma (e.g., motor vehicle accidents, natural disasters, fires, near drowning, witnessing abuse, animal attacks, gang violence, robbery, etc.). These criteria excluded 95% of the 732 initial respondents to our advertisement. Twenty-eight percent of the subjects who completed all of the prescreening instruments (N 564) had a self-reported history of childhood sexual abuse, but only 9.5% of the prescreened sample had a history of childhood sexual abuse unaccompanied by exposure to other forms of abuse.
Using these criteria 26 abused women (mean age=20.0 years, range 18–22) and 17 healthy female controls (mean age=19.4 years, range 18–22) were enrolled. Controls, selected from the same pool of respondents, had no current or past DSM-IV Axis I disorder on Structured Clinical Interviews {33} and no history of abuse or exposure to other traumatic events. Subjects were predominantly middle class or above (96%) and the two groups were similar in measures of socioeconomic status (Hollingshead index {34}: 2.3±0.9 versus 2.0±0.6; F =1.95, df=1,41, P=0.17). Subjects were paid for participation, provided written, informed consent. The study was approved and monitored by the McLean Hospital Institutional Review Board. Specific information about each subject is presented in Table I. None of the subjects recruited had more than a minimal history of drug or alcohol use, and no subject met criteria for borderline personality disorder. None of the subjects enrolled were seeking treatment.
Table I.
Subjects' History of Abuse and Psychiatric Diagnoses
| SUBJ | GROUP | AGE | SES | Age of CSA Onset | Duration Abuse | Number Perps | Perp Relation | Past Dx | Current Dx | REGIONS |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 2 | Abused | 20 | 2 | 3 | 5 | 2 | F | MDD, PTSD | DD-NOS, AD-NOS, | hipp, amyg, CC, PFC |
| 9 | Abused | 20 | 5 | 4 | 10 | 1 | F | MDD | None | hipp, amyg, CC |
| 12 | Abused | 19 | 2 | 4 | 2 | 1 | EF | MDD | None | hipp, amyg, CC, PFC |
| 13 | Abused | 18 | 3 | 11 | 3 | 1 | F | None | None | CC |
| 17 | Abused | 19 | 3 | 6 | 7 | 1 | EF | BD | PTSD, GAD | CC |
| 20 | Abused | 22 | 1 | 5 | 8 | 3 | B | MDD | None | hipp, amyg, CC, PFC |
| 22 | Abused | 22 | 2 | 4 | 2 | 1 | EF | None | None | hipp, amyg, CC, PFC |
| 24 | Abused | 19 | 3 | 5 | 3 | 1 | F | PTSD, SAD | None | CC |
| 26 | Abused | 18 | 1 | 8 | 3 | 1 | EF | MDD, OCD, DD | None | hipp, amyg, CC, PFC |
| 29 | Abused | 18 | 2 | 4 | 2 | 1 | F | None | None | hipp, amyg, CC, PFC |
| 32 | Abused | 21 | 2 | 6 | 4 | 3 | B | MDD | PTSD | hipp, amyg, CC, PFC |
| 33 | Abused | 19 | 1 | 15 | 2 | 1 | EF | None | PTSD | hipp, amyg, CC, PFC |
| 36 | Abused | 21 | 2 | 5 | 2 | 1 | EF | MDD, DD | None | hipp, amyg, CC, PFC |
| 40 | Abused | 22 | 3 | 6 | 4 | 2 | B | MDD, PTSD, OCD, ADHD | PTSD, OCD, ADHD | CC, PFC |
| 41 | Abused | 21 | 3 | 5 | 8 | 1 | F | MDD | MDD | hipp, amyg, PFC |
| 42 | Abused | 20 | 2 | 6 | 3 | 2 | EF | MDD, PTSD | None | hipp, amyg, CC, PFC |
| 44 | Abused | 19 | 2 | 6 | 5 | 2 | B | None | None | hipp, amyg, CC, PFC |
| 45 | Abused | 20 | 2 | 3* | 2 | 1 | F | None | None | hipp, amyg, CC, PFC |
| 48 | Abused | 20 | 2 | 7 | 2 | 3 | EF | MDD | None | hipp, amyg, CC |
