Abstract
This in vitro study investigated whether restoration materials and adhesives influence secondary caries formation in gaps using a short-term in vitro biofilm model. Sixty enamel–dentin blocks were restored with 6 different restoration materials with or without adhesives (n = 10 per group) with a gap: 1) Clearfil AP-X composite, 2) Clearfil AP-X composite + SE Bond, 3) Clearfil AP-X composite + ProtectBond, 4) Filtek Silorane composite, 5) Filtek Silorane composite + Silorane System adhesive, or 6) Tytin amalgam. Specimens were subjected to an intermittent 1% sucrose biofilm model for 20 days to create artificial caries lesions. Lesion progression in the enamel–dentin next to the different materials was measured in lesion depth (LD) and mineral loss (ML) using transversal wavelength independent microradiography (T-WIM). A regression analysis was used to compare the LD and ML of the different restoration materials at 4 measurement locations: 1 location at the surface of the enamel, 1 location at the wall of the enamel, and 2 locations at the wall of the dentin. A statistically significant effect of AP-X composite with Protect Bond was found for LD and ML at the WallDentin1 location, leading to less advanced wall lesions. An additional finding was that gap size was also statistically significant at the 2 wall locations in dentin, leading to increasing lesion progression with wider gaps. In conclusion, adhesives can influence wall lesion development in gaps. Protect Bond showed significantly less caries progression compared to bare restoration materials or other adhesives in this short-term in vitro biofilm model.
Keywords: dental materials, composite resins, dental amalgam, dentin bonding agents, dental caries, tooth demineralization
Introduction
Because most clinical studies show more secondary caries development for composite restorations than for amalgam restorations in high-caries-risk patients (Bernardo et al. 2007; Soncini et al. 2007; Opdam et al. 2010; Kuper et al. 2012), secondary caries has often been related to the restorative material used.
Restorative materials may influence secondary caries development in several ways. Differences in the bacterial colonization and/or retention of restorative materials may play a role. It has been reported that composite and ceramic materials show thicker biofilms than glass ionomers (de Fucio et al. 2009). Some materials can release ions with cariostatic properties that may reduce caries development through inhibition of bacterial growth. Amalgam, for instance, releases cariostatic agents, such as Ag, Cu, and Zn ions (Nunez et al. 1976; Orstavik 1985; Morrier et al. 1998). Other materials, like glass ionomer cement, release fluoride, which enhances remineralization. In vivo plaque studies have shown that levels of lactic acid–producing bacteria in the plaque present on restoration surfaces are significantly higher on resin composite restorations than on either amalgam or glass ionomer (Svanberg et al. 1990; Hansel et al. 1998; Zalkind et al. 1998).
To counteract secondary caries, manufacturers of restorative materials developed new materials with other properties. Silorane-based composites show lower polymerization shrinkage (Lien and Vandewalle 2010) and lower quantity of adhering streptococci compared with methacrylate-based composites in vitro (Buergers et al. 2009). Also, adhesives have been developed with the promise of having anticaries properties through the presence of an antibacterial monomer (e.g., 12-methacryloyloxydodecylpyridinium bromide [MDPB]; Imazato et al. 1994, 1999). In a recent in situ study evaluating secondary surface caries development next to different restoration materials with optimal adhesion and adaptation, it was shown that a microhybrid composite bonded with an MDPB adhesive showed less mineral loss than amalgam (van de Sande et al. 2014).
Secondary caries is also thought to be influenced by the marginal seal of the restoration, with a good marginal seal considered a protection against secondary caries. The marginal seal may depend on many factors, such as the site or extension of restoration, matrix placement, skills of the operator, moisture control, and amount of polymerization shrinkage. Composite placement is a more technique-sensitive method than placement of amalgam or glass ionomer, and composite shows polymerization shrinkage. Therefore, it is suspected that composite may also be more susceptible to secondary caries due to the presence of restoration gaps. In a recent in situ study (Kuper et al. 2014), it was observed that in gaps with a minimum width of 68 µm (and wider), wall lesions were able to develop.
