Abstract
Therapies to reverse tissue damage from osteolytic inflammatory diseases are limited by the inability of current tissue-engineering procedures to restore lost hard and soft tissues. There is a critical need for new therapeutics in regeneration. In addition to scaffolds, cells, and soluble mediators necessary for tissue engineering, control of endogenous inflammation is an absolute requirement for success. Although significant progress has been made in understanding natural resolution of inflammation pathways to limit uncontrolled inflammation in disease, harnessing the biomimetic properties of proresolving lipid mediators has not been demonstrated. Here, we report the use of nano-proresolving medicines (NPRM) containing a novel lipoxin analog (benzo-lipoxin A4, bLXA4) to promote regeneration of hard and soft tissues irreversibly lost to periodontitis in the Hanford miniature pig. In this proof-of-principle experiment, NPRM-bLXA4 dramatically reduced inflammatory cell infiltrate into chronic periodontal disease sites treated surgically and dramatically increased new bone formation and regeneration of the periodontal organ. These findings indicate that NPRM-bLXA4 is a mimetic of endogenous resolving mechanisms with potent bioactions that offers a new therapeutic tissue-engineering approach for the treatment of chronic osteolytic inflammatory diseases.
Keywords: periodontal disease, cardiovascular disease, bacteremia, resolution of inflammation, lipoxins, resolvins
Introduction
In the face of uncontrolled host responses, tissue engineering, regeneration, and reconstruction of diseased and injured tissues are significantly hampered (Lumelsky 2007). The ideal outcome of acute inflammation is complete resolution (Rock and Kono 2008). Uncontrolled acute inflammation can lead to tissue injury, chronic inflammation, tissue scarring, and fibrosis (Samuelsson et al. 1987; Funk 2001; Serhan et al. 2008). In any contaminated traumatic wound involving hard tissues, an uncontrolled inflammatory response causes neutrophil (PMN)–mediated tissue injury that in turn leads to irreversible bone loss. PMN are essential for protection in microbial host defense, but chronic PMN activation can release noxious materials leading to host injury and loss of organ function (Dalli et al. 2014). In chronic osteolytic inflammatory diseases such as periodontitis (Offenbacher et al. 2008; Williams 2008), sustained microbial challenge and a failure of endogenous resolution pathways result in tissue destruction (Van Dyke 2011). It is now appreciated that resolution of inflammation is an active process: an endogenous anti-inflammatory and proresolving mechanism that activates wound healing with tissue regeneration instead of fibrosis and scarring (Levy et al. 2012; Ortega-Gomez et al. 2013). Recent results reveal that endogenous control of inflammation directly affects bone healing and regeneration (Hasturk et al. 2006; Hasturk et al. 2007). Mediators of resolution of inflammation also have actions beyond control of PMN, including receptor-mediated control of osteoclast and osteoblast function in wound healing and bone regeneration (Herrera et al. 2008; Zhu et al. 2013).
Periodontitis is a significant public health problem worldwide. In the United States alone, recent reports estimate that 64.7 million adults, 47% of the adult population, suffer from periodontal disease; 38.5% have moderate to severe disease (Eke et al. 2012). Periodontitis is an inflammatory disease of bacterial etiology that results in host-mediated destruction of the supporting tissues of the teeth, including connective tissue attachment and bone. Clinically, periodontitis is difficult to treat with a high rate of recurrence. Regeneration of hard and soft tissues lost to disease is limited and not predictable (Murphy and Gunsolley 2003; Reynolds et al. 2003; Sculean et al. 2008).
Nonsoluble communication between cells has emerged as an efficient means of long-range communication beyond soluble cytokines and autacoids (Distler and Distler 2010). Membrane-shed vesicles, termed microparticles, can be employed to construct nano-proresolving medicines (NPRM) incorporating proresolving lipid mediators (LMs) that can target specific tissues without dilution or inactivation of the mediators (Dalli and Serhan 2012). Incorporation of proresolving mediators into NPRM has proven to be an effective approach for promoting survival in animal models of sepsis (Dalli et al. 2014), reducing inflammation and stimulating resolution (Norling et al. 2011). Here, we report regeneration of soft and hard tissues lost to inflammatory disease using proresolving nanomedicines.
Both lipoxin A4 and resolvin E1 (RvE1) facilitate bone regeneration in small animal models of periodontitis (Hasturk et al. 2007; Herrera et al. 2008; Van Dyke 2011). We constructed NPRM containing a stable lipoxin analog benzo-lipoxin A4 (bLXA4; Sun et al. 2009) to create NPRM-bLXA4 (Norling et al. 2011) for clinical evaluation in a large animal model of inflammation-induced bone loss. NPRM-bLXA4 is a mimetic of endogenous resolving mechanisms with potent bioactions that offers a new therapeutic tissue-engineering approach for the treatment of chronic osteolytic inflammatory diseases. Here, we report a proof-of-principle study that documents induction of periodontal regeneration by an endogenous proresolving mediator in a nonhealing large animal model of chronic periodontitis.