| 51 | Abused | 21 | 2 | 10 | 3 | 1 | F | MDD | None | hipp, amyg, CC, PFC |
| 52 | Abused | 18 | 2 | 13 | 1 | 1 | F | None | None | hipp, amyg, CC, PFC |
| 57 | Abused | 22 | 2 | 3* | 6 | 1 | F | MDD | None | hipp, amyg, CC, PFC |
| 58 | Abused | 20 | 2 | 3 | 9 | 3 | B | None | MDD | hipp, amyg, CC, PFC |
| 60 | Abused | 19 | 4 | 5 | 5 | 3 | B | CU, BN | MDD, BE | hipp, amyg, CC, PFC |
| 62 | Abused | 20 | 3 | 13 | 4 | 2 | EF | MDD, CU | None | PFC |
| 65 | Abused | 22 | 3 | 11 | 4 | 2 | EF | None | None | hipp, amyg,PFC |
| 1 | Control | 19 | 3 | hipp, amyg, CC, PFC | ||||||
| 4 | Control | 20 | 2 | hipp, amyg, CC, PFC | ||||||
| 5 | Control | 18 | 2 | hipp, amyg, CC, PFC | ||||||
| 7 | Control | 19 | 2 | hipp, amyg, CC, PFC | ||||||
| 10 | Control | 19 | 1 | hipp, amyg, CC, PFC | ||||||
| 11 | Control | 18 | 2 | hipp, amyg, CC, PFC | ||||||
| 15 | Control | 20 | 3 | hipp, amyg, CC, PFC | ||||||
| 19 | Control | 22 | 2 | CC | ||||||
| 21 | Control | 18 | 3 | hipp, amyg, CC, PFC | ||||||
| 23 | Control | 21 | 2 | PFC | ||||||
| 30 | Control | 18 | 1 | hipp, amyg, CC, PFC | ||||||
| 31 | Control | 18 | 2 | hipp, amyg, CC, PFC | ||||||
| 35 | Control | 19 | 1 | hipp, amyg, CC, PFC | ||||||
| 43 | Control | 19 | 2 | hipp, amyg, CC, PFC | ||||||
| 46 | Control | 21 | 2 | hipp, amyg, CC | ||||||
| 49 | Control | 18 | 2 | hipp, amyg, CC | ||||||
| 50 | Control | 22 | 2 | hipp, amyg, CC, PFC | ||||||
Rationale for Selection Procedure
Subjects were selected based on exposure history regardless of psychiatric outcome (except for substance abuse, which could directly affect brain development). This differs from prior studies that have focused on abused subjects with PTSD {9, 19, 22, 23}. Selecting subjects with CSA regardless of outcome facilitates the relatively unbiased assessment of the morphometric affects of abuse during specific developmental stages. Preselection of subjects based on a history of PTSD (or other psychopathologies) would bias morphometric findings toward those brain regions involved in the disorder. This distinction is important when the goal is to ascertain the possible effects of exposure to early stress at key developmental points and less relevant if the goal is to delineate the neurobiology of an underlying psychiatric disorder such as PTSD.
Imaging Methods
T1-weighted coronal sections (3-D, spoiled gradient recalled acquisition in the steady state; TR=40 ms; TE=5 ms; NEX=2; flip angle 40°; fov 24 cm; matrix 256 x 128; 124 sections of 1.5 mm thickness, no gaps) were acquired using a 1.5 T magnetic resonance scanner (Echospeed; GE Medical Systems). Raters were blind to subject identity and history, and provided results from images in which definitive measurements could be made based on distinctiveness of landmarks, borders, image quality and motion artifact.
Hippocampus and amygdala were traced in their entirety according to the method detailed by Pruessner et al. {35}. In our hands this technique yielded excellent reliability (intra-rater ICC 0.91 to 0.95, inter-rater 0.83 to 0.94), though raters rejected 20% of the sample because of ambiguity in delineating one or more borders. Manual tracing is currently considered optimal for measuring the volume of these two regions {36}.