Many studies have investigated the anticaries effect of different restorative materials with a good marginal seal; however, there is no literature showing the influence of different materials when the marginal seal is lacking due to defects or gaps at the interface. Because marginal degradation has been demonstrated to increase with time (Dickinson et al. 1993; Wendt and Leinfelder 1994), this is very clinically relevant. Moreover, most studies evaluating the effect of gaps on caries development have studied restorative materials without adhesive layers, and the effect of the adhesive is unclear.
Therefore, this study investigated whether different restoration materials and adhesives influence secondary caries formation in gaps using a short-term in vitro biofilm model.
Materials and Methods
Preparation of the Samples
Dentin–enamel block samples (3.2×3.2×2.0 mm; n = 60) were cut from bovine incisors and polished with 800 grit paper (Siawat, Abrasives, Frauenfeld, Switzerland) (Figure 1). Block samples (3.2×3.2×2.0 mm) of the following restoration materials were made:
Figure 1.
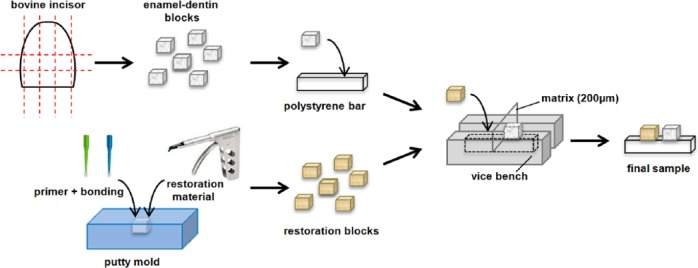
Schematic representation of samples preparation.
Clearfil AP-X composite (Kuraray Medical Inc., Okayama, Japan) (n = 10)
Clearfil AP-X composite + SE Bond (Kuraray) (n = 10)
Clearfil AP-X composite + Protect Bond (Kuraray) (n = 10)
Filtek Silorane composite (3M ESPE, St. Paul, MN, USA) (n = 10)
Filtek Silorane composite + Silorane System adhesive (3M ESPE) (n = 10)
Tytin amalgam (Kerr Corporation, Orange, CA, USA) (n = 10)
The composite resin materials were condensed in a putty mold of 3.2×3.2×2.0 mm and light cured for 20 s. If the complete adhesive system (primer + bonding) was used in combination with the composite, the primer was applied first (and cured, if necessary, according to the manufacturer’s instructions) in the mold and subsequently the bonding, resembling the clinical situation with primer toward the side of the tooth substrate. The amalgam material was mixed for 17 s and condensed in the mold with a mechanical condenser (Amal-Pak, JR Rand Corporation, Freeport, NY, USA). Excess material of the amalgam blocks was removed by grinding it with 800 grit paper 1 day after setting.
First, the enamel–dentin blocks were mounted plan parallel on polystyrene bars (Stripstyrene, Item No. 176, .100 × .125”, Evergreen scale models, Kirkland, WA, USA) of 3.2×2.5×15 mm with flowable composite (GrandioSO Flow, Voco, Cuxhaven, Germany). Then, the samples were secured in a small vice bench. In the vice bench, a matrix of 200 µm was placed perpendicularly. With the matrix in place, the restoration blocks also were mounted on the polystyrene bars with flowable composite, with the aim to create a fixed gap of about 250 µm between the restoration material and the enamel–dentin block; see Figure 1. For the purpose of the microradiographical method used, utmost care was taken to position the top surface of the enamel and the interface surface in such a way that when placed in the microradiography holder, they were parallel to the central ray of the X-ray beam.
Sterilization of the Samples
Samples were kept moist in deionized distilled water, individually sealed within thin plastic films, and placed into thin plastic bags. Subsequently, the samples were placed at 3 cm from the radiation source and sterilized with gamma irradiation from a cobalt-60 source with particle energies of 1.25 MeV and submitted to 533.53 Gy/min, with a total dose of 4.08 KGy (Theratronics, Eldorado 78, Best Theratronic LTDA, Ottawa, ON, Canada). The sterilization procedures were carried out at the Regional Center of Oncology/Radiotherapy Service, Faculty of Medicine (Federal University of Pelotas, Pelotas, RS, Brazil).
