Abstract
Salivary protein histatin 5 (Hst 5) is fungicidal toward Candida albicans, the causative agent of oropharyngeal candidiasis. However, its activity in saliva is compromised by salivary protease-mediated degradation and interaction with salivary salts. Hst 5 has also been shown to bind various metals in saliva—namely, Zn, Cu, and Ni. Surprisingly, interactions of Hst 5 with Fe have not been studied, although iron is one of the most abundant metals present in saliva. Using circular dichroism, we show that Hst 5 can bind up to 10 equivalents of iron as measured by loss of its alpha-helical secondary structure that is normally observed for it in trifluoroethylene. A significant decrease in the candidacidal ability of Hst 5 was observed upon iron binding, with increasing iron concentrations being inversely proportional to Hst 5 killing activity. Binding assays showed that the decrease in killing was likely a result of reduced binding (10-fold reduction) of Fe–Hst 5 to C. albicans cells. Protease stability analysis showed that Fe–Hst 5 was completely resistant to trypsin digestion. In contrast, zinc binding had limited effects on Hst 5 fungicidal activity or protease susceptibility. RNA sequencing results identified changes in iron uptake genes in Hst 5–treated C. albicans cells. Our findings thus suggest that consequences of Hst 5 binding iron not only affect candidacidal ability and proteolyic stability of Hst 5, but may also contribute to a novel killing mechanism involving interference with cellular iron metabolism.
Keywords: saliva, zinc, Candida albicans, antimicrobial peptides, transition elements, proteolysis
Introduction
Salivary histatin 5 (Hst 5) possesses significant fungicidal activity against Candida albicans, the causative agent of oropharyngeal candidiasis among immunocompromised individuals and denture users. Unlike many antimicrobial peptides, its mechanism of action does not include membrane lysis (reviewed in Puri and Edgerton 2014). A major barrier for the use of Hst 5 as a topical drug for oropharyngeal candidiasis is that Hsts exhibits limited fungicidal activity when added to whole saliva (WS; Helmerhorst et al. 2004). Thus, some individuals have low salivary killing activity despite high concentrations of Hsts in saliva (Conti et al. 2011). Two processes that can explain these observations are the dynamic turnover of salivary proteins balancing secretion with proteolytic degradation (Xu et al. 1993; Helmerhorst et al. 2006) and “masking” of functional activity (Helmerhorst et al. 2004) that is likely due to Hst binding with salts and with various salivary metals, including Zn and Cu (Melino et al. 1999; Grogan et al. 2001; Gusman et al. 2001; Porciatti et al. 2010). Many salivary proteins bind divalent metals, including acidic proline-rich proteins and statherin, which bind calcium, and salivary amylase, which has 2 selective metal-binding sites, 1 for calcium and 1 for copper or zinc (reviewed in Gusman et al. 2001). Fungicidal activity of salivary lactoferrin was similarly affected by the presence of divalent cations in saliva (Viejo-Diaz et al. 2004), and comparable results were obtained for Hst 5 with regard to calcium (Dong et al. 2003).
Various transitional metals, such as Zn, Ni, Cu, and Fe, are intrinsic in human saliva (Bales et al. 1990; Duggal et al. 1991; Eliades et al. 2003; Garhammer et al. 2004; Watanabe et al. 2005; Hong and Kim 2011), although there are large variations in metal concentrations among individuals. Selective amino acids in Hst 5, particularly histidine, along with tyrosine, aspartic, and glutamic acid, are all known to coordinate peptide-metal interactions, making it an ideal metal-binding peptide, or a “metallopeptide.” Not surprising, Hst 5 has been shown to bind various transition metals that can exist in a divalent state—namely, Zn, Cu, and Ni—and these binding events induce structural changes in Hst 5 (Melino et al. 1999; Grogan et al. 2001; Gusman et al. 2001; Tay et al. 2009; Porciatti et al. 2010; Kurowska et al. 2011). Hst 5 possesses a definitive Cu- and Ni-binding motif (ACTUN motif) encompassing the first 3 N-terminal amino acids (Asp-Ser-His) and a Zn-binding motif (HEXXH motif) at the C terminus (Grogan et al. 2001; Gusman et al. 2001). Stoichiometric analysis suggested the presence of at least 1 high-affinity site for both Zn and Cu, along with 2 lower-affinity interactions for each metal, and this study concluded that Hst 5 has greater specificity for copper than zinc, although it is capable of binding both metals at their physiologic concentrations in saliva (Gusman et al. 2001).
Physiologic consequences for Hst 5 metal interactions have also come to light. Zinc binding induced Hst 5 to catalyze fusion of lipid vesicles (Melino et al. 1999), and some increase in the bactericidal activity for Hst 5 has been reported for Zn–Hst 5 (Rydengard et al. 2006). However, effects of Cu binding offer a more direct significance to Hst 5 with regard to its antimicrobial activity. A high level of hydrogen peroxide production was observed in solutions with Hst 5 and Cu, in the presence of a reductant such as ascorbate (Cabras et al. 2007; Houghton and Nicholas 2009; Tay et al. 2009). This can potentially generate reactive oxygen species that have been implicated in fungicidal activity of Hst 5.
