Abstract
In temporomandibular joints (TMJs), the disc and condylar cartilage function as load-bearing, shock-absorbing, and friction-reducing materials. The ultrastructure of the TMJ disc and cartilage is different from that of hyaline cartilage in other diarthrodial joints, and little is known about their lubrication mechanisms. In this study, we performed micro-tribometry testing on the TMJ disc and condylar cartilage to obtain their region- and direction-dependent friction properties. Frictional tests with a migrating contact area were performed on 8 adult porcine TMJs at 5 different regions (anterior, posterior, central, medial, and lateral) in 2 orthogonal directions (anterior-posterior and medial-lateral). Some significant regional differences were detected, and the lateral-medial direction showed higher friction than the anterior-posterior direction on both tissues. The mean friction coefficient of condylar cartilage against steel was 0.027, but the disc, at 0.074, displayed a significantly higher friction coefficient. The 2 tissues also exhibited different frictional dependencies on sliding speed and normal loading force. Whereas the friction of condylar cartilage decreased with increased sliding speed and was independent of the magnitude of normal force, friction of the disc showed no dependence on sliding speed but decreased as normal force increased. Further analysis of the Péclet number and frictional coefficients suggested that condylar cartilage relies on interstitial fluid pressurization to a greater extent than the corresponding contact area of the TMJ disc.
Keywords: articular cartilage, fibrocartilage, friction, permeability, mandibular condyle, TMJ disc
Introduction
The temporomandibular joint (TMJ), composed of the temporal fossa, mandibular condyle, and articular disc, is the only diarthrodial joint in the human head. During physiological function, the soft tissues in the TMJ are subjected to tensile, compressive, and shear loads due to a combination of translational and rotational movements (Tanaka and van Eijden 2003; Kuroda et al. 2009). TMJ tissues are mostly subjected to dynamic loading during chewing or talking, although static and quasi-static loading arising from clenching or grinding may also be prevalent depending on personal habits (Tanaka and van Eijden 2003; Kuroda et al. 2009). Unlike hyaline cartilage in other joints, the TMJ disc and condylar cartilage are fibrocartilaginous tissues with a unique composition and structure (Tanaka and van Eijden 2003; Kuroda et al. 2009). A dense fibrous zone composed of anteroposteriorly aligned large type I collagen bundles covers the top of the condylar cartilage, resulting in a surface roughness that is an order of magnitude higher than that of hyaline cartilage in the knee joint (Chan et al. 2011). The TMJ disc is approximately 75% type I collagen by dry weight with region-dependent alignment directions.
Previous studies using a pendulum device determined the coefficient of friction (COF) of an intact porcine TMJ to be ~0.02 under physiological loading conditions (Tanaka et al. 2004; Tanaka et al. 2006), increasing with increased loading magnitude and compression duration prior to articulation. Nickel and McLachlan (1994) studied lubrication of the disc with a similar pendulum arrangement, and although friction coefficients were initially low (~0.005), they increased with loading time in a manner consistent with weeping lubrication (McCutchen 1962; Forster and Fisher 1996). More recently, the group investigated the “tractional coefficient,” which includes interfacial friction and plowing forces. They found that the plowing component, while considerable at high speeds and loads, is minimized at low speeds and loads (Nickel et al. 2004; Nickel et al. 2006; Nickel et al. 2009).
Several mechanisms have been proposed to explain the excellent lubrication properties of hyaline cartilage in diarthrodial joints. According to hydrodynamic (Wright and Dowson 1976), elastohydrodynamic (Medley et al. 1984), and boosted (Walker et al. 1968) lubrication mechanisms, contacting articular surfaces are separated by a viscous third body fluid film or gel, which can reduce friction significantly compared with solid-solid contact. In boundary lubricated contacts, cartilage surfaces are separated by a surface-bound lubricant layer (Schmidt and Sah 2007). Weeping and interstitial fluid lubrication (IFL) theories involve pressurization of the interstitial fluid in cartilage under compression (Soltz and Ateshian 1998; Park et al. 2003; Krishnan et al. 2004; Ateshian 2009). The pressurized interstitial fluid supports up to 99% of the contact force, thereby reducing the load carried by friction-causing solid-solid contacts. IFL is the mechanism that distinguishes cartilage from typical tribomaterials and is regarded as the primary mechanism of joint lubrication (McCutchen 1962).