Biofilm Model
The specimens were subjected to the biofilm model described by van de Sande et al. (2011). Human saliva was used as the inoculum, and the enamel–dentin blocks were the substratum. The nutrient growth medium used for the experiment was a chemically defined medium enriched with mucin (DMM), pH 6.8 (Wong and Sissons, 2001). Biofilms were grown under intermittent sucrose exposure.
For the experiment, fresh stimulated saliva was collected from one healthy subject (male, aged 34). Saliva was collected in the morning (during fasting), and the volunteer abstained from oral hygiene for 24 h prior to collection. The sterilized specimens were transferred aseptically into sterile wells (24-well tissue culture plate [TPP], Techno Plastic Products, Trasadingen, Switzerland), and 0.4 ml homogenized saliva was dripped into each gap of the specimens. After 1 h incubation at 37°C, the saliva was aspirated and 2.2 ml of DMM 1% sucrose was added. After 6 h, the growth medium was replaced for DMM without sucrose. DMM renewal was performed until the end of the experiment, alternating medium with (6 h) and without sucrose (18 h). The biofilms were incubated anaerobically with increased CO2 by using the Anaerobac® system (Probac do Brasil produtos Bacteriológicos Ltda, Santa Cecília, SP, Brazil) in anaerobic jars for up to 20 days at 37°C without shaking.
Transversal Wavelength Independent Microradiography (T-WIM)
T-WIM pictures of the specimens were made at baseline (T0) and after 20 days (T20) using the method of Thomas et al. (2006). The settings for the microradiography were 60 kV, 30 mA at an exposure time of 8 s. A step wedge with the same absorption coefficient as tooth material (a 94% Al–6% Zn alloy) was used for proper quantitative measurement of lesion depth (LD) and mineral loss (ML).
Film Processing and Image Measurements
After exposure, the films were developed (10 min), fixed (7 min), rinsed, and dried. A digital image of each sample was recorded with a light microscope (Leica Microsystems, Wetzlar, Germany) with a magnification of 10× and a complementary metal oxide semiconductor camera (Canon EOS 50D, Tokyo, Japan). Microradiographs were quantitatively assessed for the presence of wall lesions and surface lesions. A lesion with a progressing front parallel to the outer surface of the tooth sample was considered an outer surface lesion. A wall lesion was defined as a lesion progressing perpendicularly to the tooth–restoration interface. LD and ML for T-WIM were defined as the distance on the microradiograph between the thresholds of 8 and 78.3 vol.% mineral for enamel and between 8% and 43.2% mineral for dentin (Thomas et al. 2006). Each sample was measured at 4 locations using a software program developed in our laboratory (see Figure 2):
Figure 2.
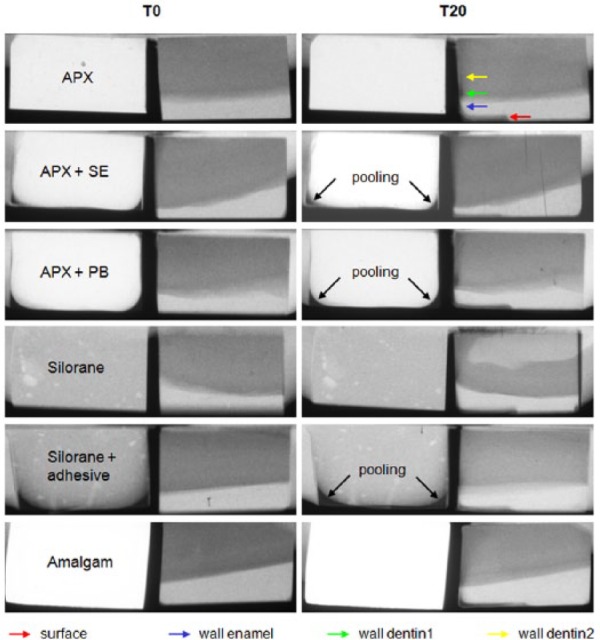
Examples of T-WIM pictures of each restoration material at baseline (T0) and after 20 days (T20) with caries development. Measurement locations are pointed out by colored arrows. This figure is available in color online at http://jdr.sagepub.com.
