Abstract
Stem cells from exfoliated deciduous teeth (SHED) possess multipotent differentiation and immunomodulatory properties. They have been used for orofacial bone regeneration and autoimmune disease treatment. In this study, we show that acetylsalicylic acid (ASA) treatment is able to significantly improve SHED-mediated osteogenic differentiation and immunomodulation. Mechanistically, ASA treatment upregulates the telomerase reverse transcriptase (TERT)/Wnt/β-catenin cascade, leading to improvement of SHED-mediated bone regeneration, and also upregulates TERT/FASL signaling, leading to improvement of SHED-mediated T-cell apoptosis and ameliorating disease phenotypes in dextran sodium sulfate–induced colitis mice. These data indicate that ASA treatment is a practical approach to improving SHED-based cell therapy.
Keywords: mesenchymal stem cells, deciduous teeth, cell therapy, telomerase, Fas ligand, Wnt pathway
Introduction
Stem cells from human exfoliated deciduous teeth (SHED) have been identified as a population of postnatal stem cells with the capacities for self-renewal and multipotent differentiation into osteogenic/odontogenic cells, adipocytes, and neural cells (Miura et al. 2003; Sakai et al. 2010; Wang et al. 2010). When implanted into immunocompromised mice, SHED were able to form new bone in vivo (Miura et al. 2003; Seo et al. 2008) and repair critical-size craniofacial defects in mice (Laino et al. 2006; Seo et al. 2008; Zheng et al. 2009). SHED also possess immunomodulatory properties, such as inhibiting T helper 17 (Th17) cells and upregulating regulatory T cells (Tregs) in vitro. When transplanted systemically, SHED ameliorated systemic lupus erythematosus (SLE)–associated disorders in MRL/lpr mice (Yamaza et al. 2010). SHED represent an easily accessible and promising resource for mesenchymal stem cell (MSC)–based therapy. For clinical applications, MSCs have to be culture expanded to obtain a sufficient number of cells. During ex vivo culture processes, SHED, like other MSCs, may partially lose their stem cell properties and show reduced therapeutic effects. Thus, it is critical to develop appropriate culture conditions to maintain the stem cell properties of SHED.
Acetylsalicylic acid (ASA) is a widely used nonsteroidal anti-inflammatory drug (NSAID) that affects multiple biological pathways, for instance, inhibiting cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX-2), and prostaglandin E2 (PGE2) activity (Smith and Willis 1971). Epidemiological study has shown that the regular use of ASA might have a moderate beneficial effect on bone mineral density in postmenopausal women (Carbon et al. 2003). Furthermore, it has been reported that ASA treatment rescued osteoporotic phenotypes in ovariectomized mice by promoting osteogenesis and inhibiting osteoclastogenesis (Yamaza et al. 2008). ASA treatment also improved MSC-based tissue regeneration via reducing the concentrations of interferon-γ (IFN-γ) and tumor necrosis factor–α (TNF-α) (Liu et al. 2011). It was also shown that ASA treatment enhanced the immunomodulatory capacity of MSCs (Chen et al. 2014). In the present study, we reveal that ex vivo ASA treatment significantly improves SHED-based bone formation and immunomodulation by regulating the telomerase reverse transcriptase (TERT)/Wnt and TERT/FASL pathways, respectively.
Materials and Methods
Animals
C57BL/6J mice were purchased from the Jackson Lab (Bar Harbor, ME). Beige nude/nude Xid (III) mice were purchased from Harlan (Indianapolis, IN). All animal experiments were performed under institutionally approved protocols for the use of animal research (University of Southern California IACUC protocols 10941, 11141, and 11327).
Antibodies and Reagents
All antibodies and reagents used in this study are described in the Appendix.
Isolation and Culture of SHED
Human exfoliated deciduous incisors were obtained as discarded biological samples from children (6–8 y old) at the Dental Clinic of the University of Southern California following the approved institutional review board guidelines. SHED were cultured as reported previously (Miura et al. 2003). SHED (1 × 106) were seeded in 100-mm culture dishes, and ASA at concentrations of 10, 50, and 200 µg/mL (Sigma-Aldrich, St. Louis, MO) was added to the culture medium for 3 d starting at 70% confluence. The cells were harvested and used directly in further experiments.
siRNA and Chemical Treatments
Detailed methods of the small interfering RNA (siRNA) and chemical treatments are described in the Appendix.
Cell Proliferation Assay
Proliferation rates of SHED were assessed using a bromodeoxyuridine (BrdU) staining kit (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocols. The detailed method is described in the Appendix.
In Vitro Osteogenic Differentiation Assay
Methods are described in detail in the Appendix.
Western Blot Analysis
Western blots were performed as described in the Appendix.