Materials and Methods
Animals
The Institutional Animal Care Committee (IACUC) at the Forsyth Institute and Pine Acre Rabbitry Farm, Inc. reviewed and approved the animal protocol prior to any study activities. This study conformed to ARRIVE guidelines for preclinical animal studies. All procedures used in this study complied with the Association for Assessment and Accreditation of Laboratory Animal Care International–accredited guidelines. Two adult (~50 kg; 18 to 24 mo old) female miniature swine (Hanford) were purchased from Sinclair Bio Resources (Windham, ME) and acclimatized for 7 d before procedures. The miniature swine, housed in metal pens separately, were maintained at 24 °C ± 2 °C and 55% relative humidity and fed a standard diet (ProLab Mini-Pig Diet; LabDiet, St. Louis, MO) and tap water ad libitum.
Surgical Procedures and Maintenance
Induction of chronic periodontal defects
Animals were premedicated with Telazol (4 to 6 mg/kg intramuscularly [IM]) and xylazine (2.2 mg/kg IM) and maintained by isoflurane 3% to 4% for induction and 0.5% to 2% for maintenance (Hasturk et al. 2011). Local anesthesia (2% lidocaine with 1:100,000 epinephrine–0.5 carpule/quadrant) was administered for hemostasis. Mucoperiosteal flaps were elevated with vertical releasing incisions. Interproximal periodontal defects were created using burs (6 mm in depth from the cemento-enamel junction [CEJ] and extending to the mid interproximal buccolingually). Sterile orthodontic wires were positioned below the CEJ and the twisted ends placed in the surgical defects in all 4 quadrants around the second and fourth premolars (Appendix Fig). Flaps were repositioned covering the ligatures using 4-0 Vicryl sutures (Ethicon, Inc., Blue Ash, OH). Animals were fed a soft diet (mixed with applesauce or water) for 6 wk to stimulate plaque accumulation. Oral examinations including digital photographs were repeated at 2 and 4 wk, and ligatures were removed at 6 wk. The sites were left untreated for 38 d to eliminate self-healing as a variable.
Treatments
At day 0 (baseline), animals were anesthetized and blood samples collected via external jugular vein. Mucoperiosteal periodontal flaps were elevated, defects debrided, and root surfaces scaled and root planed. A notch was placed at the defect base, and measurements (from CEJ to crestal bone and to the base of the defect) were recorded. Defects were treated with bLXA4 alone (10 µg of 2.1 µM solution), NPRM alone, or NPRM containing bLXA4 (NPRM-bLXA4). Surgery alone was the negative control. Four defects were treated with each regimen. Randomization was performed by quadrant to prevent spillover to adjacent teeth. The 4-0 Vicryl sutures were placed, and pain medication (buprenorphin) was administered for 2 to 3 d. Sutures were removed at 2 wk.
Maintenance
Animals were monitored monthly to provide oral hygiene and assess healing and inflammation. Periodontal probing was not performed during the healing phase. Probing depth, hard-tissue measurements, histomorphometry, serum lipidomics, and micro computed tomography (µCT) analysis were performed by trained and calibrated examiners (D.N., histology; A.K., clinical, and M.O.F., µCT) who were blinded to treatment allocations.
NPRM with bLXA4
NPRM were prepared from microparticles from isolated 4 × 108 human neutrophils from a single donor (Norling et al. 2011). Intercalation of active bLXA4 ((5S, 6R, E)-methyl 5,6-dihydroxy-8-(2-((R,E)-3-hydroxyoct-1-en-1-yl) phenyl) oct-7-enoate (Avanti Polar Lipids, Alabaster, AL) at 0.1 µg per 5 × 105 NPRM with fluorescent phospholipid was performed by aqueous energy dissemination using a sonic dismembrator (output power 15 W, 15 min, 25 °C; Fisher Scientific, Loughborough, UK) as in Norling et al. (2011). NPRM-bLXA4 was ultracentrifuged at 100,000 g to remove unbound bLXA4 and resuspended in filtered phosphate-buffered saline. Incorporation of bLXA4 was confirmed by flow cytometry (BD FACSCanto II; BD Biosciences, San Jose, CA) and liquid chromatography–tandem mass spectrometry (LC-MS-MS) as in Norling et al. (2011). One hundred microliters was delivered topically into the defects with a micropipette (Appendix Fig).
Three-Dimensional µCT Analysis and Quantification of Tissue Regeneration
Samples were placed in a standardized sample holder and scanned using high-resolution µCT (Scanco Medical, Brüttisellen, Sweden) at a spatial resolution of 5.376 μm (voxel dimension) with 1536 × 1536 pixel matrices. The 2-dimensional image data were stored in Digital Imaging and Communications in Medicine format and transferred to a computer for 3-dimensional reconstruction and analyses. Mandibular and maxillary regions were cropped from consecutive micro-tomographic slice images as volume of interest using Amira 3D software (VSG|FEI Visualization Sciences Group, Burlington, MA; Freire, Sedghizadeh, et al. 2011; Freire,You, et al. 2011). The original spatial resolution was maintained because data were not resampled.