A model surface (e.g., steel, glass, or acrylic) is often used as a counterbody to isolate and probe the properties of the cartilage surface of interest. These experiments can be subdivided into stationary contact area (SCA) and migrating contact area (MCA) testing (Caligaris and Ateshian 2008). In SCA testing, the normal pressure remains stationary relative to the cartilage surface (e.g., cartilage explant against large flat surface; McCutchen 1962). During such tests, the friction coefficient is initially low and increases over time as fluid “weeps” from the contact and load shifts from the fluid to the solid matrix (McCutchen 1962; Forster and Fisher 1996; Ateshian 2009). In MCA testing (e.g., convex body on cartilage), the contact pressure field migrates across the cartilage surface, an environment consistent with that of cartilage-cartilage contacts in joints. Interstitial fluid pressure and low friction can be maintained if the migration rate greatly exceeds the characteristic flow rate of the tissue (Pe>>1; Ateshian 2009; Spilker et al. 2009). Although there is evidence that the TMJ disc exhibits IFL (Spilker et al. 2009), it is uncertain how the maintenance and restoration of fluid pressure compare with that of hyaline cartilage. To date, it remains unclear whether the degree of IFL in TMJ tissues is comparable with hyaline cartilage in other joints, as the TMJ cartilaginous tissues display a unique ultrastructure and composition.
As has been noted before, the long-term health of diarthrodial joints depends on mitigating tissue stresses, of which friction is an important source (Nickel et al. 2001). In the TMJ, an increase in friction has been proposed as an important factor in disorders including disc displacement (Nitzan 2001) and damaging shear at the cartilage-bone interface (Koolstra 2012). However, the exact mechanism by which the TMJ achieves low friction is not well defined (Nickel et al. 2001). Thus, the aims of this study are 1) to evaluate and compare the individual frictional responses of the TMJ disc and condylar cartilage and the region and direction dependence of friction properties and 2) to examine and compare the roles of IFL in the lubrication of the TMJ disc and condylar cartilage. The specific aims were chosen to determine the level of IFL in TMJ tissues and thus evaluate the active lubrication mechanisms, to further understand the friction processes within the TMJ.
Materials and Methods
Eight porcine TMJs, 4 left and 4 right joints (Green Village, NJ), were harvested for this study; this joint was selected because of its prior use as a human TMJ model (Bermejo et al. 1993; Tanaka and van Eijden 2003). The animals were of mixed breed and sex, with an average age of 4 mo and an average weight of 90 kg. During extraction, the inferior TMJ capsule was preserved with the disc attached on the condyle head. Histology images of the central regions of disc and condylar cartilage, including hematoxylin and eosin (H&E), Picrosirius red, and Safranin O staining, were obtained to examine ultrastructure and composition. Surface roughness measurements were made on the central region of 4 samples using scanning white light interferometry (Wyko NT9100; Veeco, Camarillo, CA) after removing surface fluid with a lint-free cloth.
On the day of testing, the disc and mandibular condyle were separated (Fig. 1A) and unbound synovial fluid on the articular surface was gently removed with a phosphate-buffered saline (PBS) rinse. The tissues were left to equilibrate in 0.15 M PBS for 30 min prior to testing. Samples were then fixed on a custom-built micro-tribometer (Fig. 1B), as described previously (Bonnevie et al. 2011; Bonnevie et al. 2012). The biconcave TMJ disc was fixed on a rigid curved plastic substrate attached to the testing frame. Friction coefficients were measured by reciprocating a stainless-steel sphere (radius = 3.2 mm, roughness = 50 nm) over a 1.5-mm sliding track on the inferior surface of the TMJ disc or the condylar cartilage surface (MCA configuration). Orthogonal capacitance sensors were used to measure the normal contact force and shear force on the sphere. The friction coefficient was calculated by dividing the mean absolute value of the forward and reverse friction forces by the mean normal force, eliminating the bias error associated with sample curvature (Burris and Sawyer 2009). Friction coefficient measurements were collected over the central 10% of the track after reaching a dynamic steady state (~1–3 min) to minimize the plowing effects during transient sliding (Nickel et al. 2006).
Figure 1.