Midsagittal corpus callosum area was manually-traced using NIH image and an automated algorithm divided it into seven regions as defined by Witelson {37}. Previous studies indicated that midbody regions 3–6 were most significantly affected by abuse or neglect {9, 11, 21}. Region 3, rostral body, was selected for sensitive period analysis as this region showed the greatest overall vulnerability to CSA (regardless of age of abuse) in the present sample.
Grey matter volume (GMV) of frontal cortex was assessed using a semi-automated program for cortical surface-based analysis (FreeSurfer) {38–40}. A composite measure of average frontal lobe GMV was obtained by combining measures from all parts of the inferior frontal, middle frontal, superior frontal, orbital, suborbital, transverse, frontopolar and cingulate gyri and sulci.
Data Analysis
Statistical analyses were conducted to provide evidence for sensitive periods in a clinical population where abuse occurred over a variable number of years. Subjects with CSA had experienced abuse for an average of 4.2±2.4 years. In most cases abuse occurred in continuous years (70%). The remainder had an average gap of 5.0±2.7 years between clusters of abuse. This situation presents challenges to conventional between group designs, so alterative statistical methods were used.
The first method was multiple regression analysis (SPSS), in which the primary assumption was that abuse during different stages of development would exert additive effects on regional brain morphometry. If this assumption is true, then it is possible to determine whether abuse during one or more stages of development was associated with a particularly significant reduction in regional brain size. The reality may be more complicated, there may be interactive effects between abuse at different stages, but an additive multiple regression model provides a parsimonious initial approach, and is a good starting point if the model provides a reasonable fit to the available data.
Subjects were characterized by the density of CSA they experienced during sequential developmental stages. Density of CSA was defined as number of years of abuse experienced during the stage divided by number of years in the stage. Designated stages compared were: pre-school (3–5 years), latency (6–8 years), prepubertal (9–10 years), pubertal (11–13 years) and adolescent (14–16 years) based on the ages of exposure. Stages were selected to be relatively short, to make it possible to detect multiple distinct windows of vulnerability during the prepubertal period, as preclinical studies suggested that vulnerable periods may be brief {41}. The number of subjects who experienced abuse during sequential stages were 13, 16, 7, 10 and 7, respectively.
Additional independent variables of potential significance were: intracranial volume, midsaggital area, total gray matter volume, SES, list recall, history of depression, and history of PTSD. List recall was selected as a potential covariate for hippocampal volume given the role of the hippocampus in verbal declarative memory, and observation of a strong correlation between hippocampal volume and measures of list recall {43}. Multiple regression analyses were performed without data transformations as all of the morphometric measures were normally distributed (Kolmogorov-Smirnov test, hippocampus: z=0.457, p > 0.9, amygdala: z=0.622, p > 0.8, corpus callosum: z=0.820, p > 0.5, frontal cortex: z=0.508, p > 0.9). Path analysis was performed using structural equation modeling (SEM) with Amos Graphics (http://www.assess.com/Software/AMOS.htm) as a confirmatory statistical procedure.
Path analysis is a specific form of SEM in which there are no latent variables (all variables are directly measured). This approach makes several assumptions, particularly that there are an adequate number of known correlations or covariances as inputs to generate a sensible set of results, and that there is a unique or best solution. Path analysis was guided by the robust results of the multiple regression analysis, and made feasible with limited sample size given the strength of the associations and normality of the data. SEM provides a sophisticated analysis that accounts for the tendency of abusive experiences to carry over into subsequent stages. Further, SEM controls for problems with multiple comparisons by placing the entire analysis into a single statistical model. Full-information maximum likelihood estimation was used to minimize the discrepancy fit function defined by the available data points, pathways and coefficients.