The volume of new bone was measured within a digital box with fixed size (6 high × 4 diameter at the base) positioned identically for all samples. Tissue type was segmented using global thresholding procedures (bone tissue = 3,741). Newly regenerated bone was determined by applying a cylindrical divider at the base of the previous bone defect. Total and new bone volume is presented as mean and standard deviation of voxel units calculated by Amira. Statistical significance was determined by analysis of variance (ANOVA).
Histology
Bone blocks were fixed in formalin for 3 d; maxillary sites were decalcified using Immunocal for about 6 wk, embedded in paraffin, and 6-µm sections made from each block. To provide quantitative histological measures of local inflammation, the inflammatory cell infiltrate was determined from 4 areas (0.09 mm2 in total) of hematoxylin and eosin–stained slides at 400× magnification. Inflammatory cells were clearly distinguishable based on morphologic characteristics and enumerated using Image J software. Data were analyzed by ANOVA with Bonferroni post hoc corrections for multiple comparisons.
Miniature Swine Sera Targeted LC-MS-MS–Based Lipidomics
Blood samples were collected at baseline and at 3 mo, centrifuged at 2,300 rpm, and frozen at −80 °C until analysis. Methanol (4 volumes, 4 °C, 30 min) containing 500 pg of deuterated internal standards d4-LTB4, d8-5S-HETE, d5-LXA4, and d4-PGE2 to facilitate quantification of sample recovery were added to serum. LMs were extracted using C18-silica reverse-phase cartridges as in Dalli and Serhan (2012). Samples were eluted with 6 mL methylformate and dried using Speedvac and suspended in methanol/water for LC-MS-MS. The liquid chromatography-UV coupled with tandem mass spectrometry system includes QTrap 5500 equipped with a Shimadzu SIL-20AC auto-injector and LC-20AD binary pump. An Agilent Eclipse Plus C18 column (100 mm × 4.6 mm × 1.8 µm) was used with a gradient of methanol/water/acetic acid of 60:40:0.01 (v/v/v) to 100:0:0.01 at a 0.5-mL/min flow rate. To monitor and quantify the levels of the various LMs, we developed a multiple reaction–monitoring (MRM) method with signature ion fragments for each molecule. Identification was conducted using published criteria (Dalli and Serhan 2012). Calibration curves were obtained using synthetic and authentic LM mixtures, including d8–5S-HETE, d4-LTB4, d4-PGE2, LXA4, LXB4, LTB4, PGE2, PGD2, PGF2α, TxB2, RvE1, RvE2, RvD1, RvD2, RvD3, RvD5, PD1, and MaR1 at 12.5, 25, 50, and 100 pg. Linear calibration curves for each were obtained with r2 values in the range of 0.98 to 0.99. Quantification was based on peak area of the MRM transition and the linear calibration curve for each compound. Reverse-phase chiral LC-MS-MS was conducted as described (Dalli and Serhan 2012).
Results
There were no complications, including general anesthesia, surgical treatments, or follow-up maintenance. No adverse reactions were noted. During periodontitis induction, the animals developed significant gingival inflammation characterized by gingival edema, bleeding, and redness; however, this did not affect eating and physical activity. A mean monthly weight increase of about 1 kg corroborated this observation.
Bone Evaluation Using µCT
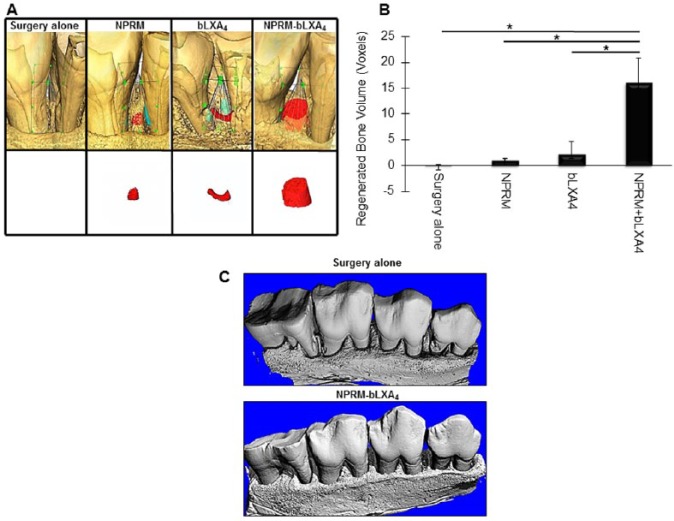
The 3-dimensional region of interest for determination of bone volume changes was defined vertically as the distance from the CEJ of the tooth to the root notch made at the time of surgery, buccolingually to the limit of the cortical bone and mesiodistally to the root surfaces (Fig. 1). Quantitative bone gain is apparent in Figure 1A, with new bone illustrated in red. NPRM-bLXA4 treatment led to a statistically significant gain of bone compared with control, bLXA4 alone, or NPRM alone (P < 0.05). Note that the notch in the root placed at the time of surgery at the bone level is no longer visible in the NPRM-bLXA4–treated samples (Fig. 1C). Quantification of bone volume (Fig. 1B) reveals markedly greater bone gain with NPRM-bLXA4 treatment. The probing pocket depths of the gingival sulcus at the chronic disease sites ranged from 6 to 8 mm presurgery. All treatments resulted in pocket depth reductions to a final probing pocket depth of 1 to 3 mm.