Mechanical and frictional testing. (A) Picture of the condylar head of a left porcine temporomandibular joint (TMJ) with the articular disc lifted, exposing the silky inferior surface of the disc. The rough surface of the condylar cartilage is covered with a layer of visible crossed collagen bundles. Collagen fibers in the disc are predominantly aligned in the anterior-posterior direction. Five testing regions (anterior, posterior, central, lateral, and medial) are marked on the condyle head. The corresponding contact regions on the TMJ disc were also tested. (B) Schematic of the custom-built micro-tribometer. Sliding of stainless-steel spherical tip on the articular surface is achieved by reciprocation of the piezoelectric bottom stage. Normal loading on the tissue is controlled by the top stage and the capacitance sensor on the cantilever beam. Friction force is recorded by another orthogonal sensor at the end of the beam. (C) A table summarizes the testing parameters. The first row includes the testing modes for all 5 regions, and the second row lists the extra tests performed on the central region to examine the effects of sliding speed and normal force on the friction coefficient. (D) Schematic of the custom-built micro-indenter employed for the indentation test. The step size of the piezoelectric motor is 60 nm (Physik Instrumente, Karlsruhe, Germany).
To probe regional properties on the articular surface, contact areas were necessarily smaller than those in vivo. We assumed physiological pressures in the TMJ in the range of 0.05 to 0.5 MPa based on a 10-mm contact radius and 10- to 170-N normal forces (Boyd et al. 1990; Nitzan 1994), which are also consistent with those measured ex situ (Nickel et al. 2004). Tests were conducted at a nominal load of 100 mN, which, according to Hertz’s contact theory, results in a mean contact pressure of 0.25 MPa when the probe radius is 3.2 mm. The friction properties of the disc and condylar cartilage were measured at 5 different regions (anterior, posterior, central, medial, and lateral) in both the anterior-posterior (AP) and lateral-medial (LM) sliding directions at the 100-mN nominal load and a nominal sliding speed of 2 mm/s (Fig. 1C). The nominal speed is on the low end of physiological values (Gallo et al. 2000), chosen to minimize the potential for hydrodynamic and plowing effects (Nickel et al. 2004; Nickel et al. 2009) while being fast enough to satisfy the condition Pe>>1 required for IFL (Ateshian 2009; Caligaris and Ateshian 2008). In addition, the central regions of the disc and condyle were tested in the AP direction at 6 normal loads (25, 50, 75, 100, 125, and 150 mN) and 7 sliding speeds (0.05, 0.1, 0.25, 0.5, 1, 2, and 5 mm/s) to investigate the potential contributions of plowing friction and IFL, respectively (Fig. 1C). The nominal values in italics above were held while the other quantity varied. The testing order was randomized and showed no effect on results. PBS supplemented with a protease inhibitor cocktail (Lu et al. 2009) was dripped abundantly over the entire tissue surface to flood the contact and maintain uniform hydration throughout the 3 to 4 h of testing for each sample.
Indentation creep tests were performed using a micro-indenter (Fig. 1D; Lu et al. 2004; Lu et al. 2009) to extract the biomechanical properties at the locations of frictional measurements. The sample was affixed in a medium chamber attached on a 3-dimensional ball head, enabling the tissue surface to be aligned perpendicular to a porous cylindrical indenter tip (ϕ = 1.6 mm). A 50-mN tare load was applied to the mandibular condyles for 5 min, followed by a 200-mN load for 1 h (Lu et al. 2009). Tests were identical for the disc, but given that it is roughly an order of magnitude softer than condylar cartilage, the tare (5 mN) and creep (20 mN) loads were reduced accordingly to maintain similar strains (Kim et al. 2003). The creep deformation under constant loading was analyzed by a biphasic curve-fitting program (Mak et al. 1987; Lu et al. 2004), which simultaneously determined Poisson’s ratio, aggregate modulus, and hydraulic permeability. The imposed compressive strains (<25%) were low enough to justify the use of linear small-strain biphasic theory (Spilker et al. 2009). Tissue thickness was measured using the needle penetration method (Hoch et al. 1983; Lu et al. 2004).
Values reported are mean ± standard deviation unless otherwise noted. One-way analysis of variance with Tukey post hoc was performed (Origin 8.1; OriginLab, Northampton, MA) to detect statistical significance among different regions for mechanical properties. Student’s t test was performed to seek significance between the orthogonal sliding directions and between roughness values for each tissue. Linear regression analysis was employed to detect correlations between mechanical properties and friction coefficients, correlations between testing conditions (speed and normal force) and friction coefficients, and correlations between frictional coefficients and Péclet number (, where V = sliding speed, h = tissue thickness, HA = aggregate modulus, k = hydraulic permeability), with significance indicated by P < 0.05.