With SEM the null hypothesis is that the model fits the data. Statistically, the absolute fit of the model is tested using a chi-square procedure in which p values<0.05 lead to the rejection of the model. With a global chi-square p value > 0.05 one can then provisionally accept the given model along with the p values indicating the significance of the individual pathways. The Tucker-Lewis Index and Comparative Fit Index served as measures of relative fit to ascertain how parsimoniously the model fit the data in comparison to other models.
Data from all subjects were used but brain sizes for all regions could not be ascertained with confidence in every subject. Thus, number of subjects for analyses were 21 CSA/16 controls for hippocampus and amygdala, 23 CSA/16 controls for corpus callosum, and 21 CSA/15 controls for frontal cortex GMV. Path analysis evaluated associations between morphometric measures and density of abuse during each stage. Density of abuse was used instead of presence or absence of abuse during each stage, as AMOS Graphics can not use simple dichotomous variables, and density of abuse was the simplest acceptable alternative, and only alternative considered.
Results
Multiple regression analysis identified three variables that were significantly associated with hippocampal volume (overall r=0.837, adjusted r2=0.580, F=5.84, df=10,25, p<0.0001; Table II). Density of abuse at index stage 3–5 (p<0.0004) was associated with reduced volume. List-recall (p<0.002) and intracranial volume (p=0.001) were positively correlated with hippocampal volume. Density of abuse at stage 11–13 (p=0.054) was marginal. No other stage of abuse significantly enhanced goodness of fit, nor did a history of PTSD or depression. List-recall on the Memory Assessment Scale {42}, was a highly significant covariate. Subjects with CSA in this sample had measures of list-recall that were as high as healthy controls (11.56 ± 1.75 vs. 10.59 ± 2.09, F=2.65, df=1,40, p > 0.1). This covariate did not compensate for group differences but reduced the degree of scatter between subjects in both groups. A measure of list recall was also found to be highly correlated with hippocampal volume by Tischler et al {43}. While including list recall in the multiple regression analysis enhanced goodness of fit, and is appropriate, the same basic results were obtained without this covariate (e.g., overall correlation: r=0.740, p=0.006; density of abuse stage 3–5: beta=−0.535, p=0.004).
Table II.
Multiple regression analysis indicating relationship between measures of regional brain size and density of abuse during different stages.
| Measure | Hippocampus | Corpus Callosum | Frontal Cortex | |||
|---|---|---|---|---|---|---|
| Beta | p-value | Beta | p-value | Beta | p-value | |
| Brain size controla | 0.415 | 0.001 | 0.508 | 0.002 | 0.655 | 0.00005 |
| Density abuse 3–5 yrs | −0.566 | 0.0004 | −0.190 | 0.25 | −0.02 | 0.90 |
| Density abuse 6–8 yrs | 0.313 | 0.17 | 0.251 | 0.33 | 0.102 | 0.62 |
| Density abuse 9–10 yrs | 0.036 | 0.83 | −0.422 | 0.03 | −0.13 | 0.45 |
| Density abuse 11–13 yrs | −0.308 | 0.054 | −0.121 | 0.50 | 0.094 | 0.55 |
| Density abuse 14–16 yrs | −0.058 | 0.67 | −0.041 | 0.80 | −0.386 | 0.009 |
| Socioeconomic Status | −0.048 | 0.77 | −0.232 | 0.20 | 0.148 | 0.28 |
| History of Depression | −0.254 | 0.18 | −0.141 | 0.47 | 0.112 | 0.58 |
| History of PTSD | 0.011 | 0.93 | 0.031 | 0.85 | −0.11 | 0.43 |
| List Recall | 0.452 | 0.002 | ||||
| Overall correlation | 0.837 | 0.00002 | 0.691 | 0.01 | 0.798 | 0.0005 |
Intercranial volume, midsaggital area, total gray matter volume, respectively
Multiple regression analysis failed to identify any index stage that was associated with a significant effect on total volume of the amygdala.
Two variables were associated with reduced callosal area (r=0.691, adjusted r2=0.321, F=3.05, df=9,30, p<0.01). These were midsaggital area (p<0.002) and density of abuse at index stage 9–10 (p=0.03).