Figure 1.
NPRM-bLXA4 induces bone regeneration. (A) Micro–computed tomography (µCT) images of the 4 treatment conditions: (–) sham surgery, unloaded NPRM, bLXA4 alone, NPRM-bLXA4 (red = new bone; n = 4/group). (B) NPRM-bLXA4 induces greater bone formation (*P < 0.001, analysis of variance). (C) Three-dimensional reconstruction of µCT of surgery alone and NPRM-bLXA4–treated sites to provide visual references for changes induced by lipoxin treatment. Note that bone irregularities are resolved and root notches placed at the time of surgery are covered by new bone in the NPRM-bLXA4–treated specimen.
Histology/Histomorphometry
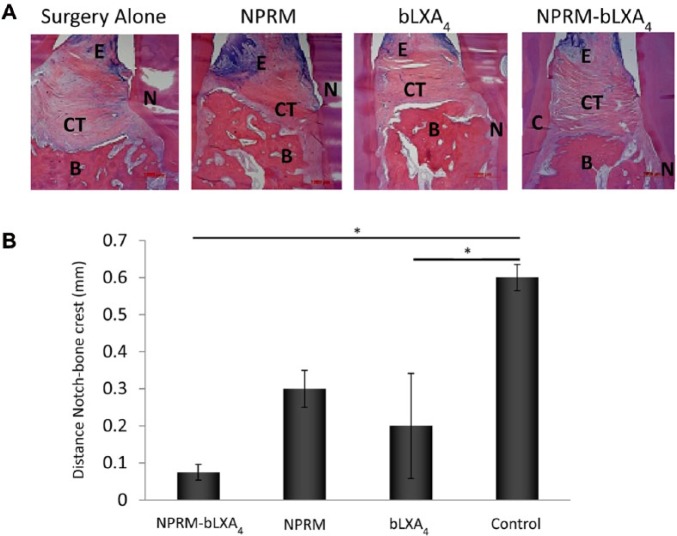
To differentiate between bone growth and regeneration of the periodontal organ (new root surface cementum, periodontal ligament formation, and new bone), qualitative and quantitative histology results in Figures 2 and 3 reveal that NPRM-bLXA4–treated sites showed well-organized and newly formed interproximal bone coronal to the root notch placed at the time of surgery with supracrestal connective tissue fibers that are parallel to the newly formed bone crest and anchored to new cementum on the root surface, confirming the µCT findings and suggesting periodontal regeneration. By contrast, in the surgery alone group, the notch is clearly present coronal to the bone level filled with disorganized granulation tissue with little to no bone regeneration. Linear histomorphometric measurements made using the notch as the reference revealed that sites treated with NPRM-bLXA4 showed statistically significant (P = 0.012) coronal regeneration of alveolar bone without regeneration in the surgery-alone sites. An intermediate response was noted for sites treated with NPRM alone (P = 0.075) and bLXA4 applied in solution phase alone (P = 0.027).
Figure 2.
Histology confirms bLXA4 regeneration of the periodontal organ. (A) NPRM-bLXA4 induces regeneration of new bone, new connective tissue attachment, and new cementum; evidence for regeneration of the entire periodontal organ (N = notch, CT = connective tissue, B = bone, E = epithelium). (B) NPRM-bLXA4 and bLXA4 alone regenerate periodontium quantified as distance from the bone crest to the root notch created at the time of surgery (*P < 0.05, analysis of variance; n = 4/group).
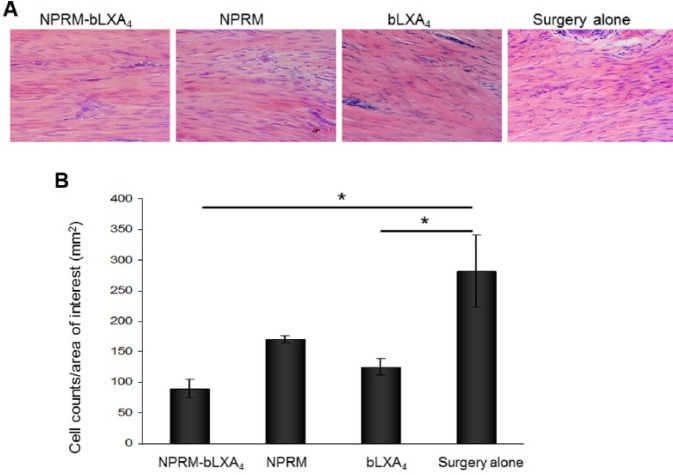
Figure 3.
NPRM-bLXA4 and bLXA4 alone limit inflammatory cell infiltrate. (A) Histological images captured from hemotoxylin and eosin–stained slides at 400× magnification at the subepithelial connective tissue at 3 mo. (B) Inflammatory cell counts were determined using Image J. (*P < 0.05, analysis of variance; n = 4/group). Three captures were performed for each site, and the averages were used for analysis.