Results
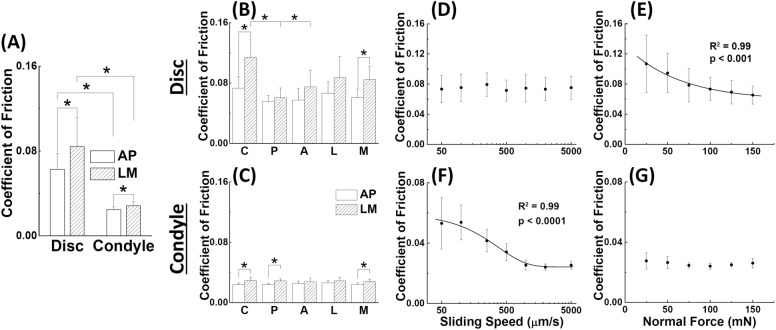
Friction on the disc was significantly higher than that of the condyle when tested against stainless steel (0.074 ± 0.025 vs 0.027 ± 0.004 with data from all regions, n = 80, P < 0.0001). Both tissues demonstrated higher friction in the LM direction compared with the AP direction, and this effect was more pronounced in the disc (0.063 ± 0.015 vs 0.084 ± 0.028 for the disc and 0.025 ± 0.003 vs 0.028 ± 0.004 for the condyle, n = 40, P < 0.0001; Fig. 2A). The central region of the disc (0.114 ± 0.023) demonstrated significantly higher friction coefficients than the anterior (0.075 ± 0.022, P = 0.011) and posterior (0.061 ± 0.013, P = 0.0004) regions in the LM direction (Fig. 2B), but no regional differences were detected for the AP direction in the disc or for either direction on the condyle (Fig. 2C). The disc and condyle exhibited distinct load- and speed-dependent frictional properties, detected by linear regression. Friction on the disc was independent of speed (P = 0.98; Fig. 2D) but decreased exponentially (R2 = 0.99) from 0.107 ± 0.038 to 0.065 ± 0.012 (P < 0.001) as load increased from 25 to 150 mN (Fig. 2E). Friction on the condyle displayed a significant exponential decrease (R2 = 0.99) from 0.053 ± 0.017 to 0.025 ± 0.003 (P < 0.0001; Fig. 2F) as speed increased from 0.05 to 5 mm/s but showed no dependence on load (P = 0.23; Fig. 2G).
Figure 2.
Friction coefficient of the temporomandibular joint (TMJ) disc and condylar cartilage. (A) Comparison of frictional coefficients of disc and condyle in 2 perpendicular sliding directions. Data from all 5 regions were combined for each tissue (n = 40). (B, C) Regional- and directional-dependent friction coefficients of disc and condylar head (n = 8). On the disc, the central region in the lateral-medial direction has a higher coefficient of friction than the anterior and posterior regions. No regional difference was detected on condylar cartilage. (D–G) Frictional coefficient versus sliding speed and normal load, respectively, for disc (D–E) and condyle (F–G). The normal load (100 mN) or sliding speed (2 mm/s) was fixed while the other quantity varied. Linear regression was performed to detect correlations between the friction coefficient and either sliding speed or normal load. In all plots, the asterisk (*) denotes P < 0.05. All error bars indicate 1 standard deviation.
A set of typical indentation creep data and biphasic fitting curves is shown in Figure 3A. The disc’s aggregate modulus was 1 order of magnitude lower than that of the condyle (0.036 ± 0.009 MPa vs 0.25 ± 0.06 MPa; Fig. 3B, C), and its permeability was 1 order higher (10.2 ± 8.8 × 10−15 m4/N·s vs 1.10 ± 0.41 × 10−15 m4/N·s; Fig. 3D, E). No significant difference in mechanical properties was detected among regions (P > 0.065 for every pair comparison).
Figure 3.

Biomechanical properties of the temporomandibular joint (TMJ) disc and condylar cartilage. (A) A set of typical creep deformation curves generated by constant loading on TMJ disc and condylar cartilage. Higher strain was observed in the disc, although the loading on it is smaller than that on condylar cartilage (20 mN vs 200 mN). Biphasic mixture theory curve fits are shown as solid lines. (B, C) Regional aggregate moduli for the disc and condylar cartilage. (D, E) Regional permeability for the disc and condylar cartilage. All mechanical properties were determined by curve fitting the creep deformation with linear biphasic theory. No significant regional differences were detected for either tissue or property (n = 8, P < 0.05).