Multiple regression analysis identified two variables that were associated with frontal cortex GMV (r=0.793, adjusted r2=0.512, F=5.08, df=9, 26, p< 0.001). The first was total GMV (p<0.0001) and the second density of abuse at index stage 14–16 (p<0.01).
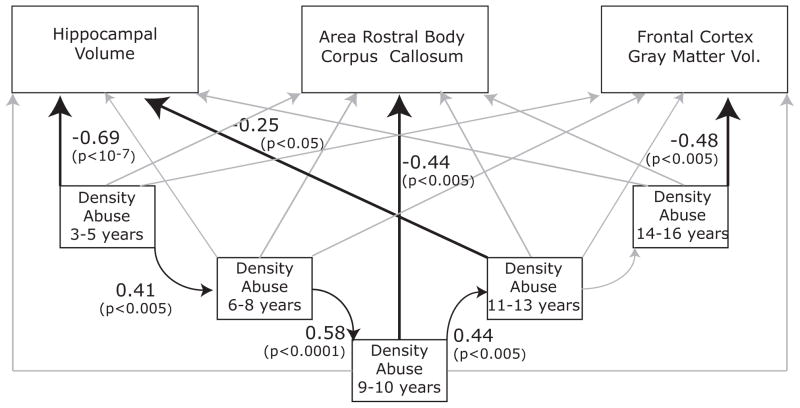
Figure 1 illustrates the composite path analysis derived from SEM. The model provides a satisfactory fit to the data ( χ2=13.82, df=9, p>0.1). Hippocampal volume was significantly influenced by density of abuse at stages 3–5 (p<0.0001) and 11–13 (p<0.05) years. Corpus callosum was influenced by density of abuse at 9–10 years of age (p<0.005), and frontal cortex GMV by abuse at 14–16 years (p<0.005). There was also a strong tendency for abuse (or lack of abuse) to carry over from one stage to another up until 13 years of age (all p values<0.005). Altogether, this model accounted for 52%, 32% and 37% of the variance in the covaried measures of hippocampal volume, corpus callosum area and frontal cortex GMV, respectively. A simpler model that included only paths with p values<0.05 from the multiple regression analysis provided a robust fit (χ2=22.13, df=20, p>0.3) with a reasonable degree of parsimony (Tucker-Lewis=0.94 and Comparative Fit Index=0.96).
Figure 1.
Path analysis indicating relationships between density of abuse during different stages of development and measures of brain size derived from structural equation modeling. Path analysis examined two main components. The first was that CSA (or absence of CSA) during one period would predict CSA (or absence of CSA) during the subsequent period. The second component examined the association between density of CSA during each stage and all morphometric measures. Numerical values represent standardized beta-weights and their associated p-values. Light gray lines were evaluated in the model but were not significantly predictive of any relationship between the variables. Morphometric measures for corpus callosum and frontal cortex gray matter volume (GMV) were covaried by midsaggital area and total GMV, respectively. Hippocampal volume was covaried by intracranial volume and list recall, based on results of the multiple regression analyses (see Table II).
These statistical approaches indicated that hippocampus, corpus callosum and frontal cortex were maximally affected by abuse at 3–5, 9–10 and 14–16 years, respectively. Table III provides volume or area measures in subjects with CSA during these stages versus controls and subjects with CSA during all other stages.
Table III.
Adjusted size of brain regions for controls and abused subjects at stages of greatest vulnerability to childhood sexual abuse.
| Region | Stage (years) | Abused at Index Stage(s) | Abused but Not at Index Stage(s) | Controls |
|---|---|---|---|---|
| Hippocampus (cm3) | 3–5 | 3.030 ± 0.215 (12) | 3.175 ± 0.213 (9) | 3.371 ± 0.217 (15) |
| Corpus Callosum | 9–10 | 70.311 ± 19.792 (6) | 93.334 ± 18.443 (18) | 95.958 ± 18.896 (16) |
| Frontal Cortex | 14–16 | 83.589 ± 4.322 (7) | 90.499 ± 4.355 (15) | 88.428 ± 4.359 (14) |
Discussion
Episodes of repeated CSA were associated with alterations in regional brain size during specific stages. Both analytic techniques lead to the same conclusions. Hippocampal volume was most strongly related to abuse reported to occur between 3–5 years of age, and secondly to abuse between 11–13 years. In contrast, corpus callosum area was associated with abuse reported to have occurred between ages 9–10, and frontal cortex by abuse between ages 14–16. It is worth emphasizing that regional differences in sensitivity across age occurred in the same group of subjects.