Treatment with either the NPRM-bLXA4 or bLXA4 alone resulted in histologically healthy connective tissue and vascularization with minimal inflammatory cell infiltration compared with control NPRM or surgery alone (Fig. 3). NPRM-bLXA4 treatment was specifically associated with increased density of fibroblasts. NPRM without LX analog resulted in an increased inflammatory infiltrate especially in subepithelial areas, while surgical treatment alone showed dense inflammatory cell infiltration both in the subepithelial/supracrestal and interproximal areas (Fig. 3A). Statistical analysis revealed that NPRM-bLXA4 and bLXA4 alone markedly reduced the inflammatory infiltrate; the difference was statistically significant compared with surgery alone (Fig. 3B, P = 0.012 and 0.027, respectively). Control NPRM without bLXA4 also showed reduced inflammatory infiltrates compared with surgery alone; however, the difference was not statistically significant (P = 0.067).
Systemic LMs
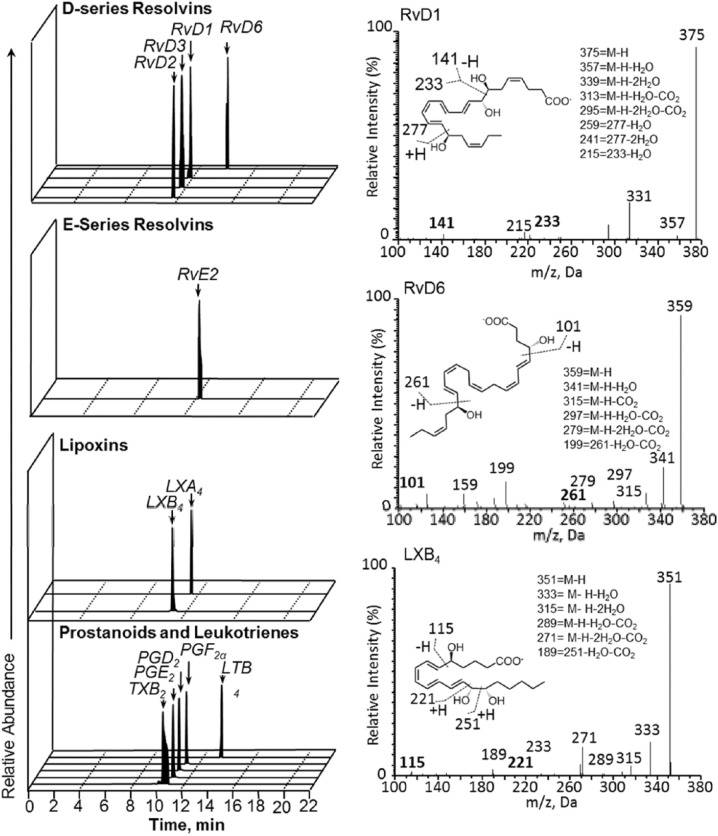
To determine the potential impact of local NPRM-bLXA4 administration on peripheral blood systemic LM levels, whole blood was obtained at the indicated intervals posttreatment. Since both animals received all treatments in this split-jaw design, the changes in LM levels with time were analyzed as an independent variable. LM levels were investigated using LM metabololipidomics and leukotriene B4, Prostaglandins (PGD2, PGE2, PGF2α), lipoxins (LXA4, LXB4, LX pathway markers), and D-series resolvins (RvD1-RvD6) serum levels were measured (Fig. 4; Table). These revealed that local treatment with the proresolving mediator analog (bLXA4) markedly up-regulates systemic expression of other proresolving mediators (Table). Notably, both arachidonic acid and ω-3 polyunsaturated fatty acid–derived mediators were increased, including endogenous lipoxins and resolvins. Concurrently, proinflammatory prostaglandins PGE2 and PGD2 were decreased. A spike in systemic prostaglandins at week 12 (Table) appears attributable in large part to an increase in PGF2α, an anabolic mediator associated with bone formation.
Figure 4.
Regulation of peripheral blood lipid mediator profiles by bLXA4 in periodontal disease. Whole blood was obtained at the indicated intervals post-treatment and serum isolated by centrifugation. Lipid mediator (LM) levels were investigated using LM metabololipidomics (see the Materials and Methods section for details). (Left) Representative multiple reaction–monitoring chromatograms of the identified lipid mediators in the peritoneal exudates. (Right) Accompanying tandem mass spectrometry spectra employed for identification.
Table.