Histologically, the ultrastructure of the disc is homogenous across its depth, consisting mainly of collagen fibers and a small amount of proteoglycans (Fig. 4A). In contrast, condylar cartilage consists of 3 distinct, hyaline-like layers interwoven with large collagen fibers, which is covered by an extra ~400-µm-thick fibrous mat of collagen (Fig. 4A). Interferometry images (Fig. 4B) report a mean surface roughness of 12.81 ± 2.34 µm for the condyle versus 8.53 ± 2.75 µm on the TMJ disc, but the difference is not significant (n = 4, P = 0.085).
Figure 4.

Structural analysis of temporomandibular joint (TMJ) tissues. (A) Histology images of TMJ disc and condylar cartilage stained with hematoxylin and eosin, Picrosirius red, and Safranin O, respectively. The TMJ disc has a relatively homogenous structure across its depth with little proteoglycans. A thick fibrous mat of collagen fibers exists in the top zone of condylar cartilage, covering the proliferative zone in the middle-deep layer. The images were obtained from the central region of the tissues, parallel to the anterior-posterior direction. (B) Scanning white light interferometry images of the central region of disc and condylar cartilage produced with a scan length of 50 µm. Data restore was used to interpolate over regions of missing data smaller than 35 pixels, with the entire image comprising 640 × 480 pixels, representing an area of 1 mm2. Anterior-posterior alignment of collagen fibers is visible in the disc. In each tissue, the anterior-posterior direction runs vertically.
Linear regression analysis detected no statistically significant correlations between material properties and friction coefficients (P > 0.1 in all cases; Fig. 5A–D). In addition, no significant correlation between Péclet number and friction coefficient was observed for the disc (P = 0.65; Fig. 5E). However, a relationship between the Péclet number and COF was observed for the condyle (P < 0.0005; Fig. 5F). As the Péclet number increased from 1,000 to 100,000, the friction coefficient on the condyle decreased exponentially (R2 = 0.22) from 0.06 to 0.03.
Figure 5.
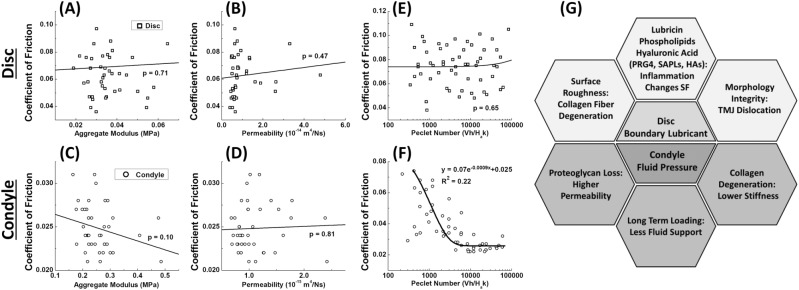
Correlations between frictional coefficient and mechanical properties. (A–D) Correlations between mechanical properties (aggregate modulus and permeability) and coefficient of friction for both disc (A, B) and condyle (C, D) were analyzed by linear regression, with the anterior-posterior (AP) coefficient of friction reported. No statistically significant relationship was observed. Similar results (not shown) were obtained for COF in the lateral-medial direction. (E, F) Correlation between Péclet number and coefficient of friction, measured in the AP direction. A strong exponential relationship between Péclet number and friction coefficient (R2 = 0.22) can be seen for condylar cartilage. (G) Biomechanical factors necessary to maintain functional lubrication in the TMJ disc and condylar cartilage.
Discussion
This study revealed unexpected differences between the friction properties of the TMJ disc and condylar cartilage. The friction coefficient of the disc was significantly higher than that of condylar cartilage. Speed and load dependencies offer some insight into the responsible mechanisms. Whereas condylar cartilage exhibited an exponential reduction in friction with increased sliding speed, consistent with IFL theory (Ateshian 2009), the disc showed no evidence of localized IFL despite having comparably large Péclet numbers. While other studies clearly demonstrated bulk IFL for the disc (Nickel et al. 2004), our results suggest that migrating contact alone cannot restore TMJ disc hydration after exudation. This supports the notion that the biphasic nature of the tissue and Pe>>1 are necessary but not sufficient to maintain IFL.