Implantation of SHED into Immunocompromised Mice
In total, 6.0 × 106 SHED were mixed with 40 mg hydroxyapatite/tricalcium phosphate (HA/TCP) ceramic powder (Zimmer, Inc., Warsaw, IN) and then transplanted into the dorsal surface of 10-week-old immunocompromised mice as previously described (Miura et al. 2003). These procedures were performed in accordance with specifications of an approved small animal protocol (USC IACUC 10874). The transplants were harvested after 8 wk post-transplantation, fixed in 4% paraformaldehyde, and then decalcified with 10% EDTA (pH 8.0) for paraffin embedding. Paraffin sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin (H&E). For quantification of new bone regeneration in vivo, we used 10 representative images from different regions of the SHED implants to calculate the area of bone formation using ImageJ software (National Institutes of Health, Bethesda, MD). This assay was repeated with 3 independent implants for each experimental group.
T-Lymphocyte Apoptosis Assay
T-lymphocyte apoptosis assay was performed as described in the Appendix.
Dextran Sulfate Sodium–Induced Mouse Colitis and Treatment with SHED
Acute colitis was induced in C57BL/6J mice. Methods are described in detail in the Appendix.
Telomerase Activity Assay
SHED (0.5 × 106/well) were seeded in 6-well culture plates with siRNA or chemicals at the indicated concentrations. For the telomerase activity assay, a TeloTAGGG Telomerase PCR ELISA kit (Roche, Indianapolis, IN) was used with cell lysates, according to the manufacturer’s instructions.
Methylthiazolyldiphenyl–Tetrazolium Bromide Assay
The methylthiazolyldiphenyl–tetrazolium bromide (MTT) analysis was performed as described in the Appendix.
Statistics
SPSS 13.0 (SPSS, Inc., Chicago, IL) was used to perform the statistical analysis. Comparisons between 2 groups were analyzed using independent 2-tailed Student’s t tests, and comparisons between more than 2 groups were analyzed using 1-way analysis of variance (ANOVA). P values less than 0.05 were considered statistically significant.
Results
ASA Treatment Improves Osteogenic Differentiation of SHED
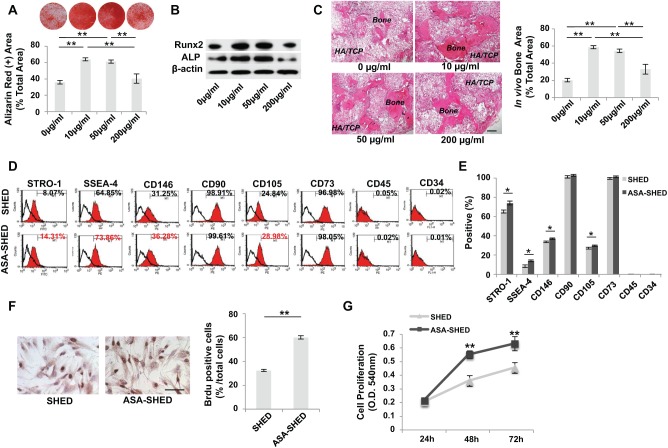
To select an optimal concentration of ASA to treat cultured SHED, different doses (10–200 µg/mL) of ASA were used to treat SHED for 3 d. We found that low doses of ASA (10/50 µg/mL) significantly improved osteogenic differentiation of SHED in vitro, while a high dose of ASA (200 µg/mL) had no significant effects, as indicated by mineralized nodule formation and expression of osteogenic markers Runx2 and ALP (Fig. 1A, B). We further confirmed that 10/50-µg/mL doses of ASA significantly improved SHED-mediated bone formation in vivo when SHED were implanted into immunocompromised mice (Fig. 1C). Therefore, we used 10 µg/mL ASA to treat cultured SHED in this study. However, new bone formed after SHED and ASA-treated SHED (ASA-SHED) treatment lacked hematopoietic marrow elements, as reported previously (Miura et al. 2003; Seo et al. 2008). Furthermore, we used human-specific anti–nuclear antibody staining to confirm that both SHED and ASA-SHED differentiated into osteocytes and generated new bone (Appendix Fig. 1). These data indicate that ASA treatment is able to improve SHED-mediated osteogenic differentiation.
Figure 1.