Regulation of Peripheral Blood Lipid Mediator Profiles by NPRM-bLXA4 in Periodontal Disease.a
| Week 0 |
Week 2 |
Week 4 |
Week 8 |
Week 12 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Pig 1 | Pig 2 | Pig 1 | Pig 2 | Pig 1 | Pig 2 | Pig 1 | Pig 2 | Pig 1 | Pig 2 | |
| DHA bioactive metabolome | ||||||||||||
| RvD1 | 375 | 141 | * | 2.2 | 4.4 | 5.0 | 5.6 | 4.8 | 2.4 | 8.3 | 2.1 | 2.4 |
| RvD2 | 375 | 215 | * | 5.5 | 10.9 | 12.2 | 13.8 | 11.9 | 5.8 | 20.5 | 5.1 | 5.8 |
| RvD3 | 375 | 147 | * | * | * | * | * | 2.6 | 0.5 | * | 0.9 | 0.7 |
| RvD5 | 359 | 199 | * | * | * | * | * | * | * | * | * | * |
| RvD6 | 359 | 159 | 17.5 | 20.4 | 0.4 | 77.6 | 17.2 | 59.8 | 29.3 | 21.0 | 95.6 | 19.6 |
| DHA | 329 | 285 | 11,636.5 | 8,935.0 | 7,795.2 | 13,587.3 | 7,246.8 | 9,869.2 | 7,023.3 | 9,482.8 | 9,327.7 | 9,440.0 |
| EPA bioactive metabolome | ||||||||||||
| RvE1 | 349 | 195 | * | * | * | * | * | * | * | * | * | * |
| RvE2 | 333 | 199 | * | 6.4 | 6.9 | 7.4 | 5.9 | 7.4 | 7.2 | 1.9 | 1.3 | 17.0 |
| RvE3 | 333 | 245 | * | * | * | * | * | * | * | * | * | * |
| EPA | 301 | 257 | 10,489.0 | 5,370.3 | 6,125.2 | 7,194.1 | 5,542.4 | 4,032.6 | 5,861.9 | 4,767.2 | 9,853.8 | 6,706.3 |
| AA bioactive metabolome | ||||||||||||
| LXA4 | 351 | 115 | 0.5 | 0.8 | 0.2 | 1.1 | 0.7 | 3.9 | 1.4 | 0.3 | 1.8 | 1.3 |
| LXB4 | 351 | 221 | 24.4 | 35.9 | 100.5 | 49.8 | 12.1 | 84.4 | 26.7 | 11.7 | 26.2 | 69.5 |
| LTB4 | 335 | 195 | 31.2 | 3.2 | 102.5 | 1.6 | 17.3 | 0.4 | 1052.1 | 82.7 | 274.0 | 4.1 |
| PGE2 | 351 | 189 | 18.9 | 7.6 | 2.8 | 7.3 | 5.8 | 7.2 | 7.8 | 3.4 | 19.2 | 15.8 |
| PGD2 | 351 | 233 | 128.4 | 62.5 | 13.9 | 35.8 | 24.1 | 22.1 | 24.2 | 9.4 | 101.8 | 124.7 |
| PGF2α | 353 | 193 | 1,646.7 | 494.0 | 45.1 | 327.6 | 41.1 | 34.2 | 93.5 | 20.4 | 1,180.0 | 1,086.1 |
| TxB2 | 369 | 169 | 132.1 | 223.5 | 46.0 | 192.4 | 123.1 | 56.5 | 68.1 | 32.4 | 620.7 | 533.9 |
| AA | 303 | 259 | 152,248.6 | 68,793.8 | 79,177.1 | 72,991.1 | 70,407.7 | 63,948.9 | 68,914.9 | 49,290.0 | 102,885.2 | 73,402.1 |
Whole blood was obtained at the indicated intervals post-treatment and serum isolated by centrifugation. Lipid mediator (LM) levels were investigated using LM metabololipidomics (see the Materials and Methods section for details). Specific bioactive lipid mediator and precursor/pathway markers: Q1, M-H (parent ion); Q3, diagnostic ion in the MS-MS (daughter ion). Results are representative of n = 2 pigs and 12 determinations and are expressed as pg/mL of serum. The detection limit was ~1 pg. *Below detection limit.
Discussion
The current report demonstrates that new NPRM-bLXA4 activates distinct osteogenic responses in the treatment of chronic inflammatory osteolytic periodontitis when compared with surgery alone and either bLXA4 or NPRM alone. NPRM-bLXA4 treatment in this large animal model enhanced hard- and soft-tissue regeneration and reformation of the periodontal organ. NPRM-bLXA4 treatment was also found to regulate systemic proresolving mediators, where it promoted endogenous biosynthesis of other resolvins and lipoxins and dampened production of proinflammatory mediators. In addition, NPRM-bLXA4 stimulated production of eicosanoids that are known to favor bone formation systemically (Yao et al. 2009). These findings support the hypothesis that NPRM constructed with bLXA4 potentiates the anabolic actions of bLXA4 in regeneration of tissues lost to inflammatory disease by activating distinct proresolving and tissue protective pathways.
Periodontal disease is a bacterial biofilm–induced chronic inflammatory disease resulting in loss of connective tissue attachment to the teeth and osteoclast-mediated alveolar bone resorption (Taubman et al. 2005). Leukocytes play multiple roles in the progression of periodontal disease, including phagocytosis and killing of bacteria, secretion of inflammatory cytokines, mounting of specific immune response, and activation of osteoclasts (Graves et al. 2011). Leukocytes are essential in the host defense against oral pathogens; however, in susceptible individuals, unable to resolve the inflammatory lesion, chronic inflammation in the periodontium causes periodontal bone loss (Van Dyke 2011).
As in other osteolytic inflammatory diseases such as arthritis, current tissue-engineering procedures remain limited. There is a critical need for new therapeutics in regeneration. The classic triad of regenerative medicine (scaffold, cells, and soluble mediators) is insufficient because of our inability to control inflammation during regeneration. In rabbit periodontitis, agonists of resolution of inflammation, including lipoxins (LXA4) and resolvins (RvE1), prevent periodontal bone loss (Hasturk et al. 2006) and in treatment of existing periodontitis permit periodontal regeneration (Hasturk et al. 2007), emphasizing the negative impact of uncontrolled inflammation.