Although interstitial fluid has more paths to escape the disc, due to the lack of subchondral bone, this is not a significant factor in the lack of disc IFL. In contrast with the present results, the meniscus, another fibrous joint tissue with no impermeable subchondral bone, showed even lower friction coefficients than those of knee hyaline cartilage in our previous experiments with the same device and setup (Baro et al. 2012). While no dependence of friction on speed was detected, the friction coefficient of the disc was proportional to force to the −0.328 power (Fig. 2F; best power-law fit not shown), which is in line with the theoretical −1/3 power predicted for a Hertzian contact lubricated by a boundary lubricant of fixed shear strength. The disc is distinguished from hyaline cartilage and meniscus in its inability to promote detectable IFL in MCA sliding.
Although condylar cartilage displayed significant evidence of IFL, this tissue is also functionally different from hyaline cartilage in the knee. Our past studies of knee cartilage yielded frictional variations on the order of 10× over the speed range studied here, while friction of TMJ cartilage varied by less than 2×, a change indicative of ~50% interstitial load support compared with 80% to 90% in knee cartilage under identical testing conditions (Bonnevie et al. 2011). The observation of limited cartilage IFL here may be related to the lack of proteoglycans in the 400-µm-thick superficial fibrous zone, which we would expect to increase permeability (Fig. 4A). Deformations during MCA testing in this study were limited to ~100 µm, and thus contact stresses were largely contained within this superficial layer. This also helps explain the poor correlation between frictional and mechanical properties (Fig. 5A–D). Whereas the frictional response was mainly driven by superficial zone properties, the indentation response was likely more sensitive to the proteoglycan-rich subsurface domain. In addition, both TMJ tissues exhibited unexpectedly small values of equilibrium friction coefficient of ~0.07 (toward the limit of zero speed). Measurements of the equilibrium friction coefficients for knee cartilage and meniscus under the same conditions produced values an order of magnitude larger (Bonnevie et al. 2011). The results suggest that the TMJ is not as reliant on IFL as the knee and may be better equipped for sliding following long-term static loading, potentially because of improved boundary lubricity. These 2 interesting results (minimal time-dependent rise in friction and low equilibrium friction) are consistent with literature reporting that friction of the TMJ increased only marginally with loading time, rising merely 1.5× after 45 min of loading (Tanaka et al. 2004), while friction of hyaline cartilage increased by 20× to 60× over comparable durations (Forster and Fisher 1996; Caligaris and Ateshian 2008).
The results imply some clinically relevant factors regarding the maintenance of healthy lubrication in the TMJ (Fig. 5G). Condylar cartilage relies on interstitial fluid pressurization, for which the mechanical integrity of the collagen network and the existence of a dense fibrous top zone with high tensile strength are crucial (Krishnan et al. 2003; Hosseini et al. 2014). While the TMJ disc does not promote detectable IFL under the present testing conditions, our results indicated that boundary lubrication can be crucial for the disc. Therefore, the morphological integrity, surface roughness, and efficacy of joint lubricants appear to be critical in minimizing disc friction. In addition, the importance of boundary lubricants in mitigating disc friction may lend support to the debated practice of intra-articular lubricant injections (de Souza et al. 2012).
There are several limitations in this work. First, flushing the joints with PBS was performed to ensure a consistent testing environment, although this condition differs from that in vivo. Second, the mixed background of the tested animals likely increased variability and discouraged detection of regional differences previously reported in the literature (Kim et al. 2003; Lu et al. 2009). Nevertheless, greater variability improves generalization of results. In addition, although the MCA configuration maintains IFL as would be the case with motion in vivo, it also introduces an unknowable plowing force. However, we used low speeds, low loads, steady-state conditions, and measurements from the center 10% of the track, all of which attenuate the contribution from plowing (Nickel et al. 2009). Our previous study using similar conditions revealed no significant difference in friction coefficients when the probe radius varied by a factor of 2 (Bonnevie et al. 2011), which would increase the plowing component by increasing the frontal area per normal area. Furthermore, increased load in this study caused decreased friction, despite increasing the plowing component for the same reason given above. Lastly, the experiments in this study used microscale contact in MCA sliding to probe the regions of each tissue that are mated in vivo. This necessarily limits the nature of any conclusions regarding the intact joint, as components such as the retrodiscal tissue were not considered or tested. Although the present results indicate that only condylar cartilage employs IFL, and to a lesser extent than hyaline cartilage, this does not preclude the possibility that IFL occurs within the intact TMJ. The larger contact area ensures that the proteoglycan-rich bulk participates in load support, which we believe would enhance IFL in condylar cartilage.