Acetylsalicylic acid (ASA) treatment improves osteogenic differentiation of stem cells from exfoliated deciduous teeth (SHED). (A) Alizarin red staining showed that low doses of ASA (10/50 µg/mL) treatment promoted mineralized nodule formation compared with untreated SHED, while a high dose of ASA (200 µg/mL) had no significant effects. (B) Western blot analysis showed that low doses of ASA (10/50 µg/mL) treatment, but not a high dose (200 µg/mL), upregulated the expression levels of Runx2 and ALP compared with untreated SHED. (C) When implanted into immunocompromised mice using hydroxyapatite/tricalcium phosphate (HA/TCP) as a carrier, low doses of ASA (10/50 µg/mL) treatment increased SHED-mediated new bone regeneration, while a high dose of ASA (200 µg/mL) failed to elevated SHED-mediated bone formation. (D, E) Flow cytometric analysis showed that ASA treatment increased expression levels of STRO-1, SSEA-4, CD146, and CD105 but not CD90 or CD73. SHED failed to express CD45 and CD34. (F) BrdU labeling assay showed an increased proliferation rate in ASA-SHED compared with the control SHED. Proliferation rate is indicated as a percentage of BrdU+ nuclei out of the total number of nuclear cells. (G) MTT assay showed that ASA treatment elevated SHED proliferation rate compared with untreated SHED. n = 5 in each group. Scale bar = 200 µm. *P < 0.05. **P < 0.01. ***P < 0.005. Error bars: mean ± SD.
To identify the role of ASA in regulating the stem cell function of SHED, we examined the surface molecule expression and proliferation rate of SHED after ASA treatment for 3 d. Flow cytometric analysis showed that expression levels of some stem cell surface markers, including STRO-1, SSEA-4, CD146, and CD105, were increased in the ASA-treated group in comparison to the control group, while the hematopoietic lineage markers CD34 and CD45 were absent in both groups (Fig. 1D, E). BrdU-labeling assay showed that ASA treatment was able to increase the proliferation rate of SHED (Fig. 1F). MTT analysis was used to confirm that ASA-SHED have an increased proliferation rate relative to that of untreated SHED (Fig. 1G).
ASA Treatment Enhances Immunomodulatory Properties of SHED
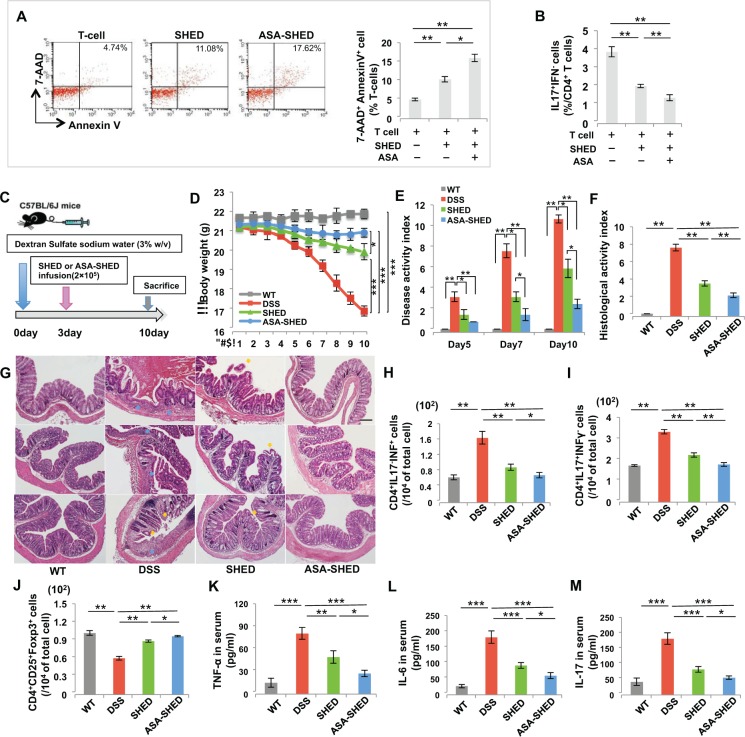
SHED display immunomodulatory functions also seen in other types of MSCs: they are able to induce T-cell apoptosis and lead to immune tolerance (Yamaza et al. 2010). To learn whether and how ASA regulates the immunomodulatory properties of SHED, we used a SHED/T-cell coculture system to show that ASA-SHED had a significantly increased capacity to induce AnnexinV+7AAD+ double-positive apoptotic T cells and inhibit CD4+IL17+IFN– Th17 cells, as assessed by flow cytometric analysis (Fig. 2A, B). Furthermore, we used dextran sodium sulfate (DSS)–induced experimental colitis mice to examine the immunotherapeutic effects of ASA-SHED. C57BL/6J mice were orally administered 3% DSS for 10 d to induce acute colitis. ASA-SHED or control SHED (2 × 105) were systemically transplanted into the mice on day 3, followed by sacrifice of the mice on day 10 (Fig. 2C). The body weight of the colitis mice was significantly reduced compared with that of the control C57BL/6J mice. Although both ASA-SHED and control SHED could partially restore body weight in colitis mice, ASA-SHED showed significantly improved therapeutic effects (Fig. 2D). An overall assessment of disease activity index (DAI) was based on the presence of weight loss, diarrhea, and bleeding (Alex et al. 2009). ASA-SHED infusion induced a more significant reduction in DAI score from 7 to 10 d than the control SHED treatment (Fig. 2E). Histologically, loss of the epithelial layer and infiltration of inflammatory cells were observed in the intestines of DSS-induced colitis mice, and systemic infusion of either ASA-SHED or control SHED could recover impaired intestinal structure (Fig. 2G). However, ASA-SHED had superior ability to recover the epithelial structure and eliminate the inflammatory cells, as assessed by the histologic activity index (HAI) (Fig. 2F). Furthermore, we used flow cytometric analysis to reveal that both ASA-SHED and control SHED infusion upregulated the levels of CD4+CD25+Foxp3+ regulatory T cells (Tregs) and downregulated the levels of CD4+IL17–IFN+ T helper 1 (Th1) and Th17 cells (Fig. 2H–J). However, ASA-SHED showed more significant upregulation of Tregs and downregulation of Th1 and Th17 than did the control SHED (Fig. 2H–J). Colitis mice showed significantly increased serum levels of TNF-α, IL-6, and IL-17, as assessed by enzyme-linked immunosorbent assay (ELISA) (Fig. 2L–N). ASA-SHED induced more significant reductions in the levels of TNF-α, IL-6, and IL-17 in colitis mice compared with control SHED (Fig. 2K–M). To follow the fate of systemically infused ASA-SHED, we used immunofluorescence staining to show that PKH26+ ASA-SHED were detectable in the bone marrow (BM), peripheral blood (PB), lungs, liver, and spleen but not in the kidneys or colon at 1 d postinfusion. Very few ASA-SHED were found in the lung and spleen at 3 d postinfusion, and they became undetectable at 7 d postinfusion (Appendix Fig. 2). Interestingly, we failed to detect PKH26+ ASA-SHED in the colon of colitis mice at 1, 3, and 7 d postinfusion. Therefore, SHED-mediated systemic immunomodulation, rather than homing of SHED, may play an important role in ameliorating the disease phenotype in colitis mice. Taken together, these pieces of experimental evidence suggest that ASA-SHED had an elevated capacity for immunotherapy in the colitis mice.
Figure 2.
Acetylsalicylic acid (ASA) treatment enhances immunomodulatory properties of stem cells from exfoliated deciduous teeth (SHED). (A) ASA treatment significantly increased SHED-mediated T-cell apoptosis, as indicated by increased numbers of AnnexinV+7AAD– and AnnexinV+7AAD+ double-positive apoptotic T cells in an in vitro coculture system. (B) Th17 induction assay showed that ASA treatment significantly increased the capacity of SHED to downregulate the levels of Th17. (C) Schema showing SHED infusion in dextran sodium sulfate (DSS)–induced experimental colitis mice. (D) Colitis mice showed significantly reduced body weight from 5 to 10 d after DSS induction. Both SHED and ASA-SHED transplantation rescued body weight loss compared with the nontransplant group at 10 d post-DSS induction. However, ASA-SHED showed more significant rescue of body weight loss than did untreated SHED. (E) Disease activity index (DAI) was significantly increased in mice with colitis compared with the C57BL/6J control mice from 5 to 10 d post-DSS induction. Although both SHED and ASA-SHED infusion significantly reduced the DAI scores, ASA-SHED showed more capacity to reduce DAI scores than did untreated SHED. (F, G) Hematoxylin and eosin (H&E) staining showed the infiltration of inflammatory cells (blue arrows) in the colon, with destruction of the epithelial layer (yellow arrows) in mice with colitis. ASA-SHED transplantation produced more significant rescue of disease phenotypes in the colon and reduction of histologic activity index (HAI) compared with the untreated control SHED group. (H, I) The levels of Th1 and Th17 were significantly elevated in colitis mice compared with C57BL/6J control mice at 10 d post-DSS induction. Compared with untreated SHED, ASA-SHED infusion resulted in significantly reduced levels of Th1 and Th17 cells in colitis mice at 10 d post-DSS induction. (J) The levels of Tregs were significantly reduced in mice with colitis compared with C57BL/6J control mice at 10 d post-DSS induction. ASA-SHED infusion exhibited a greater ability to upregulate Tregs levels in mice with colitis than did untreated SHED. (K–M) The levels of tumor necrosis factor–α (TNF-α), interleukin (IL)–6, and IL-17 in serum were markedly increased in colitis mice compared with C57BL/6J control mice at 10 d post-DSS induction, and ASA-SHED infusion significantly downregulated serum levels of TNF-α, IL-6, and IL-17. n = 5 in each group. Scale bar = 200 µm. *P < 0.05. **P < 0.01. ***P < 0.005. Error bars: mean ± SD.