Earlier observations revealed that in addition to the proresolving and anti-inflammatory actions of lipoxins, these receptor agonists have direct anabolic actions on bone consistent with the observed increases in bone regeneration. In experiments including transgenic rabbits systems (Serhan et al. 2003), topical application of lipoxin stable analogs dramatically reduced leukocyte infiltration, bone loss, and inflammation. LX-enriched humanized NPRM likewise reduced PMN influx in murine peritonitis, accelerated healing, and protected against temporomandibular joint inflammation (Norling et al. 2011; Jones et al. 2012), indicating that LX-enriched NPRM offer new therapeutic approaches as mimetics of endogenous resolution of inflammation mechanisms with potent beneficial bioactions in vivo.
The advantage of loading bLXA4 into NPRM is 2-fold. First, NPRM display anti-inflammatory properties through ALX/FPR2, the receptor for bLXA4 an Annexin 1, which targets the delivery to inflamed tissues (Dalli et al. 2008). Second, many nanoparticle systems cause nanotoxicity by uptake of nanoparticles and activation of dendritic cells (Hess and Tseng 2007). NPRM provide a new, well-documented means to deliver locally and activate proresolving pathways. Thus, NPRM-bLXA4 nanomedicines exhibit agonist actions in resolution by activating endogenous ALX receptors and releasing precursors for proresolving lipids, and they deliver their bLXA4 cargo at the site of inflammation or surgical intervention (Norling et al. 2011).
Domesticated miniature pigs (miniature swine) are a marked improvement over other animal systems for human translational research because of their smaller size and adaptability to laboratory housing. The pig has more similarities to humans than other commonly used laboratory animals in anatomy, physiology, immunology, and oral structures and is specifically suitable for regenerative studies (Harding et al. 2013). For these reasons, it is widely used in cardiovascular, digestive tract, and skin studies (Forster et al. 2010). Here, microbe-associated inflammation, leukocyte-mediated bone destruction (periodontitis), and regeneration of hard and soft tissues lost to inflammatory disease were evaluated. In addition, the nonhealing, chronic periodontal lesion in the Hanford miniature swine mimics the human situation including an omnivorous diet and jaw motions during chewing. The miniature swine represent a significant advance in translational research that targets novel approaches to inflammatory diseases (e.g., periodontitis and arthritis, in which inflammation and bone destruction are characteristics of the disease).
This proof-of-principle study does not contain enough animals to be generalizable, but the data are robust and the analysis is valid despite the small N. Four teeth were analyzed for each treatment between 2 pigs and statistics corrected for within-pig comparisons. In addition, the development of a large-animal periodontitis model with nonhealing lesions significantly advances the ability to test new treatments in a model significantly closer to the human condition.
In conclusion, the present results provide evidence for a new mechanism in regeneration of bone lost to inflammatory disease, as in periodontitis, using NPRM-bLXA4 demonstrated here in a large-animal model. NPRM-bLXA4 is functionally transferred to bone cell serum membrane receptors in addition to leukocytes modulating their responses to promote bone formation. In the complex, ongoing remodeling of bone, the cellular events initiated by bLXA4 lead to efficient control of the biofilm environment, leading to the elimination of pathogens, up-regulation of endogenous proresolving LMs (e.g., lipoxins and D-series resolvins), as well as direct actions on bone cells that affect osteogenesis, resulting in significant regeneration of bone and connective tissue and reestablishment of the periodontal organ.
Author Contributions
T.E. Van Dyke, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; H. Hasturk, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript; A. Kantarci, M.O. Freire, J. Dalli, C.N. Serhan, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; D. Nguyen, contributed to data acquisition, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors of this study thank the veterinary and technical personnel of the Forsyth Institute Animal Center and Pine Acre Research Farm for assistance during animal handling and experimental procedures and the Organic Synthesis Core of P50-DE016191 (to C.N.S.) for preparing NPRM and bLXA4.
Footnotes
This study was partly supported by the USPHS National Institute of Dental and Craniofacial Research (NIDCR) grants (DE19938 and DE15566 to T.E. Van Dyke; DE18917 to H. Hasturk).