The apparent vulnerability of the hippocampus to early stress is consistent with preclinical observations that exposure of the immature hippocampus to corticotropin-releasing hormone (CRH), a key limbic stress modulator, results in a delayed and progressive affect on cell survival and dendritic branching {44}. Further, there is a special population of cells in the immature hippocampus, but not in the adult hippocampus, that can release CRH in response to stress {45}, potentially explaining the heightened sensitivity of the hippocampus to abuse during early childhood. Early effects of abuse on the hippocampus are consistent with evidence from humans and primates that the hippocampus matures rapidly and is functional very early in childhood {46}. This is also consistent with morphometric measures that show that the hippocampus has obtained about 85% of adult volume by four years of age {47}. In contrast, functional ontogeny of the prefrontal cortex may not emerge until puberty {31}, or may go through continuous changes during childhood associated with pruning and strengthening of synaptic connections {48}. Volumetrically, the prefrontal cortex grows at a slow rate until about 8 years of age, and then has a rapid growth spurt between ages 8–14. The lack of apparent effect of exposure to early CSA on young adult frontal cortex volume is consistent with finding in rodents, which showed vulnerability of the hippocampus but not prefrontal cortex to early isolation stress {30}.
The observation that the corpus callosum was vulnerable to the effects of CSA between 9–10 years of age is consistent with a diffusion tensor imaging study that showed substantial changes in fractional anisotropy and diffusivity occurring between 8–12 years of age {49}.
Lack of a discernible sensitive period for the amygdala is consistent with several other studies that have failed to find an effect of childhood abuse on the volume of this region {9, 21–23, 25}. The amygdala is at full adult size in females at four years of age {47}, and may have an earlier sensitive period than we were able to assess.
This study is limited by the relatively small number of subjects who experienced abuse during each stage. However, finding distinctly different stages of vulnerability for these brain regions, within the same sample, provides support for the sensitive period hypothesis. It should be noted that the association between abuse and morphometric change is correlational and does not provide direct evidence of a cause-effect relationship.
Another limitation is that although we focused on exposure to CSA and excluded subjects with exposure to other forms of abuse, such as witnessing domestic violence, we did not specifically exclude subjects who may have experienced neglect or other types of emotional maltreatment such as exposure to parental verbal aggression {10}. This could lead to an error in interpretation if subjects exposed to CSA during one stage had a greater degree of exposure to neglect or emotional maltreatment than subjects exposed to CSA during a different stage. This was not the case. First, perpetrators of abuse were rarely parents or step-parents (3 cases). Second, subjects were in upper middle-class families and were all enrolled in college. This upbringing may have protected them from some of the complexities borne by others exposed to CSA, as none of the subjects in this carefully selected sample with CSA had any significant history of neglect. Third, only 9 subjects were exposed to levels of parental verbal aggression (PVA) that we have come to define as abusive (PVA> 40, {10}). There were no differences in average PVA score based on stage of exposure to CSA (F=0.15, df =1,22, p > 0.9). Hence, it seems unlikely that morphometric effects related to CSA during specific stages were an artifact of differential exposure to other forms of maltreatment.
Childhood abuse has been associated with vulnerability to a host of psychiatric disorders and behavioral problems. Based on the present findings, there may be different abuse-related syndromes associated with particular ages of abuse and specific regional brain changes. For example, we found using regression analysis that current symptoms of depression on the Kellner Symptom Questionnaire {50} were specifically associated with abuse during stage 3–5 years (r=0.459, p=0.001), but with no other stage. In contrast, PTSD-like symptoms as measured by the revised Mississippi Civilian PTSD scale {51} were only associated with abuse during stage 9–10 years (r=0.349, p=0.02). In will be useful in future studies to ascertain if a sensitive period approach can more specifically delineate morphometric changes associated with psychopathology.