Surprisingly, interactions of Hst 5 with Fe have not been examined, although iron is one of the most abundant metals present in saliva (Duggal et al. 1991; Eliades et al. 2003; Garhammer et al. 2004; Watanabe et al. 2005) and is an important component of human diet. Saliva contains both free and bound iron (Mukherjee et al. 1997), and the total iron-binding capacity of WS ranges from 0.3 to 0.15 mg of Fe per milliliter (Reilly et al. 1968). Lactoferrin is the most well-characterized high-affinity iron-binding protein in saliva, although calculations of the total iron-binding capacity of saliva showed the existence of other unidentified iron-binding proteins (Mukherjee et al. 1997) that are likely to include Hsts. Lactoferrin creates localized iron-depleted sites exerting a bacteriostatic effect (Fine and Furgang 2002; Sherman et al. 2004); however, its activity is pH dependent (reviewed in Baker and Baker 2004) so that its iron-binding ability is modulated at sites of infection and inflammation. Here we show for the first time that Hst 5 can bind iron and that this binding affects its candidacidal ability—both in vitro and in WS.
Materials and Methods
Media and Growth Conditions
C. albicans CAI4 was cultured in YPD (yeast extract peptone dextrose) medium with uridine (50 μg/mL).
Circular Dichroism
Hst 5 (60 μM) in 95% trifluoroethylene (TFE; Sigma-Aldrich, St Louis, MO, USA), alone or after titration with increasing concentrations of iron (ferric chloride hexahydrate) or zinc (zinc chloride) up to 10 equivalents of was analyzed in a fluorimeter cell (Starna Cells, Inc, Atascadero, CA, USA) with a 1-mm path length at 25°C. Far ultraviolet spectra (190-280 nm) were collected with a Jasco 815 circular dichroism (CD) spectrophotometer (Easton, MD, USA) and a thermostatic water bath. Data were processed with Jasco software and analyzed with Prism 5.0.
Candidacidal Assays
The susceptibility of C. albicans cells to Hst 5 was measured with microdilution plate assays in triplicate as previously described (Li et al. 2003), with some modifications for experiments with metals and/or unstimulated WS. A detailed description is provided in the Appendix.
Flow Cytometry for Hst 5 Binding
To measure the binding of FITC-labeled Hst 5 (FHst 5; synthesized by Genemed Synthesis, San Antonio, TX, USA), 1-mL cells (106 cells/mL in sodium phosphate buffer) were preincubated on ice for 1 h to prevent Hst 5 uptake. FHst 5 alone or after incubation with different molar equivalents of metal for 1 h at 37°C was added to cells for 15 min, followed by washing with phosphate buffered saline to remove unbound FHst 5, with final concentration of FHst 5 in the binding assay being 30 μM. Flow cytometry analysis was performed with fluorescence-activated cell-sorting Calibur flow cytometry and Cellquest Pro Software (BD-Biosciences; San Jose, CA, USA) whereby 10,000 cells were collected and analyzed.
Proteolytic Stability
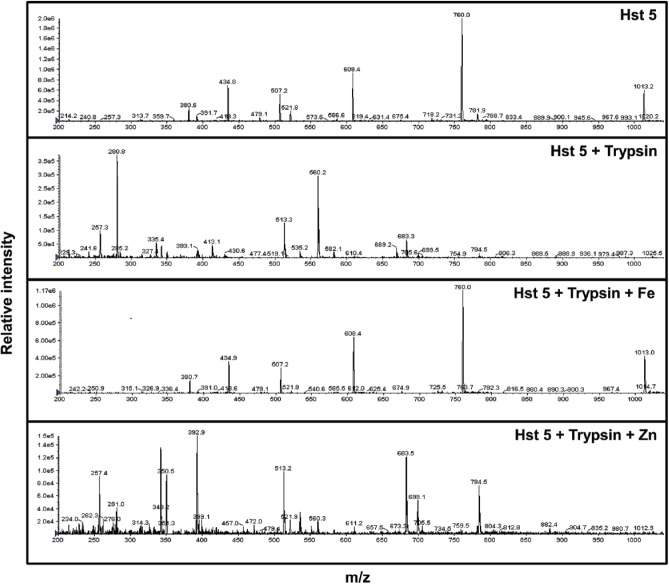
For metal-bound species, Hst 5 was incubated with 10 equivalents of iron or zinc for 60 min at 37°C. Hst 5, Fe:Hst 5, and Zn:Hst 5 were then incubated with trypsin (protease: peptide = 1:30) for 60 min at 37°C. Samples were diluted to 50× in water with 2% TFA (trifluoroacetic acid) to 40 µg/mL of peptide and analyzed by liquid chromatography/mass spectrometry via a C18 column and an API3000 triple-quadrupole mass spectrometer (Applied Biosystems, USA). The mass spectrum of Hst 5 gave a distribution of charge states, with the most abundant being the M+4 at 760 Da.