Location Surface, surface lesion in enamel: at 400 µm distance from the tooth restoration gap
Location Wall Enamel, wall lesion in enamel: 200 µm above the enamel–dentin junction (EDJ)
Location Wall Dentin1, wall lesion in dentin: 200 µm below the EDJ
Location Wall Dentin2, wall lesion in dentin: 800 µm below the EDJ
Baseline measurements (T0) were subtracted from measurements after 20 days (T20) to estimate true lesion depth and mineral loss at T20. The subtracted values were used in the statistical analysis.
From previous experience, we knew that gaps made under standardized circumstances may still vary in size, so gap sizes were measured on the baseline T-WIM picture.
Statistical Analysis
A regression analysis was used to compare the LD and ML of the different restoration materials at the 4 measurement locations. Differences in gap size between restoration materials were corrected for, and amalgam was used as the reference material.
Results
From the 60 specimens, 2 specimens (n = 1 AP-X-SE; and n = 1 Silorane) were discarded because of fracture of the enamel–dentin block of the polystyrene bar. One specimen (n = 1, Silorane + system adhesive) could not be measured as accidentally no baseline T-WIM picture was made. From one specimen (n = 1, amalgam), only the LD and ML of the dentin locations could be analyzed because of insufficient enamel thickness.
Visual assessment of the T-WIM pictures, of which typical examples are shown in Figure 2, showed that surface lesions and wall lesions developed in all groups.
The mean gap sizes, as measured on the microradiographs for the different restoration materials, are amalgam = 305 µm (SD = 79 µm); AP-X = 329 µm (SD = 61 µm); AP-X + SE = 384 µm (SD = 80 µm); AP-X + PB = 358 µm (SD = 44 µm); Silorane = 373 µm (SD = 33 µm); and Silorane + adhesive system = 388 µm (SD = 61 µm). The overall range of the gap sizes was 213–578 µm. There were no significant differences in gap size among the different groups.
In Figure 3, the mean values of LD and ML of each material at the 4 different locations are shown in a bar chart. AP-X + PB shows the lowest LD and ML at all 4 locations, except for location Wall Dentin2, where the LD of AP-X is the lowest. In general, lesion depth was higher in dentin than in enamel at wall locations.
Figure 3.
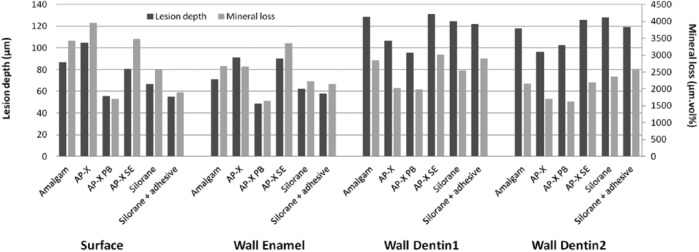
Bar chart showing the mean lesion depth and mineral loss values of each restoration material at the 4 different measurement locations.
The Table shows the estimated effect of the various restorative materials on LD and ML increment at the 4 locations after multiple linear regression analysis. Amalgam was used as the reference material for analysis. A statistically significant effect of AP-X-composite with Protect Bond was found for LD and ML at the Wall Dentin1 location, leading to less advanced wall lesions. The effect of the gap size on lesion progression was statistically significant at the Wall Dentin1 and Wall Dentin2 locations with effects of 0.24 and 0.30 for LD, respectively, resulting in an extra 24 or 30 µm of lesion depth for every 100-µm increase in gap size.
Table.