The Forsyth Institute has filed patent applications.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Dalli J, Norling LV, Montero-Melendez T, Federici Canova D, Lashin H, Pavlov AM, Sukhorukov GB, Hinds CJ, Perretti M. 2014. Microparticle alpha-2-macroglobulin enhances pro-resolving responses and promotes survival in sepsis. EMBO Mol Med. 6(1):27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. 2008. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 112(6):2512–2519. [DOI] [PubMed] [Google Scholar]
- Dalli J, Serhan CN. 2012. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 120(15):e60–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler JH, Distler O. 2010. Inflammation: microparticles and their roles in inflammatory arthritides. Nat Rev Rheumatol. 6(7):385–386. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ; CDC Periodontal Disease Surveillance workgroup. 2012. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 91(10):914–920. [DOI] [PubMed] [Google Scholar]
- Forster R, Bode G, Ellegaard L, van der Laan JW. 2010. The RETHINK project on minipigs in the toxicity testing of new medicines and chemicals: conclusions and recommendations. J Pharmacol Toxicol Methods. 62(3):236–242. [DOI] [PubMed] [Google Scholar]
- Freire MO, Sedghizadeh PP, Schaudinn C, Gorur A, Downey JS, Choi JH, Chen W, Kook JK, Chen C, Goodman SD, et al. 2011. Development of an animal model for Aggregatibacter actinomycetemcomitans biofilm-mediated oral osteolytic infection: a preliminary study. J Periodontol. 82(5):778–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire MO, You HK, Kook JK, Choi JH, Zadeh HH. 2011. Antibody-mediated osseous regeneration: a novel strategy for bioengineering bone by immobilized anti-bone morphogenetic protein-2 antibodies. Tissue Eng Part A. 17(23-24):2911–2918. [DOI] [PubMed] [Google Scholar]
- Funk CD. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294(5548):1871–1875. [DOI] [PubMed] [Google Scholar]
- Graves DT, Oates T, Garlet GP. 2011. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J Oral Microbiol. 2011;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J, Roberts RM, Mirochnitchenko O. 2013. Large animal models for stem cell therapy. Stem Cell Res Ther. 4(2):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Ghattas M, Schmidt M, Giordano RA, Ashman A, Diekwisch TG, Van Dyke T. 2011. The use of light/chemically hardened polymethylmethacrylate, polyhydroxyethylmethacrylate, and calcium hydroxide graft material in combination with polyanhydride around implants in minipigs: part I: immediate stability and function. J Periodontol. 82(9):1339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. 2007. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 179(10):7021–7029. [DOI] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. 2006. RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J. 20(2):401–403. [DOI] [PubMed] [Google Scholar]
- Herrera BS, Ohira T, Gao L, Omori K, Yang R, Zhu M, Muscara MN, Serhan CN, Van Dyke TE, Gyurko R. 2008. An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. Br J Pharmacol. 155(8):1214–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess H, Tseng Y. 2007. Active intracellular transport of nanoparticles: opportunity or threat? ACS Nano. 1(5):390–392. [DOI] [PubMed] [Google Scholar]
- Jones CN, Dalli J, Dimisko L, Wong E, Serhan CN, Irimia D. 2012. Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proc Natl Acad Sci U S A. 109(50):20560–20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Vachier I, Serhan CN. 2012. Resolution of inflammation in asthma. Clin Chest Med. 33(3):559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumelsky NL. 2007. Commentary: engineering of tissue healing and regeneration. Tissue Eng. 13(7):1393–1398. [DOI] [PubMed] [Google Scholar]
- Murphy KG, Gunsolley JC. 2003. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects: a systematic review. Ann Periodontol. 8(1):266–302. [DOI] [PubMed] [Google Scholar]
- Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. 2011. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 186(10):5543–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S, Barros SP, Beck JD. 2008. Rethinking periodontal inflammation. J Periodontol. 79(8 suppl):1577–1584. [DOI] [PubMed] [Google Scholar]
- Ortega-Gomez A, Perretti M, Soehnlein O. 2013. Resolution of inflammation: an integrated view. EMBO Mol Med. 5(5):661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. 2003. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects: a systematic review. Ann Periodontol. 8(1):227–265. [DOI] [PubMed] [Google Scholar]
- Rock KL, Kono H. 2008. The inflammatory response to cell death. Annu Rev Pathol. 3:99–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. 1987. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 237(4819):1171–1176. [DOI] [PubMed] [Google Scholar]
- Sculean A, Nikolidakis D, Schwarz F. 2008. Regeneration of periodontal tissues: combinations of barrier membranes and grafting materials—biological foundation and preclinical evidence: a systematic review. J Clin Periodontol. 35(8 suppl):106–116. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, et al. 2003. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 171(12):6856–6865. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Yacoubian S, Yang R. 2008. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 3:279–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YP, Tjonahen E, Keledjian R, Zhu M, Yang R, Recchiuti A, Pillai PS, Petasis NA, Serhan CN. 2009. Anti-inflammatory and pro-resolving properties of benzo-lipoxin A(4) analogs. Prostaglandins Leukot Essent Fatty Acids. 81(5-6):357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman MA, Valverde P, Han X, Kawai T. 2005. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 76(11 suppl):2033–2041. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE. 2011. Proresolving lipid mediators: potential for prevention and treatment of periodontitis. J Clin Periodontol. 38(suppl 11):119–125. [DOI] [PubMed] [Google Scholar]
- Williams RC. 2008. Understanding and managing periodontal diseases: a notable past, a promising future. J Periodontol. 79(8 suppl):1552–1559. [DOI] [PubMed] [Google Scholar]
- Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, Narumiya S. 2009. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 15(6):633–640. [DOI] [PubMed] [Google Scholar]
- Zhu M, Van Dyke TE, Gyurko R. 2013. Resolvin E1 regulates osteoclast fusion via DC-STAMP and NFATc1. FASEB J. 27(8):3344–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]