Author Contributions
B.K. Zimmerman, X.L. Lu, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; E.D. Bonnevie, contributed to conception, design, and data interpretation, critically revised the manuscript; M. Park, contributed to design, data acquisition, and interpretation, critically revised the manuscript; Y. Zhou, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; L. Wang, contributed to conception and data interpretation, critically revised the manuscript; D.L. Burris, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work was supported by the National Institutes of Health (grant P20RR016458) and University of Delaware Research Foundation. B.K.Z. and E.D.B. acknowledge support from University of Delaware Undergraduate Research Program.
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ateshian GA. 2009. The role of interstitial fluid pressurization in articular cartilage lubrication. J Biomech. 42(9):1163–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baro VJ, Bonnevie ED, Lai X, Price C, Burris DL, Wang L. 2012. Functional characterization of normal and degraded bovine meniscus: rate-dependent indentation and friction studies. Bone. 51(2):232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo A, Gonzalez O, Gonzalez JM. 1993. The pig as an animal model for experimentation on the temporomandibular articular complex. Oral Surg Oral Med Oral Pathol. 75(1):18–23. [DOI] [PubMed] [Google Scholar]
- Bonnevie ED, Baro V, Wang L, Burris DL. 2011. In-situ studies of cartilage microtribology: roles of speed and contact area. Tribol Lett. 41(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnevie ED, Baro VJ, Wang L, Burris DL. 2012. Fluid load support during localized indentation of cartilage with a spherical probe. J Biomech. 45(6):1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd RL, Gibbs CH, Mahan PE, Richmond AF, Laskin JL. 1990. Temporomandibular joint forces measured at the condyle of Macaca arctoides. Am J Orthod Dentofacial Orthop. 97(6):472–479. [DOI] [PubMed] [Google Scholar]
- Burris DL, Sawyer WG. 2009. Addressing practical challenges of low friction coefficient measurements. Tribol Lett. 35(1):17–23. [Google Scholar]
- Caligaris M, Ateshian GA. 2008. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthritis Cartilage. 16(10):1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SM, Neu CP, Komvopoulos K, Reddi AH. 2011. Dependence of nanoscale friction and adhesion properties of articular cartilage on contact load. J Biomech. 44(7):1340–1345. [DOI] [PubMed] [Google Scholar]
- de Souza RF, Lovato da, Silva CH, Nasser M, Fedorowicz Z, Al-Muharraqi MA. 2012. Interventions for the management of temporomandibular joint osteoarthritis. Cochrane Database Syst Rev. 4:CD007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster H, Fisher J. 1996. The influence of loading time and lubricant on the friction of articular cartilage. Proc Inst Mech Eng. H210(2):109–119. [DOI] [PubMed] [Google Scholar]
- Gallo LM, Nickel JC, Iwasaki LR, Palla S. 2000. Stress-field translation in the healthy human temporomandibular joint. J Dent Res. 79(10):1740–1746. [DOI] [PubMed] [Google Scholar]
- Hoch DH, Grodzinsky AJ, Koob TJ, Albert ML, Eyre DR. 1983. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1(1):4–12. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, Wu Y, Ito K, van Donkelaar CC. 2014. The importance of superficial collagen fibrils for the function of articular cartilage. Biomech Model Mechanobiol. 13(1):41–51. [DOI] [PubMed] [Google Scholar]
- Kim KW, Wong ME, Helfrick JF, Thomas JB, Athanasiou KA. 2003. Biomechanical tissue characterization of the superior joint space of the porcine temporomandibular joint. Ann Biomed Eng. 31(8):924–930. [DOI] [PubMed] [Google Scholar]
- Koolstra JH. 2012. Biomechanical analysis of the influence of friction in jaw joint disorders. Osteoarthritis Cartilage. 20(1):43–48. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Kopacz M, Ateshian GA. 2004. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J Orthop Res. 22(3):565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan R, Park S, Eckstein F, Ateshian GA. 2003. Inhomogeneous cartilage properties enhance superficial interstitial fluid support and frictional properties, but do not provide a homogeneous state of stress. J Biomech Eng. 125(5):569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Tanimoto K, Izawa T, Fujihara S, Koolstra JH, Tanaka E. 2009. Biomechanical and biochemical characteristics of the mandibular condylar cartilage. Osteoarthritis Cartilage. 17(11):1408–1415. [DOI] [PubMed] [Google Scholar]