RNA Seq
RNA was isolated as described previously (Kumar et al. 2011) from C. albicans cells treated with 30 μM Hst 5 (or with phosphate buffered saline as control) for 60 min, and RNA-seq was performed as described in supplemental information.
Results
Hst 5 Can Bind up to 10 Equivalents of Iron
To investigate whether Hst 5 binds iron, we titrated 60 μM Hst 5 with increasing concentrations of iron, followed by CD measurements. Ligand binding–induced secondary structural changes as recorded by CD can provide evidence for peptide-metal binding. Hst 5 (in 100% trifluoroethylene) showed typical secondary structural features of an α-helix (black line, Fig. 1A and B), with conspicuous bands at 208 and 222 nm. Titration with Fe led to a loss of these spectral features, starting with subtle changes at 1 equivalent of iron (dark dashed line, Fig. 1A) that steadily progressed to a further loss in secondary structure with each subsequent titration (solid, dashed, dotted, and dot-dashed black and gray lines representing 0-3 and 4-7 equivalents, respectively; solid, dashed, and dotted light gray lines representing 8-10 equivalents). This change saturated at 10 equivalents of iron (light gray dotted line, Fig. 1A), resulting in an almost complete loss in secondary structure. Hence, CD results clearly show that Hst 5 can bind up to 10 equivalents of Fe, as determined by ligand binding–induced structural changes upon titration with iron.
Figure 1.
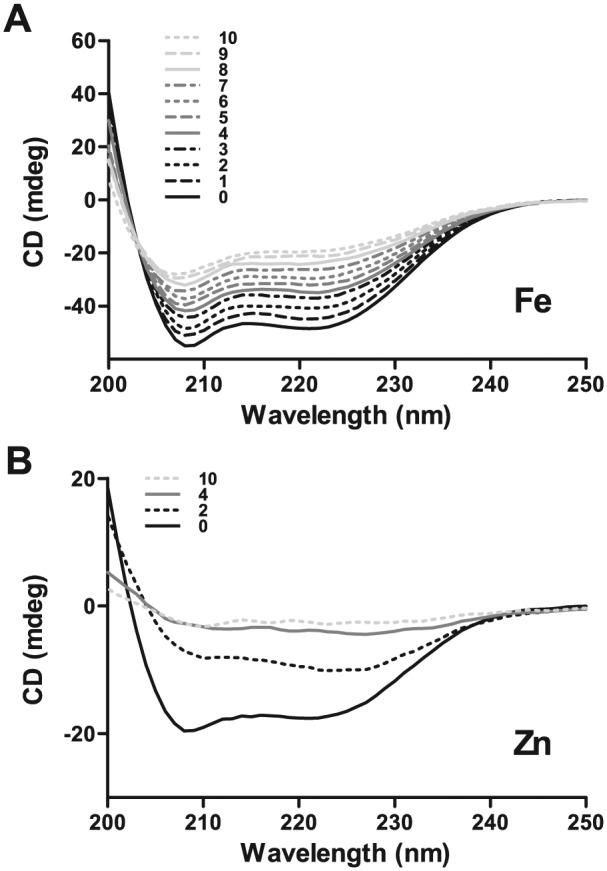
Circular dichroism (CD) spectra of histatin 5 titrated with Fe3+ or Zn2+ showed changes in secondary structure upon metal binding. Hst 5 (250 μL of 60 μM) in 95% trifluoroethylene was titrated with 7.5-mM solution of ferric chloride or zinc chloride equivalents in increments of 2 μL to achieve a Hst 5:metal ratio ranging from 1:1 to 1:10; spectra was recorded between 190 and 280 nm. (A) Hst 5 can bind up to 10 equivalents of iron, resulting in complete loss of secondary structure at 10 equivalents (solid, dashed, dotted, and dot-dashed black and gray lines represent 0-3 and 4-7 equivalents, respectively; solid, dashed, and dotted light gray lines represent 8-10 equivalents of iron). (B) Hst 5 shows saturation in changes in secondary structure upon binding 4 equivalents of zinc, with negligible additional change at 10 equivalents (solid black, dotted black, solid gray, and dotted light gray lines represent 0, 2, 4, and 10 equivalents of Zn, respectively).
We also examined zinc binding by Hst 5 (Fig. 1B) and compared it with iron binding as measured by CD (Fig. 1A). Only 2 equivalents of zinc (dark dotted line, Fig. 1B) led to a drastic loss in secondary structure, while 4 equivalents of Zn (light gray solid line, Fig. 2B) induced complete loss of secondary structure. Increasing Zn from 4 to 10 equivalents (light gray solid line and light gray dashed line, respectively, Fig. 1B) did not further alter Hst 5 structure, as CD profiles were almost identical, pointing toward saturation at 4 equivalents of Zn. This contrasts with gradual changes in secondary structure with increasing amounts of iron, suggesting that Hst 5 has much higher binding potential (at least 10 equivalents of iron) for iron and zinc induces more profound changes in secondary structure upon its binding.
Figure 2.