Multiple Linear Regression Analysis Results Showing the Estimated Effect of Materials on Lesion Depth (LD) and Mineral Loss (ML) Increment, Also Considering Gap Size.
| Measurement Location | Material | Effect on LD (µm) | P Value | 95% CI of Effect |
Effect on ML (µm vol.%) | P Value | 95% CI of Effect |
||
|---|---|---|---|---|---|---|---|---|---|
| Upper | Lower | Upper | Lower | ||||||
| Surface Enamel | Constant* | 99.54 | 27.69 | 171.39 | 4074 | 649 | 7500 | ||
| APX | 18.89 | 0.386 | −24.51 | 62.29 | 585 | 0.572 | −1484 | 2654 | |
| APX-PB | −28.97 | 0.196 | −73.34 | 15.41 | −1620 | 0.130 | −3735 | 496 | |
| APX-SE | −3.10 | 0.895 | −49.90 | 43.70 | 213 | 0.849 | −2018 | 2444 | |
| Silorane | −17.23 | 0.459 | −63.64 | 29.18 | −713 | 0.520 | −2926 | 1500 | |
| Silorane + adhesive | −27.96 | 0.243 | −75.49 | 19.58 | −1352 | 0.236 | −3618 | 914 | |
| Gap(µm) | −0.04 | 0.688 | −0.25 | 0.17 | −2.11 | 0.671 | −12.07 | 7.84 | |
| Wall Enamel | Constant* | 40.12 | −23.58 | 103.81 | 1684 | −706 | 4074 | ||
| APX | 18.16 | 0.347 | −20.31 | 56.64 | −73 | 0.920 | −1516 | 1371 | |
| APX-PB | −27.21 | 0.171 | −66.55 | 12.13 | −1182 | 0.114 | −2658 | 294 | |
| APX-SE | 12.00 | 0.564 | −29.49 | 53.49 | 456 | 0.559 | −1101 | 2012 | |
| Silorane | −15.43 | 0.455 | −56.57 | 25.72 | −650 | 0.402 | −2194 | 894 | |
| Silorane + adhesive | −21.31 | 0.315 | −63.45 | 20.83 | −796 | 0.316 | −2378 | 785 | |
| Gap (µm) | 0.10 | 0.285 | −0.09 | 0.29 | 3.18 | 0.362 | −3.76 | 10.13 | |
| Wall Dentin1 | Constant* | 56.29 | −6.14 | 118.72 | 895 | −790 | 2579 | ||
| APX | −27.96 | 0.144 | −65.76 | 9.85 | −980 | 0.059 | −2000 | 40 | |
| APX-PB | −45.65 | 0.022 | −84.47 | −6.83 | −1205 | 0.025 | −2253 | −157 | |
| APX-SE | −15.65 | 0.448 | −56.75 | 25.46 | −326 | 0.557 | −1436 | 783 | |
| Silorane | −20.94 | 0.307 | −61.67 | 19.80 | −751 | 0.176 | −1850 | 349 | |
| Silorane + adhesive | −27.16 | 0.198 | −68.96 | 14.65 | −502 | 0.376 | −1630 | 626 | |
| Gap (µm) | 0.24 | 0.013 | 0.05 | 0.42 | 6.40 | 0.013 | 1.40 | 11.40 | |
| Wall Dentin2 | Constant* | 26.51 | −33.74 | 86.76 | 413 | −937 | 1763 | ||
| APX | −28.47 | 0.123 | −64.96 | 8.02 | −592 | 0.152 | −1410 | 225 | |
| APX-PB | −31.22 | 0.100 | −68.69 | 6.24 | −828 | 0.053 | −1668 | 11 | |
| APX-SE | −14.83 | 0.456 | −54.51 | 24.84 | −397 | 0.374 | −1286 | 492 | |
| Silorane | −10.93 | 0.579 | −50.25 | 28.38 | −194 | 0.659 | −1076 | 687 | |
| Silorane + adhesive | −24.57 | 0.227 | −64.92 | 15.78 | −63 | 0.890 | −967 | 842 | |
| Gap (µm) | 0.30 | 0.001 | 0.12 | 0.48 | 5.72 | 0.006 | 1.71 | 9.73 | |
CI, confidence interval.