ASA Treatment Improves Osteogenesis of SHED via TERT/Wnt/β-Catenin Pathway
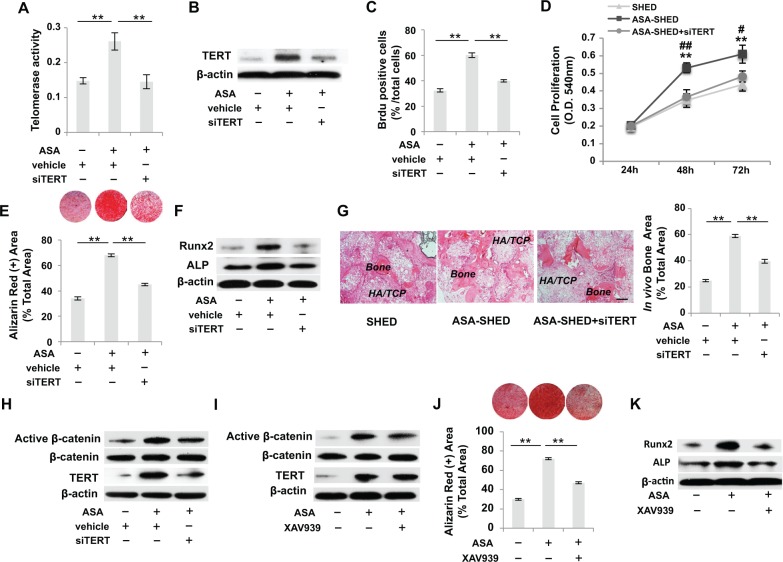
Since telomerase plays an important role in stem cell self-renewal and MSC-mediated tissue regeneration (Hiyama and Hiyama 2007; Yamaza et al. 2008; Liu et al. 2011), we examined the levels of TERT in SHED and ASA-SHED. Interestingly, we found that TERT expression was significantly increased in SHED treated with low doses of ASA (10/50 µg/mL), while TERT expression did not change significantly in SHED treated with a high dose of ASA (200 µg/mL), as assessed by a telomeric repeat amplification protocol (TRAP)–ELISA assay and Western blot analysis (Appendix Fig. 3A, B). To examine the role of TERT in SHED-mediated therapies, we used TERT siRNA to knockdown TERT expression in ASA-SHED (Fig. 3A, B). Knockdown of TERT in ASA-SHED induced a significant decrease in proliferation rate compared with regular ASA-SHED (Fig. 3C, D). Furthermore, we found that TERT siRNA transfection blocked the ASA treatment-induced increase of in vitro mineralized nodule formation and expression of Runx2 and ALP, as assessed by alizarin red staining and Western blot, respectively (Fig. 3E, F). When implanted into immunocompromised mice, knockdown of TERT in ASA-SHED also abolished the ASA treatment-induced increase of new bone formation (Fig. 3G). These data suggest that the increase in bone formation in ASA-SHED implants is due to elevated telomerase activity.
Figure 3.
Acetylsalicylic acid (ASA) treatment improves osteogenesis of stem cells from exfoliated deciduous teeth (SHED) via telomerase reverse transcriptase (TERT)/Wnt/β-catenin pathway. (A) Telomeric repeat amplification protocol (TRAP)–enzyme-linked immunosorbent assays (ELISAs) showed that ASA-SHED exhibited increased telomerase activity compared with the untreated group, and this effect could be blocked by TERT small interfering (siRNA) treatment. (B) Western blot analysis showed that ASA-SHED had a higher expression level of TERT, which could be blocked by TERT siRNA treatment. (C) BrdU labeling assay showed that TERT siRNA treatment inhibited ASA treatment-induced upregulation of the proliferation rate of SHED. (D) MTT assay showed that ASA-SHED have a higher proliferation rate than untreated SHED, which could be blocked by TERT siRNA treatment. (E) ASA-SHED showed increased mineralized nodule formation compared with the control group, as assessed by alizarin red staining, while TERT siRNA blocked ASA treatment-induced elevation of mineralized nodule formation. (F) Western blot analysis showed that TERT siRNA treatment downregulated the ASA treatment-induced elevated expression of Runx2 and ALP when cultured under osteogenic inductive conditions. (G) When implanted into immunocompromised mice using hydroxyapatite/tricalcium phosphate (HA/TCP) as a carrier, ASA-SHED exhibited an increased capacity to generate new bone, which could be blocked by TERT siRNA treatment. (H) Western blot analysis showed that increased levels of active β-catenin, but not total β-catenin, in ASA-SHED could be blocked by TERT siRNA treatment. (I) Western blot analysis showed that Wnt inhibitor (XAV939) treatment reduced elevated levels of active β-catenin in ASA-SHED, but XAV939 treatment had no effects on the expression of TERT. (J) ASA-SHED showed increased mineralized nodule formation, as assessed by alizarin red staining, while Wnt inhibitor XAV939 treatment inhibited ASA-induced elevated mineralized nodule formation. (K) Western blot analysis showed that Wnt inhibitor XAV939 treatment downregulated ASA-induced elevation of Runx2 and ALP when cultured under osteogenic inductive conditions. n = 5 in each group. Scale bar = 200 µm. *P < 0.05. **P < 0.01. ***P < 0.005. Error bars: mean ± SD. Vehicle: scrambled siRNA-treated SHED.