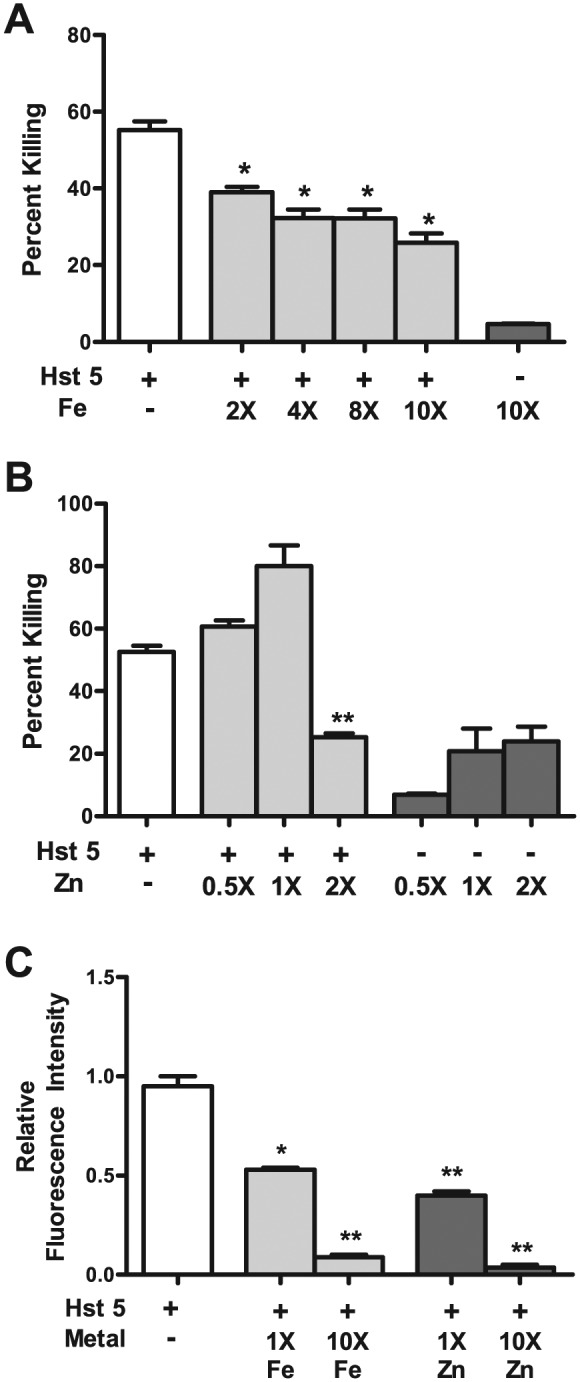
Histatin 5 (Hst 5) killing and cellular binding are affected upon metal binding. Hst 5 (30 μM) with or without metals was used to perform killing assays via a microdilution plate method. (A) Killing abilities of Hst 5 are significantly reduced upon binding with iron, starting at 2 equivalents of iron, with incremental decrease upon addition of further equivalents (white bar, Hst 5 alone; light gray bars, Fe:Hst 5; and dark gray bar, 300 μM iron alone). (B) Killing abilities of Hst 5 (30 μM) are significantly reduced upon binding with 2 equivalents of Zn; however, Zn alone had toxicity (white bar, Hst 5 alone; light gray bars, Zn:Hst 5; and dark gray bars, zinc alone). (C) Cellular binding of Hst 5 is affected upon its binding to iron and zinc. X is the number of times that the concentration is of Hst 5 alone (30 μM).
Hst 5 Candidacidal Activity and Fungal Cell Wall Binding Are Affected by Iron and Zinc
To analyze the effect of metal binding on killing of Hst 5, we performed candidacidal assays using the microdilution method. For metal binding, Hst 5 (30 μM) was incubated with respective metal equivalents and incubated at 37°C for 1 h to facilitate peptide-metal binding before the assay was performed. C. albicans cells treated with Hst 5 alone exhibited almost 55% killing, while there were significant reductions in killing when Hst 5 was bound with iron (Fig. 2A). Hst 5 bound to 2 equivalents of iron showed 39% killing, which was subsequently reduced to only 25% killing for Hst 5 bound to 10 equivalents of iron (Fig. 2A), showing that binding iron negatively affects the killing abilities of Hst 5. Iron alone had negligible toxicity against C. albicans cells in the killing assay when tested at the maximal concentration of 300 μM used in the assay (Fig. 2A).
We compared the effects observed for Fe–Hst 5 interactions with that of Zn–Hst 5 interactions; however, in line with the CD results in Figure 1B, we tested lower-range concentrations for Zn, since 4 equivalents almost saturated the change in CD spectrum. We did not observe any significant change in Hst 5 killing ability at 0.5 and 1 equivalents of Zn, in spite of the fact that Zn itself showed 20% killing activity at 30 μM (1×). Killing of Hst 5 was significantly reduced (25%) at 2 Zn equivalents; however, at this and higher Zn concentrations tested (data not shown), there was greater killing with Zn itself, thus interfering with the assay.