Amalgam is used as the reference material for analysis.
Discussion
In this short-term in vitro biofilm study, we found that adhesives might influence secondary caries formation in gaps. In comparison with amalgam, AP-X composite with a layer of Protect Bond showed significantly less caries development in dentin. The other restoration materials and adhesives did not differ significantly from amalgam in caries development. These findings are similar to the findings of the in situ study of van de Sande et al. (2014), which also showed a decrease in caries development next to AP-X composite with Protect Bond. In the study of van de Sande et al., however, only surface lesions (in dentin) were analyzed, in contrast to this study where both surface (enamel) and wall lesions (enamel + dentin) were analyzed. If we compare the LD and ML of the surface lesions of both studies, a similar pattern was seen between all the restoration materials and adhesives of the 2 studies. In both studies, AP-X + PB showed the lowest caries development, followed by Silorane + adhesive, then AP-X + SE, and finally amalgam.
Because we also analyzed caries lesion progression next to AP-X composite without Protect Bond and we did not observe a significant difference in LD or ML, we can conclude that the anticaries properties come from Protect Bond. Protect Bond is known to contain a bacterial inhibitor, the monomer MDPB. MDPB is a quaternary ammonium compound that is known to have antibacterial effects due to cationic binding to cell wall components, which disturbs the membrane function and subsequently induces leakage of cytoplasmic material (Scheie 1989). Protect Bond incorporated MDPB in the primer and exhibited bactericidal effects by the similar mechanism as described above in unpolymerized form. In our study, Protect Bond was cured, as described by the manufacturer’s instructions, and during the curing process it is estimated that the degree of cure of dentin bonding components is about 70% (Imazato et al. 1997). So, probably, most of the bactericidal effect comes from the residual portion of uncured MDPB. In this study, Protect Bond might have shown a positive anticaries effect because this study lasted only a short period of 3 weeks. The potential antimicrobial effect from MDPB is likely to get diluted quickly under clinical conditions. Amalgam, however, had a disadvantage in anticaries effects in this present study because corrosive products, which are believed to contribute to the cariostatic properties, do not form in a 3-week period. For these reasons, future research should evaluate the long-term anticaries effect of aged restorative materials in a clinical setting.
In this study, there were some issues in making the samples. From previous experience, we knew that intentional gaps might end up around 60 µm larger than intended. Gaps in this study were on average 150 µm larger than intended, probably because dentin–enamel blocks and restoration material blocks were made separately from each other and then fixed together onto a polystyrene bar. We also observed some pooling of the primer–bonding agent at the bottom of the mold, which can be seen in Figure 2 on the surface of the restoration block. Because we wanted to simulate the clinical situation, we applied primer and bonding agent in the whole mold (like in a box), after which this mold was filled with the restoration material. Pooling is a phenomenon also seen in clinical situations, especially at box preparation angles (Pamir et al. 2010). We would like to emphasize that there was also primer and bonding agent present at the wall sides.
A secondary finding of this study was that at the dentin wall locations, a significant gap size effect was present. In a previous in situ study (Kuper et al. 2014), we could not observe any clear trend for increasing lesion progression with wider gaps. Gap sizes of 50, 100, 200, and 400 µm were compared in the in situ study, and only the 50-µm gap group showed a slight indication (not significant) of reduced lesion depth. Extrapolating the results of the present study, we would have expected a difference in lesion depth in the Kuper 2014 study between the 200-µm and 400-µm gap group of about 48 to 60 µm, a difference that would have been statistically significant. We explain these differences in findings by the patient factor present in the in situ study, which was not present in the in vitro study. We assume that, clinically, the caries susceptibility of the patient may override the effect of gap size on secondary caries development.