Identifying sensitive periods may also provide insight into key ages at which stimulation or environmental enrichment may optimally benefit development of specific brain regions. Theoretically, periods of maximal sensitivity to early stress could occur during phases of rapid development, during times of high plasticity, or during times when systems are programmed to establish enduring set-points {52}.
Acknowledgments
This study was supported by RO1 awards from the National Institute of Mental Health (MH-53636, MH-66222) and National Institute of Drug Abuse (DA-016934, DA-017846) to MHT. We thank Dr. Ross J. Baldessarini for constructive comments. Cynthia E. McGreenery, Danielle Webster, R.N., M.S., C.S., and Hanako Suzuki provided assistance with subject recruitment, diagnostic interviews, and data management, respectively.
Footnotes
This research was presented at the New York Academy of Science, Mount Sinai Medical School, September 11, 2005, American Academy of Child and Adolescent Psychiatry, Toronto, Ontario, Canada, October 23, 2005, International Society for Developmental Psychobiology, Washington DC, November 9, 2005, Society of Biological Psychiatry, May 18, 2006, and International Society for Traumatic Stress Studies, November 5, 2006.
References
- 1.Edwards VJ, Holden GW, Felitti VJ, et al. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160(8):1453–60. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med. 2004;34(8):1475–82. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- 3.Post RM, Leverich GS, Xing G, et al. Developmental vulnerabilities to the onset and course of bipolar disorder. Dev Psychopathol. 2001;13(3):581–98. doi: 10.1017/s0954579401003091. [DOI] [PubMed] [Google Scholar]
- 4.Sansone RA, Sansone LA, Wiederman M. The prevalence of trauma and its relationship to borderline personality symptoms and self-destructive behaviors in a primary care setting. Arch Fam Med. 1995;4(5):439–42. doi: 10.1001/archfami.4.5.439. [DOI] [PubMed] [Google Scholar]
- 5.Navalta CP, Polcari A, Webster DM, et al. Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. J Neuropsychiatry Clin Neurosci. 2006;18(1):45–53. doi: 10.1176/jnp.18.1.45. [DOI] [PubMed] [Google Scholar]
- 6.Teicher MH, Andersen SL, Polcari A, et al. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1–2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 7.Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–4. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 8.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 9.De Bellis MD, Keshavan MS, Clark DB, et al. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45(10):1271–84. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 10.Teicher MH, Samson JA, Polcari A, et al. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am J Psychiatry. 2006;163(6):993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- 11.Teicher MH, Dumont NL, Ito Y, et al. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56(2):80–5. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci. 2006;1071:313–23. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- 13.Hubel DH, Wiesel TN. Early exploration of the visual cortex. Neuron. 1998;20(3):401–12. doi: 10.1016/s0896-6273(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 14.Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 15.Eggermont JJ, Ponton CW. Auditory-evoked potential studies of cortical maturation in normal hearing and implanted children: correlations with changes in structure and speech perception. Acta Otolaryngol. 2003;123(2):249–52. doi: 10.1080/0036554021000028098. [DOI] [PubMed] [Google Scholar]
- 16.Sininger YS, Doyle KJ, Moore JK. The case for early identification of hearing loss in children. Auditory system development, experimental auditory deprivation, and development of speech perception and hearing. Pediatr Clin North Am. 1999;46(1):1–14. doi: 10.1016/s0031-3955(05)70077-8. [DOI] [PubMed] [Google Scholar]
- 17.Goodyear BG, Nicolle DA, Menon RS. High resolution fMRI of ocular dominance columns within the visual cortex of human amblyopes. Strabismus. 2002;10(2):129–36. doi: 10.1076/stra.10.2.129.8140. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman J, Plotsky PM, Nemeroff CB, et al. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48(8):778–90. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y, Teicher MH, Glod CA, et al. Preliminary evidence for aberrant cortical development in abused children: a quantitative EEG study. Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10:298–307. doi: 10.1176/jnp.10.3.298. [DOI] [PubMed] [Google Scholar]
- 20.Teicher MH, Ito Y, Glod CA, et al. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Annals of the New York Academy of Sciences. 1997;821:160–75. doi: 10.1111/j.1749-6632.1997.tb48277.x. [DOI] [PubMed] [Google Scholar]