Internalization of Hst 5 is necessary for killing of C. albicans cells since all Hst 5 targets are intracellular (reviewed in Puri and Edgerton 2014). Therefore, to investigate whether effects on killing by iron- or zinc-bound Hst 5 are a result of reduced cellular binding, we performed binding assays using fluorescence-activated cell sorting to quantitate binding of fluorescein isothiocyanate–labeled Hst 5 (FHst 5) to C. albicans cells. FHst 5 was preincubated with 1 or 10 equivalents of iron or zinc, respectively, and binding was compared with FHst 5 without any bound metal. As seen in Figure 2C, FHst 5 bound to 1 equivalent of either iron or zinc showed a 2-fold reduction in cellular binding, which increased to a 20-fold reduction for FHst 5 bound to 10 equivalents of either metal. This strongly suggests that reduced killing upon metal binding (Fig. 2) is a result of its reduced cellular binding (Fig. 2C) that potentially affects subsequent uptake.
Metals Affect the Candidacidal Activity of Native and Exogenously Added Hst 5 in WS
To examine whether metals affect Hst 5 function in its native environment of saliva, we performed candidacidal assays with WS to which either metal only or metal-bound Hst 5 was added (Fig. 3). WS itself (left light gray bar) or WS with Hst 5 (30 μM; left dark gray bar) showed lower candidacidal activity than Hst 5 alone (white bar; Fig. 3A). Taking the average concentration of Hst 5 in saliva at 30 μM, we added 2 or 10 equivalents of iron (60 μM and 300 μM, middle and right light gray bars, respectively) to WS or added iron-bound Hst 5 (Hst 5:Fe at 1:2 or 1:10, middle and right dark gray bars, respectively). Addition of 60 μM iron lowered the activity of native Hst 5 slightly; however, a 5-fold reduction was observed when 300 μM was added (Fig. 3A, light gray bars). Similarly, Hst 5 bound to 10 iron equivalents showed significant reduction in its activity as compared to Hst 5:Fe at 1:2 molar ratio (dark gray bars). In contrast, no significant changes were observed with Zn on the activity of either native or added Hst 5 in WS (Fig. 3B).
Figure 3.
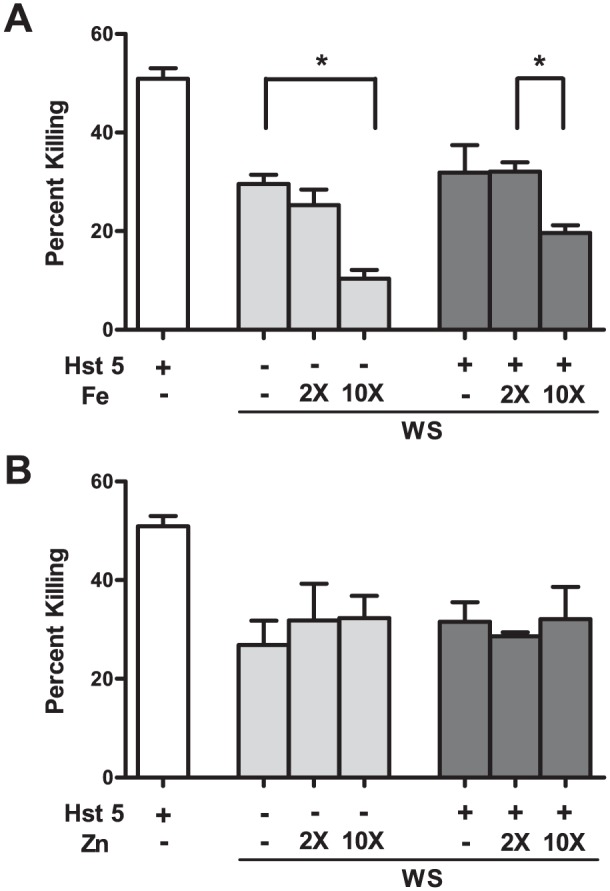
Presence of iron but not zinc in whole saliva (WS) significantly affects the killing ability of native or exogenously added Hst 5. WS and phosphate buffered saline were mixed (1:1 by volume), and respective metals and/or exogenous histatin 5 (Hst 5; 30 μM) were added to the mixture before the killing assay was performed. (A) Addition of 300 μM iron (10 times the expected concentration of Hst 5 in WS) significantly reduced the candidacidal activity of WS, as well as activity of exogenously added Hst 5 in WS. (B) Addition of up to 300 μM zinc (10 times the expected concentration of Hst 5 in WS) did not affect the candidacidal ability of native or exogenously added Hst 5 in WS. White bars represent the killing ability of Hst 5 alone in vitro, while light and dark gray bars represent the killing abilities of native or exogenously added Hst 5, respectively, in WS. X is the number of times that the concentration is of Hst 5 alone (30 μM).