In this study, we chose to use the saliva of only one person as the inoculum, because this saliva was used in previous studies and we knew that it reproducibly induced caries. This means, however, that there was no interpersonal variation in the saliva composition. Therefore, the anticaries effect of Protect Bond shown in this in vitro study should also be studied in clinical studies because the caries susceptibility factors of the patient may also modify the effect of Protect Bond.
Enamel–dentin samples were used in this study. It was observed that just beneath the enamel, the caries wall lesion was deepest. We hypothesize that this is due to the effect that when dentin and enamel are demineralizing in close proximity, the dentin, which is more soluble, provides the surrounding fluid with dissolution products, which reduces the dissolution of the enamel by raising the saturation level (Lynch and Ten Cate 2006). Lateral spread of caries lesions underneath enamel due to structural differences of the tissues was shown to be an unlikely explanation (Ekstrand et al. 1998).
In conclusion, within the limitations of the present study, adhesives can influence wall lesion development in gaps. In this biofilm model, amalgam did not show reduced secondary caries progression in dentin compared with composite materials. Also, there was no significant difference for caries development among different composite materials. AP-X composite with Protect Bond, however, showed significantly less caries progression compared with bare restoration materials or other adhesives in this short-term in vitro biofilm model.
Author Contributions
N.K. Kuper, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; F.H. van de Sande, contributed to conception, design, and data acquisition, critically revised the manuscript; N.J.M. Opdam, M.S. Cenci, contributed to conception and design, critically revised the manuscript; E.M. Bronkhorst, J.J. de Soet, contributed to data analysis and interpretation, critically revised the manuscript; M.C.D.N.J.M. Huysmans, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This study was funded by the National Institutes for Health (246 NIH), grant number 1R01DE021383-01, under call RFA-DE-10-005 Increasing the service life of dental resin composites. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitão J, DeRouen TA. 2007. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc. 138(6):775–783. [DOI] [PubMed] [Google Scholar]
- Buergers R, Schneider-Brachert W, Hahnel S, Rosentritt M, Handel G. 2009. Streptococcal adhesion to novel low-shrink silorane-based restorative. Dent Mater. 25(2):269–275. [DOI] [PubMed] [Google Scholar]
- de Fucio SB, Puppin-Rontani RM, de Carvalho FG, Mattos-Graner Rde O, Correr-Sobrinho L, Garcia-Godoy F. 2009. Analyses of biofilms accumulated on dental restorative materials. Am J Dent. 2(3):131–136. [PubMed] [Google Scholar]
- Dickinson GL, Gerbo LR, Leinfelder KF. 1993. Clinical evaluation of a highly wear resistant composite. Am J Dent. 6(2):85–87. [PubMed] [Google Scholar]
- Ekstrand KR, Ricketts DN, Kidd EA. 1998. Do occlusal carious lesions spread laterally at the enamel-dentin junction? A histolopathological study. Clin Oral Investig. 2(1):15–20. [DOI] [PubMed] [Google Scholar]
- Hansel C, Leyhausen G, Mai UE, Geurtsen W. 1998. Effects of various resin composite (co)monomers and extracts on two caries-associated micro-organisms in vitro. J Dent Res. 77(1):60–67. [DOI] [PubMed] [Google Scholar]
- Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. 1994. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 73(8):1437–1443. [DOI] [PubMed] [Google Scholar]
- Imazato S, Kinomoto Y, Tarumi H, Torii M, Russell RR, McCabe JF. 1997. Incorporation of antibacterial monomer MDPB into dentin primer. J Dent Res. 76(3):768–772. [DOI] [PubMed] [Google Scholar]