- 21.De Bellis MD, Keshavan MS, Shifflett H, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52(11):1066–78. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 22.Bremner JD, Randall P, Vermetten E, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein MB. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27(4):951–9. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 24.Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57(12):1115–22. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 25.Vythilingam M, Heim C, Newport J, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159(12):2072–80. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrion VG, Weems CF, Eliez S, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):943–51. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 27.Tupler LA, De Bellis MD. Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol Psychiatry. 2006;59(6):523–9. doi: 10.1016/j.biopsych.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 28.De Bellis MD, Keshavan MS, Spencer S, et al. N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am J Psychiatry. 2000;157(7):1175–7. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- 29.Liu D, Diorio J, Day JC, et al. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3(8):799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 30.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29(11):1988–93. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- 31.Alexander GE, Goldman PS. Functional development of the dorsolateral prefrontal cortex: an analysis utilizing reversible cryogenic depression. Brain Res. 1978;143(2):233–49. doi: 10.1016/0006-8993(78)90566-8. [DOI] [PubMed] [Google Scholar]
- 32.Herman JL, van der Kolk BA. Traumatic Antecedents Questionnaire. Cambridge, MA: The Cambridge Hospital; 1990. [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV axis I disorders - clinician version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 34.Hollingshead AB. Hollingshead two factor index of social position, occupational categories (1965) In: Guy W, Rockville MD, editors. ECDEU assessment manual for psychopharmacology. U.S. National Institute of Health, Psychopharmacology Research Branch; 1976. pp. 516–520. Rev. [Google Scholar]
- 35.Pruessner JC, Li LM, Serles W, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10(4):433–42. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 36.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 1. Review of methodologies currently employed. Mol Psychiatry. 2005;10(2):147–59. doi: 10.1038/sj.mp.4001580. [DOI] [PubMed] [Google Scholar]
- 37.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 38.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 39.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 40.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 41.Kehoe P, Shoemaker WJ, Triano L, et al. Adult rats stressed as neonates show exaggerated behavioral responses to both pharmacological and environmental challenges. Behav Neurosci. 1998;112(1):116–25. doi: 10.1037//0735-7044.112.1.116. [DOI] [PubMed] [Google Scholar]
- 42.Williams JM. Memory Assessment Scales: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 43.Tischler L, Brand SR, Stavitsky K, et al. The relationship between hippocampal volume and declarative memory in a population of combat veterans with and without PTSD. Ann N Y Acad Sci. 2006;1071:405–9. doi: 10.1196/annals.1364.031. [DOI] [PubMed] [Google Scholar]
- 44.Brunson KL, Eghbal-Ahmadi M, Bender R, et al. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci U S A. 2001;98(15):8856–61. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Bender RA, Brunson KL, et al. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101(44):15782–7. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaldy Z, Sigala N. The neural mechanisms of object working memory: what is where in the infant brain? Neurosci Biobehav Rev. 2004;28(2):113–21. doi: 10.1016/j.neubiorev.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Giedd JN, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366(2):223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1–3):241–57. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 49.Snook L, Paulson LA, Roy D, et al. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26(4):1164–73. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Kellner R. A symptom questionnaire. Journal of Clinical Psychiatry. 1987;48(7):268–273. [PubMed] [Google Scholar]
- 51.Norris FH, Perilla JL. The revised Civilian Mississippi Scale for PTSD: reliability, validity, and cross-language stability. J Trauma Stress. 1996;9(2):285–98. doi: 10.1007/BF02110661. [DOI] [PubMed] [Google Scholar]
- 52.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]