Iron Binding Provides Proteolytic Stability to Hst 5
Histatins in saliva are constantly subjected to proteolytic degradation by salivary proteases, especially those with trypsin-like activity (Xu et al. 1993). Depending on the extent of cleavage (given that Hst 5 has many potential cleavage sites for trypsin), its degradation can generate more or less active fragments of Hst 5. Since binding to iron and, to some extent, zinc affected the candidacidal abilities of Hst 5 as well as its secondary structure, we tested metal binding as a means of conferring proteolytic stability to Hst 5. Hst 5 or Hst 5 bound to 10 equivalents of iron or zinc was treated with trypsin at 1:30 (protease:peptide) ratio, and the reaction was subjected to chromatographic separation, followed by mass spectroscopy analysis. Hst 5 alone when treated with protease showed signature masses for the degradation products based on potential trypsin cleavage sites (Fig. 4). Interestingly, binding to iron offered nearly absolute protection against proteolytic degradation since the mass profile of trypsin-treated Hst 5:Fe was identical to that of Hst 5 without enzymatic treatment (Fig. 4). In contrast, zinc binding to Hst 5 offered very little protection from proteolytic degradation and yielded a mass spectrum comparable to Hst 5 that had undergone tryptic digestion. Thus, binding iron but not zinc provides substantial proteolytic stability to Hst 5.
Figure 4.
Mass spectrometry of trypsin-digested Hst 5 shows proteolytic stability in the presence of iron. Solutions of Hst 5 (6.2 mM) and respective metals (12.4 mM) were mixed in a total volume of 100 μL to achieve a Hst 5:metal ratio of 1:10, to which 6.3 μg of trypsin in 100 μL volume was added such that Hst 5:trypsin equaled 30:1. Hst 5 alone had a characteristic mass/charge (m/z) peak of 760, while Hst 5 treated with trypsin showed signature masses for the degradation products based on potential trypsin cleavage sites and disappearance of the 760 peak. The profile of Hst 5 in the presence of iron and trypsin was similar to that of Hst 5 alone showing iron binding–mediated proteolytic stability. The profile of Hst 5 in the presence of zinc and trypsin was similar to that of trypsin alone, showing that zinc binding does not offer proteolytic stability to Hst 5.
Hst 5 Treatment Affects the Expression of C. albicans Iron Homeostasis Genes
Given that Hst 5 can bind up to 10 equivalents of iron (Fig. 1A), we hypothesized that Hst 5 upon reaching the fungal cytosol may be able to interfere with cellular iron homeostasis of C. albicans. To evaluate this possibility, we performed RNA seq on C. albicans cells treated with Hst 5 (30 μM) and looked for changes in expression of genes involved in iron-related processes. We identified 7 such genes (Table) that showed more than 1.5-fold change at P < 0.05 at both 30 and 60 min of Hst 5 exposure. Interestingly, genes involved in iron uptake, such as fre30 and fre7, which encode for proteins with known and probable ferric reductase functions, respectively, were downregulated, while sfu1, which encodes for a transcriptional repressor of iron uptake, was upregulated. Copper transporters work concomitantly with iron uptake in C. albicans (Cheng et al. 2013), and interestingly, we also observed downregulation of copper transporter gene ctr1 as well as mac1, which encodes for its inducer (Table).
Table.
Iron-related Genes Affected in Histatin 5–Treated Candida albicans Cells.
| Gene | Function | Fold Change |
|---|---|---|
| SFU1 | Represses Fe uptake | 1.6 |
| orf 19.1195 | Role in cellular Fe homeostasis | 1.6 |
| FRE30 | Probable ferric reductase | −1.8 |
| FRE7 | Cu regulated ferric reductase | −1.9 |
| MAC1 | Inducer of CTR1 | −3.0 |
| Orf 19.7077 | Ferric reductase induced by MAC1 | −3.0 |
| CTR1 | Copper transporter | −3.1 |
Discussion
Salivary metallomics is still a nascent field, and the effect of various salivary metals on microbial growth, on activity of salivary antimicrobial peptides and various salivary enzymes, as well as on food digestion and taste perception is largely unknown. Iron is the one of the most abundant transition metal ions in saliva, and its levels are expected to vary significantly, in part due to availability from dietary consumption. Unbound iron is an excellent source for generation of free radicals, leading to oxidative stress; therefore, most iron is present in a bound state in the human body. Also, sequestering iron creates nutritional starvation for invading pathogens, as shown for lactoferrin (Fine and Furgang 2002; Sherman et al. 2004). Given our results, we propose that Hst 5 shares this task of binding free iron in saliva and may have evolved to do so independent of its candidacidal abilities. This is corroborated with our finding that iron binding negatively affects Hst 5 candidacidal abilities (Figs. 2, 3). This seems to be the result of 2 factors: first, change in secondary structure upon iron binding (Fig. 1A) that affects its binding to the C. albicans cell wall (Fig. 2C) and, potentially, its cellular uptake; second, iron-bound Hst 5 that is proteolytically stable (Fig. 4), which may prevent its degradation into more active smaller fragments. Interestingly, lactoferrin is also resistant to proteolytic degradation by trypsin and trypsin-like enzymes, and this resistance is proportional to degree of iron saturation (Brock et al. 1976).