Recently, evidence has suggested that TERT can regulate the Wnt/β-catenin pathway (Choi et al. 2008; Park et al. 2009). It is well known that the Wnt signaling pathway plays an important role in bone formation (Westendorf et al. 2004; Gaur et al. 2005; Krishnan et al. 2006). To investigate the downstream mechanism of TERT-activated osteogenesis, we examined the expression level of Wnt/β-catenin in SHED, ASA-SHED, and TERT knockdown ASA-SHED. Western blot analysis showed that the expression of active β-catenin (nonphosphorylated) was markedly increased in ASA-SHED compared with control SHED. While knockdown of TERT in ASA-SHED resulted in reduced expression of active β-catenin, the resulting expression levels were similar to those observed in the control SHED (Fig. 3H). Wnt signaling inhibitor (XAV939) treatment significantly inhibited expression levels of active β-catenin but not TERT in ASA-SHED (Fig. 3I). We found that XAV939 treatment blocked ASA treatment-induced elevation of osteogenesis, as indicated by decreased mineralized nodule formation and reduced expression of Runx2 and ALP (Fig. 3J, K). Collectively, our studies show that ASA treatment elevates telomerase activity and thus upregulates expression of Wnt/β-catenin, leading to increased osteogenesis.
ASA Treatment Promotes Immunomodulation of SHED via TERT/FASL Pathway
Since TERT is associated with MSC-mediated immunomodulation (Chen et al. 2014), we hypothesized that ASA treatment might promote immunomodulation of SHED by elevating telomerase activity. To test this hypothesis, we used siRNA to knock down TERT expression in ASA-SHED and found it resulted in a reduced capacity to induce activated T-cell apoptosis in a coculture system compared with the scrambled siRNA control group (Fig. 4A). Furthermore, we confirmed the role of TERT in ASA-improved immunomodulation of SHED in DSS-induced colitis mice. When SHED, ASA-SHED, and TERT knockdown ASA-SHED were systemically infused into the colitis mice (Fig. 4B), we found that TERT siRNA knockdown significantly reduced the therapeutic effects of ASA-SHED compared with the control group, as assessed by loss of body weight, increased DAI, exacerbation of colonic inflammation, and increased colitis HAI (Fig. 4C–F). Flow cytometric analysis showed that TERT-knockdown ASA-SHED had reduced capacities to downregulate the levels of Th1 and Th17 cells and upregulate the levels of Tregs compared with ASA-SHED (Fig. 4G–I). Furthermore, TERT-knockdown ASA-SHED showed reduced ability to downregulate serum levels of TNF-α, IL-6, and IL-17 in colitis mice compared with ASA-SHED (Fig. 4J–L). These data suggest that ASA treatment-induced upregulation of immunomodulation in SHED is dependent on TERT levels.
Figure 4.
Acetylsalicylic acid (ASA) treatment promotes immunomodulatory properties of stem cells from exfoliated deciduous teeth via the telomerase reverse transcriptase (TERT)/FASL pathway. (A) ASA treatment significantly increased the amount of SHED-induced AnnexinV+7AAD– and AnnexinV+7AAD+ double-positive T-cell apoptosis in an in vitro coculture system. Knockdown of TERT expression by siRNA treatment inhibited SHED-induced T-cell apoptosis. (B) Schema showing systemic infusion of SHED into dextran sodium sulfate (DSS)–induced experimental colitis mice. (C–F) Knockdown of TERT by small interfering RNA (siRNA) treatment blocked ASA treatment-induced upregulation of immunomodulatory effects of SHED, as assessed by loss of body weight, increased disease activity index (DAI), exacerbation of colonic inflammation, and colitis histologic activity index (HAI). (G, H) ASA-SHED induced significantly reduced levels of Th1, Th17 cells in colitis mice compared with untreated SHED, which could be blocked by TERT siRNA treatment. (I) Systemically infused ASA-SHED exhibited an increased capacity to upregulate the levels of Tregs in mice with colitis compared with untreated SHED. This effect could be blocked by TERT siRNA treatment. (J–L) ASA-SHED infusion significantly downregulated elevated serum levels of tumor necrosis factor–α (TNF-α), interleukin (IL)–6, and IL-17 in colitis mice, which could be blocked by TERT siRNA treatment. (M) Western blot analysis showed increased levels of FASL in ASA-SHED compared with untreated SHED, and this increase could be blocked by TERT siRNA treatment. (N) Western blot analysis showed that FASL siRNA treatment reduced elevated levels of FASL in ASA-SHED, without altering TERT expression. (O) Knockdown of FASL by siRNA treatment blocked the increase in AnnexinV+7AAD− and AnnexinV+7AAD+ double-positive apoptotic T cells induced by ASA-SHED in an in vitro coculture system. n = 5 in each group. Scale bar = 200 µm. *P < 0.05. **P < 0.01. ***P < 0.005. Error bars: mean ± SD. Vehicle: scrambled siRNA-treated SHED.