- Imazato S, Ebi N, Tarumi H, Russell RR, Kaneko T, Ebisu S. 1999. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials. 20(9):899–903. [DOI] [PubMed] [Google Scholar]
- Kuper NK, Opdam NJ, Bronkhorst EM, Huysmans MC. 2012. The influence of approximal restoration extension on the development of secondary caries. J Dent. 40(3):241–247. [DOI] [PubMed] [Google Scholar]
- Kuper NK, Opdam NJ, Ruben JL, de Soet JJ, Cenci MS, Bronkhorst EM, Huysmans MC. 2014. Gap Size and Wall Lesion Development Next to Composite. J Dent Res. 93(7 suppl):S108–S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien W, Vandewalle KS. 2010. Physical properties of a new silorane-based restorative system. Dent Mater. 26(4):337–344. [DOI] [PubMed] [Google Scholar]
- Lynch RJ, Ten Cate JM. 2006. The effect of adjacent dentine blocks on the demineralisation and remineralisation of enamel in vitro. Caries Res. 40(1):38–42. [DOI] [PubMed] [Google Scholar]
- Morrier JJ, Suchett-Kaye G, Nguyen D, Rocca JP, Blanc-Benon J, Barsotti O. 1998. Antimicrobial activity of amalgams, alloys and their elements and phases. Dent Mater. 14(2):150–157. [DOI] [PubMed] [Google Scholar]
- Nunez LJ, Schmalz G, Hembree J. 1976. Influence of amalgam, alloy, and mercury on the in vitro growth of Streptococcus mutans: I. Biological test system. J Dent Res. 55(2):257–261. [DOI] [PubMed] [Google Scholar]
- Opdam NJ, Bronkhorst EM, Loomans BA, Huysmans MC. 2010. 12-year survival of composite vs. amalgam restorations. J Dent Res. 89(10):1063-1067. [DOI] [PubMed] [Google Scholar]
- Orstavik D. 1985. Antibacterial properties of and element release from some dental amalgams. Acta Odontol Scand. 43(4):231–239. [DOI] [PubMed] [Google Scholar]
- Pamir T, Kaya AD, Baksi BG, Sen BH, Boyacioglu H. 2010. The influence of bonding agents on the decision to replace composite restorations. Oper Dent. 35(5):572–578. [DOI] [PubMed] [Google Scholar]
- Scheie AA. 1989. Modes of action of currently known chemical anti-plaque agents other than chlorhexidine. J Dent Res. 68(Spec Iss):1609–1616. [Google Scholar]
- Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. 2007. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: findings From the New England Children’s Amalgam Trial. J Am Dent Assoc. 138(6):763–772. [DOI] [PubMed] [Google Scholar]
- Svanberg M, Mjör IA, Orstavik D. 1990. Mutans streptococci in plaque from margins of amalgam, composite, and glass-ionomer restorations. J Dent Res. 69(3):861–864. [DOI] [PubMed] [Google Scholar]
- Thomas RZ, Ruben JL, de Vries J, ten Bosch JJ, Huysmans MC. 2006. Transversal wavelength-independent microradiography, a method for monitoring caries lesions over time, validated with transversal microradiography. Caries Res. 40(4):281–291. [DOI] [PubMed] [Google Scholar]
- van de Sande FH, Azevedo MS, Lund RG, Huysmans MC, Cenci MS. 2011. An in vitro biofilm model for enamel demineralization and antimicrobial dose-response studies. Biofouling. 27(9):1057–1063. [DOI] [PubMed] [Google Scholar]
- van de Sande FH, Opdam NJ, Truin GJ, Bronkhorst EM, de Soet JJ, Cenci MS, Huysmans MC. 2014. The influence of different restorative materials on secondary caries development in situ. J Dent. 42(9):1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt SL, Jr, Leinfelder KF. 1994. Clinical evaluation of a posterior resin composite: 3-year results. Am J Dent. 7(4):207–211. [PubMed] [Google Scholar]
- Wong L, Sissons C. 2001. A comparison of human dental plaque microcosm biofilms grown in an undefined medium and a chemically defined artificial saliva. Arch Oral Biol. 46(6):477–486. [DOI] [PubMed] [Google Scholar]
- Zalkind MM, Keisar O, Ever-Hadani P, Grinberg R, Sela MN. 1998. Accumulation of Streptococcus mutans on light-cured composites and amalgam: an in vitro study. J Esthet Dent. 10(4):187–190. [DOI] [PubMed] [Google Scholar]