Our discovery of high iron-binding capacity of Hst 5 also provides a fresh perspective to its candidacidal mechanism. RNA seq results (Table) showed changes in the expression of genes related to iron metabolism in Hst 5–treated C. albicans cells. While we expected that Hst 5–mediated iron chelation may induce a state of iron starvation, we instead observed a decrease in expression of genes involved in iron uptake, suggesting that Hst 5 treatment somehow creates a perception of iron “excess” for the cell. Hst 5 localizes in the vacuole and mitochondria (reviewed in Puri and Edgerton, 2014), 2 organelles that are intricately involved in iron trafficking and metabolism in C. albicans (Bai et al. 2005; Thomas et al. 2013). It is possible that Hst 5 could bind intracellular iron and redistribute iron reserves within the cell, leading to malfunctioning of cellular perception of iron levels. Alternatively, Hst 5 could create a similar situation by transporting higher levels of extracellular iron into the cell than what would have normally been permitted by regulated cellular uptake systems. Localization of iron around the mitochondria might also explain the role of reactive oxygen species and mitochondrial dysfunction that have been implicated in the candidacidal mechanism of Hst 5 (reviewed in Puri and Edgerton, 2014), since mitochondria are very sensitive to iron levels. A similar role has been proposed for Hst 5 interaction with copper (Tay et al. 2009).
We conclude here that Hst 5 is a metallopeptide that binds various transition metals, and among them, Fe binding has significant ability to modulate its function. Thus, salivary metal levels may interfere with the outcome of antifungal therapy with Hst 5, and their role may need consideration in clinical application of Hst 5. More studies are needed to determine whether salivary iron levels influence oral fungal carriage or the incidence of oral candidiasis.
Author Contributions
S. Puri, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; R. Li, D. Ruszaj, S. Tati, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; M. Edgerton, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by grants R01DE010641 and R01DE 022720 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, as well as by the Pharmaceutical Instrumentation Facility at the University at Buffalo, as funded by Shared Instrumentation Grant S10RR027232 from the National Center for Research Resources, National Institutes of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bai C, Chan FY, Wang Y. 2005. Identification and functional characterization of a novel Candida albicans gene CaMNN5 that suppresses the iron-dependent growth defect of Saccharomyces cerevisiae aft1Delta mutant. Biochem J. 389(Pt 1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker HM, Baker EN. 2004. Lactoferrin and iron: structural and dynamic aspects of binding and release. Biometals. 17(3):209–216. [DOI] [PubMed] [Google Scholar]
- Bales CW, Freeland-Graves JH, Askey S, Behmardi F, Pobocik RS, Fickel JJ, Greenlee P. 1990. Zinc, magnesium, copper, and protein concentrations in human saliva: age- and sex-related differences. Am J Clin Nutr. 51(3):462–469. [DOI] [PubMed] [Google Scholar]
- Brock JH, Arzabe F, Lampreave F, Piñeiro A. 1976. The effect of trypsin on bovine transferrin and lactoferrin. Biochim Biophys Acta. 446(1):214–225. [DOI] [PubMed] [Google Scholar]
- Cabras T, Patamia M, Melino S, Inzitari R, Messana I, Castagnola M, Petruzzelli R. 2007. Pro-oxidant activity of histatin 5 related Cu(II)-model peptide probed by mass spectrometry. Biochem Biophys Res Commun. 358(1):277–284. [DOI] [PubMed] [Google Scholar]
- Cheng X, Xu N, Yu Q, Ding X, Qian K, Zhao Q, Wang Y, Zhang B, Xing L, Li M. 2013. Novel insight into the expression and function of the multicopper oxidases in Candida albicans. Microbiology. 159(Pt 6):1044–1055. [DOI] [PubMed] [Google Scholar]
- Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, Edgerton M, Gaffen SL. 2011. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 4(4):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Vylkova S, Li XS, Edgerton M. 2003. Calcium blocks fungicidal activity of human salivary histatin 5 through disruption of binding with Candida albicans. J Dent Res. 82(9):748–752. [DOI] [PubMed] [Google Scholar]
- Duggal MS, Chawla HS, Curzon ME. 1991. A study of the relationship between trace elements in saliva and dental caries in children. Arch Oral Biol. 36(12):881–884. [DOI] [PubMed] [Google Scholar]
- Eliades T, Trapalis C, Eliades G, Katsavrias E. 2003. Salivary metal levels of orthodontic patients: a novel methodological and analytical approach. Eur J Orthodont. 25(1):103–106. [DOI] [PubMed] [Google Scholar]
- Fine DH, Furgang D. 2002. Lactoferrin iron levels affect attachment of Actinobacillus actinomycetemcomitans to buccal epithelial cells. J Periodontol. 73(6):616–623. [DOI] [PubMed] [Google Scholar]