It was previously reported that systemic infusion of MSCs induced T-cell apoptosis via the FASL/FAS pathway, leading to recipient immune tolerance (Akiyama et al. 2012). To examine whether TERT-activated immunomodulation mediated by SHED is associated with FASL expression, the expression levels of FASL were examined in control SHED, ASA-SHED, and TERT knockdown ASA-SHED by Western blot. We found that FASL expression was significantly upregulated in ASA-SHED in comparison to the control SHED, while the TERT siRNA knockdown significantly inhibited FASL expression (Fig. 4M). Next, we used siRNA to knock down the expression of FASL in ASA-SHED. Western blot analysis showed that FASL siRNA knockdown markedly decreased the expression of FASL in ASA-SHED but failed to affect TERT expression (Fig. 4N). In addition, FASL knockdown ASA-SHED had a significantly reduced capacity to induce T-cell apoptosis, as assessed by flow cytometric analysis (Fig. 4O). These data demonstrate that ASA treatment promotes the immunomodulatory properties of SHED via the TERT/FASL pathway.
Discussion
Telomerase is a ribonucleoprotein reverse transcriptase responsible for maintaining and extending telomere length at the end of chromosomes to avoid replicative senescence (Nakamura et al. 1997; Bodnar et al. 1998). TERT plays a key role in progenitor cell survival and stem cell self-renewal (Shay and Bacchetti 1997; Maser and DePinho 2002; Smogorzewska and de Lange 2004). Our previous studies have revealed that telomerase can extend the life span of MSCs as well as promote osteogenic differentiation and immunomodulation of MSCs (Shi et al. 2002; Gronthos et al. 2003; Yamaza et al. 2008; Liu et al. 2011; Chen et al. 2014). Here, we showed that TERT activation could significantly improve SHED-mediated new bone formation and enhance the immunomodulatory properties of SHED. Interestingly, expression levels of MSC surface markers, including STRO-1, SSEA-4, CD146, and CD105, were markedly upregulated in TERT-activated SHED. Thus, TERT may contribute to maintenance or improvement of stem cell properties of SHED. Telomerase activity in most human postnatal stem cells seems to be absent or only present at a very low level, and TERT is rapidly downregulated in MSCs during in vitro expansion (Shi et al. 2002; Hiyama and Hiyama 2007). Certain levels of telomerase activity are important for MSC-based tissue engineering and cell therapy. In this study, we found that ASA, a widely used anti-inflammatory drug, was able to activate the TERT pathway in a dose-dependent manner to affect proliferation, differentiation, and immunomodulation of SHED. Low doses of ASA (10/50 µg/mL), but not high dose (200 µg/mL), significantly elevated telomerase activity. Low-dose ASA therapy is therefore a feasible and efficient pharmacologic approach for activating telomerase activity to avoid replicative senescence and improve stem cell functions.
It has been reported that ASA treatment can promote MSC-based bone regeneration via reducing the local levels of IFN-γ and TNF-α (Liu et al. 2011) and ameliorate osteoporosis phenotypes in ovariectomized mice by rescuing osteogenic differentiation of MSCs (Yamaza et al. 2008). We also tested the effects of ASA on the canonical WNT signaling pathway, which plays an important role in normal skeletal development and in osteogenic differentiation of MSCs (Westendorf et al. 2004; Gaur et al. 2005; Krishnan et al. 2006). In this study, we showed that ASA elevated expression levels of TERT and active β-catenin, with Wnt/β-catenin serving as a downstream target of TERT.
MSCs possess great promise for clinical treatment of autoimmune diseases due to their extensive immunomodulatory properties. The FASL/FAS-mediated cell death pathway represents a typical mechanism of apoptotic signaling in many cell types (Hohlbaum et al. 2000; Pluchino et al. 2005; Zhang et al. 2008). Systemic infusion of MSCs can induce T-cell apoptosis via the FASL/FAS pathway, leading to upregulation of Tregs and immune tolerance (Akiyama et al. 2012). SHED showed elevated immunomodulatory function in inhibiting Th17 cells compared with human MSCs (Yamaza et al. 2010). In the present study, we showed that ASA treatment significantly enhanced the immunoregulatory function of SHED by activating telomerase, leading to upregulation of T-cell apoptosis and downregulation of Th17 cells. Importantly, ASA-SHED showed markedly increased therapeutic effects in DSS-induced colitis mice compared with untreated SHED. Our previous studies have revealed that TERT serves as a transcriptional modulator to regulate FASL expression in MSCs (Chen et al. 2014). Here, we confirmed that TERT improved the immunomodulatory properties of SHED by regulating the expression of FASL.