- Lu XL, Mow VC, Guo XE. 2009. Proteoglycans and mechanical behavior of condylar cartilage. J Dent Res. 88(3):244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XL, Sun DD, Guo XE, Chen FH, Lai WM, Mow VC. 2004. Indentation determined mechanoelectrochemical properties and fixed charge density of articular cartilage. Ann Biomed Eng. 32(3):370–379. [DOI] [PubMed] [Google Scholar]
- Mak AF, Lai WM, Mow VC. 1987. Biphasic indentation of articular cartilage—I. Theoretical analysis. J Biomech. 20(7):703–714. [DOI] [PubMed] [Google Scholar]
- McCutchen CW. 1962. The frictional properties of animal joints. Wear. 5(1):1–17. [Google Scholar]
- Medley JB, Dowson D, Wright V. 1984. Transient elastohydrodynamic lubrication models for the human ankle joint. Eng Med. 13(3):137–151. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Iwasaki LR, Beatty MW, Marx DB. 2004. Laboratory stresses and tractional forces on the TMJ disc surface. J Dent Res. 83(8):650–654. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Iwasaki LR, Beatty MW, Moss MA, Marx DB. 2006. Static and dynamic loading effects on temporomandibular joint disc tractional forces. J Dent Res. 85(9):809–813. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Iwasaki LR, Feely DE, Stormberg KD, Beatty MW. 2001. The effect of disc thickness and trauma on disc surface friction in the porcine temporomandibular joint. Arch Oral Biol. 46(2):155–162. [DOI] [PubMed] [Google Scholar]
- Nickel JC, McLachlan KR. 1994. In vitro measurement of the frictional properties of the temporomandibular joint disc. Arch Oral Biol. 39(4):323–331. [DOI] [PubMed] [Google Scholar]
- Nickel J, Spilker R, Iwasaki L, Gonzalez Y, McCall WD, Ohrbach R, Beatty MW, Marx D. 2009. Static and dynamic mechanics of the temporomandibular joint: plowing forces, joint load and tissue stress. Orthod Craniofac Res. 12(3):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzan DW. 1994. Intraarticular pressure in the functioning human temporomandibular joint and its alteration by uniform elevation of the occlusal plane. J Oral Maxillofac Surg. 52(7):671–679. [DOI] [PubMed] [Google Scholar]
- Nitzan DW. 2001. The process of lubrication impairment and its involvement in temporomandibular joint disc displacement: a theoretical concept. J Oral Maxillofac Surg. 59(1):36–45. [DOI] [PubMed] [Google Scholar]
- Park S, Krishnan R, Nicoll SB, Ateshian GA. 2003. Cartilage interstitial fluid load support in unconfined compression. J Biomech. 36(12):1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TA, Sah RL. 2007. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage. 15(1):35–47. [DOI] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. 1998. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 31(10):927–934. [DOI] [PubMed] [Google Scholar]
- Spilker RL, Nickel JC, Iwasaki LR. 2009. A biphasic finite element model of in vitro plowing tests of the temporomandibular joint disc. Ann Biomed Eng. 37(6):1152–1164. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Dalla-Bona DA, Iwabe T, Kawai N, Yamano E, van Eijden T, Tanaka M, Miyauchi M, Takata T, Tanne K. 2006. The effect of removal of the disc on the friction in the temporomandibular joint. J Oral Maxillofac Surg. 64(8):1221–1224. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Kawai N, Tanaka M, Todoh M, van Eijden T, Hanaoka K, Dalla-Bona DA, Takata T, Tanne K. 2004. The frictional coefficient of the temporomandibular joint and its dependency on the magnitude and duration of joint loading. J Dent Res. 83(5):404–407. [DOI] [PubMed] [Google Scholar]
- Tanaka E, van Eijden T. 2003. Biomechanical behavior of the temporomandibular joint disc. Crit Rev Oral Biol Med. 14(2):138–150. [DOI] [PubMed] [Google Scholar]
- Walker PS, Dowson D, Longfield MD, Wright V. 1968. “Boosted lubrication” in synovial joints by fluid entrapment and enrichment. Ann Rheum Dis. 27(6):512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright V, Dowson D. 1976. Lubrication and cartilage. J Anat. 121(Pt 1):107–118. [PMC free article] [PubMed] [Google Scholar]