- Garhammer P, Hiller KA, Reitinger T, Schmalz G. 2004. Metal content of saliva of patients with and without metal restorations. Clin Oral Invest. 8(4):238–242. [DOI] [PubMed] [Google Scholar]
- Grogan J, McKnight CJ, Troxler RF, Oppenheim FG. 2001. Zinc and copper bind to unique sites of histatin 5. FEBS Lett. 491(1–2):76–80. [DOI] [PubMed] [Google Scholar]
- Gusman H, Lendenmann U, Grogan J, Troxler RF, Oppenheim FG. 2001. Is salivary histatin 5 a metallopeptide? Biochim Biophys Acta 1545(1–2):86–95. [DOI] [PubMed] [Google Scholar]
- Helmerhorst EJ, Alagl AS, Siqueira WL, Oppenheim FG. 2006. Oral fluid proteolytic effects on histatin 5 structure and function. Arch Oral Biol. 51(12):1061–1070. [DOI] [PubMed] [Google Scholar]
- Helmerhorst EJ, Flora B, Troxler RF, Oppenheim FG. 2004. Dialysis unmasks the fungicidal properties of glandular salivary secretions. Infect Immun. 72(5):2703–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Kim KO. 2011. Operationally defined solubilization of copper and iron in human saliva and implications for metallic flavor perception. Eur Food Res Technol. 233(6):973–983. [Google Scholar]
- Houghton EA, Nicholas KM. 2009. In vitro reactive oxygen species production by histatins and copper(I,II). J Biol Inorg Chem. 14(2):243–251. [DOI] [PubMed] [Google Scholar]
- Kumar R, Chadha S, Saraswat D, Bajwa JS, Li RA, Conti HR, Edgerton M. 2011. Histatin 5 uptake by Candida albicans utilizes polyamine transporters Dur3 and Dur31 proteins. J Biol Chem. 286(51):43748–43758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska E, Bonna A, Goch G, Bal W. 2011. Salivary histatin-5, a physiologically relevant ligand for Ni(II) ions. J Inorg Biochem. 105(9):1220–1225. [DOI] [PubMed] [Google Scholar]
- Li XS, Reddy MS, Baev D, Edgerton M. 2003. Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J Biol Chem. 278(31):28553–28561. [DOI] [PubMed] [Google Scholar]
- Melino S, Rufini S, Sette M, Morero R, Grottesi A, Paci M, Petruzzelli R. 1999. Zn(2+) ions selectively induce antimicrobial salivary peptide histatin-5 to fuse negatively charged vesicles: identification and characterization of a zinc-binding motif present in the functional domain. Biochemistry. 38(30):9626–9633. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Crawford JM, McClear N, Tsang A. 1997. A longitudinal study of unsaturated iron-binding capacity and lactoferrin in unstimulated parotid saliva. Biol Trace Elem Res. 57(1):1–8. [DOI] [PubMed] [Google Scholar]
- Porciatti E, Milenković M, Gaggelli E, Valensin G, Kozlowski H, Kamysz W, Valensin D. 2010. Structural characterization and antimicrobial activity of the Zn(II) complex with P113 (demegen), a derivative of histatin 5. Inorg Chem. 49(19):8690–8698. [DOI] [PubMed] [Google Scholar]
- Puri S, Edgerton M. 2014. How does it kill: understanding the candidacidal mechanism of salivary Histatin 5. Eukaryot Cell. 13(8):958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly PL, Davis PS, Deler DJ. 1968. Iron binding properties of saliva. Nature. 217(5123):68. [DOI] [PubMed] [Google Scholar]
- Rydengard V, Andersson Nordahl E, Schmidtchen A. 2006. Zinc potentiates the antibacterial effects of histidine-rich peptides against Enterococcus faecalis. FEBS J. 273(11):2399–2406. [DOI] [PubMed] [Google Scholar]
- Sherman MP, Bennett SH, Hwang FF, Yu C. 2004. Neonatal small bowel epithelia: enhancing anti-bacterial defense with lactoferrin and Lactobacillus GG. Biometals. 17(3):285–289. [DOI] [PubMed] [Google Scholar]
- Tay WM, Hanafy AI, Angerhofer A, Ming LJ. 2009. A plausible role of salivary copper in antimicrobial activity of histatin-5: metal binding and oxidative activity of its copper complex. Bioorg Med Chem Lett. 19(23):6709–6712. [DOI] [PubMed] [Google Scholar]
- Thomas E, Roman E, Claypool S, Manzoor N, Pla J, Panwar SL. 2013. Mitochondria influence CDR1 efflux pump activity, Hog1-mediated oxidative stress pathway, iron homeostasis, and ergosterol levels in Candida albicans. Antimicrob Agents Chemother. 57(11):5580–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viejo-Diaz M, Andrés MT, Fierro JF. 2004. Modulation of in vitro fungicidal activity of human lactoferrin against Candida albicans by extracellular cation concentration and target cell metabolic activity. Antimicrob Agents Chemother. 48(4):1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Asatsuma M, Ikui A, Ikeda M, Yamada Y, Nomura S, Igarashi A. 2005. Measurements of several metallic elements and matrix metalloproteinases (MMPs) in saliva from patients with taste disorder. Chem Senses. 30(2):121–125. [DOI] [PubMed] [Google Scholar]
- Xu L, Lal K, Santarpia RP, 3rd, Pollock JJ. 1993. Salivary proteolysis of histidine-rich polypeptides and the antifungal activity of peptide degradation products. Arch Oral Biol. 38(4):277–283. [DOI] [PubMed] [Google Scholar]