In summary, we demonstrate that ASA treatment improves stem cell functions of SHED, including upregulating proliferation, osteogenesis, and immunomodulation by elevating telomerase activity. ASA treatment may be a feasible and efficient pharmacologic approach to enhance therapeutic effects of SHED.
Author Contributions
Y. Liu, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; C. Chen, contributed to data analysis and interpretation, critically revised the manuscript; S. Liu, D. Liu, X. Xu, contributed to data acquisition and analysis, critically revised the manuscript; X. Chen, contributed to data analysis, critically revised the manuscript; S. Shi, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work was supported by grants from the US National Institute of Dental and Craniofacial Research (NIDCR), National Institutes of Health (NIH), Department of Health and Human Services (R01DE017449 and R01DE019932 to S.S.), and a training grant from the School of Stomatology, China Medical University, Shenyang, China.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. 2012. Mesenchymal stem cell induced immunoregulation involves FAS-ligand/FAS mediated T cell apoptosis. Cell Stem Cell. 10(5):544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. 2009. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 15(3):341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science. 279(5349):349–352. [DOI] [PubMed] [Google Scholar]
- Chen C, Akiyama K, Yamaza T, You YO, Xu X, Li B, Zhao Y, Shi S. 2014. Telomerase governs immunomodulatory properties of mesenchymal stem cells by regulating FAS ligand expression. EMBO Mol Med. 6(3):322–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Southworth LK, Sarin KY, Venteicher AS, Ma W, Chang W, Cheung P, Jun S, Artandi MK, Shah N, et al. 2008. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 4(1):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, et al. 2005. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 280(39):33132–33140. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Chen S, Wang CY, Robey PG, Shi S. 2003. Telomerase accelerates ostoegenesis of bone marrow stromal stem cells by upregulation of CBFA1, osterix, and osteocalcin. J Bone Miner Res. 18(4):716–722. [DOI] [PubMed] [Google Scholar]
- Hiyama E, Hiyama K. 2007. Telomere and telomerase in stem cells. Br J Cancer. 96(7):1020–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlbaum AM, Moe S, Marshak-Rothstein A. 2000. Opposing effects of transmembrane and soluble Fas ligand expression on inflammation and tumor cell survival. J Exp Med. 191(7):1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Bryant HU, Macdougald OA. 2006. Regulation of bone mass by Wnt signaling. J Clin Invest. 116(5):1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laino G, Graziano A, d’Aquino R, Pirozzi G, Lanza V, Valiante S, De Rosa A, Naro F, Vivarelli E, Papaccio G. 2006. An approachable human adult stem cell source for hard-tissue engineering. J Cell Physiol. 206(3):693–701. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. 2011. Mesenchymal stem cell based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 17(12):1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, DePinho RA. 2002. Connecting chromosomes, crisis, and cancer. Science. 297(5581):565–569. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. 2003. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 100(10):5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. 1997. Telomerase catalytic subunit homologs from fission yeast and human. Science. 277(5328):955–959. [DOI] [PubMed] [Google Scholar]
- Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, et al. 2009. Telomerase modulates Wnt signaling by association with target gene chromatin. Nature. 460(7251):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, et al. 2005. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 436(7048):266–271. [DOI] [PubMed] [Google Scholar]
- Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, Santos CF, Nör JE. 2010. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 89(8):791–796. [DOI] [PubMed] [Google Scholar]
- Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, Lee JS, Shi S. 2008. SHED repair critical-size calvarial defects in mice. Oral Dis. 14(5):428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, Bacchetti S. 1997. A survey of telomerase activity in human cancer. Eur J Cancer. 33(5):787–791. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang CY. 2002. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 20(6):587–591. [DOI] [PubMed] [Google Scholar]
- Smith JB, Willis AL. 1971. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 231(25):235–237. [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, de Lange T. 2004. Regulation of telomerase by telomeric proteins. Annu Rev Biochem. 73:177–208. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, Wang S. 2010. Stem cells from human exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 19(9):1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf JJ, Kahler RA, Schroeder TM. 2004. Wnt signaling in osteoblasts and bone diseases. Gene. 341:19–39. [DOI] [PubMed] [Google Scholar]
- Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, Wang S, Shi S. 2010. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 1(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K, Sonoyama W, Patel V, Gutkind S, Young M, Gronthos S, et al. 2008. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS One. 3(7):e2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu G, Zhang L, Roberts AI, Shi Y. 2008. Th17 cells undergo Fas-mediated activation-induced cell death independent of IFN-gamma. J Immunol. 181(1):190–196. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Liu Y, Zhang CM, Zhang HY, Li WH, Shi S, Le AD, Wang SL. 2009. Stem cells from deciduous tooth repair mandibular defect in swine. J Dent Res. 88(3):249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]