Abstract
Background
Use of radiopharmaceutical may improve the survival time of patients with castrate-resistant prostate cancer and bone metastases. Whether androgen-deprivation therapy (ADT) combined with bone-targeted therapy provides clinical benefit for patients with advanced castrate-sensitive prostate cancer has not been investigated.
Methods
A total of 80 male patients were enrolled, of whom 79 were randomized: 40 to the control arm and 39 to the Sr-89 arm. After randomization, patients in both study arms received ADT, doxorubicin, and zoledronic acid. Kaplan-Meier methodology was used to evaluate progression-free survival (PFS) time. Multivariate Cox proportional hazards regression was used to evaluate the effects of Sr-89 after controlling for the number of bone metastases.
Results
Median follow-up time for the 29 patients alive at the last follow-up was 76.9 (range: 0.07 – 103.4) months. Median PFSs were 18.5 months (95% CI: (9.7, 49.4)) in the control arm and 12.9 months (95% CI: (8.9, 72.5)) in the Sr-89 arm (p = 0.86). No patient developed myelodysplastic syndrome or hematologic malignancy. Unplanned subgroup analysis suggested increased efficacy of bone-targeted therapy for greater extent of bone involvement (ie, >6 vs 6 bone metastases on the bone scan).
Conclusions
Our data showed that bone-targeted therapy using one dose of Sr-89 combined with chemohormonal ablative therapy did not favorably impact the PFS of patients with castrate-sensitive prostate cancer. The combined therapy was feasible and safe. Whether such bone-targeted therapy provides a favorable outcome for those patients with greater tumor burden in the bone warrants further investigation.
Keywords: Strontium-89, prostate cancer, bone metastasis, radiopharmaceutical, phase-2
INTRODUCTION
Prostate cancer is the most common cancer among North American men,1 and androgen deprivation therapy (ADT) is an effective treatment for advanced prostate cancer.2 Unfortunately, ADT has not cured prostate cancer but merely converted it from a primarily local disease to a predominantly bone disease3 and patients with advanced prostate cancer and bone metastases have a dismal prognosis.4, 5 The unique relationship between prostate cancer and bone metastasis suggests that the missing link in a cure for prostate cancer must be in the bone and that bone-targeted therapy will play a pivotal role in prostate cancer treatment.
Current treatment of bone metastasis in prostate cancer is inadequate. ADT rarely produces complete remission in the osseous metastases of patients with advanced prostate cancer.6 Adding chemotherapy to ADT may improve clinical outcomes in these patients by treating both castrate-sensitive and castrate-resistant prostate cancer. However, whether chemohormonal therapy improves the survival time in these patients still has not been established.7, 8 Recently, interim analysis of a randomized Phase III trial (E3805) suggested a survival time advantage for patients with prostate cancer metastatic to bone who received combined therapy with docetaxel and ADT over survival time for patients who received ADT alone (http://www.nih.gov/news/health/dec2013/nci-05.htm). It is of interest whether combined treatment adding bone-targeted radiopharmaceuticals to chemohormonal therapy may further improve clinical outcome.With the clinical introduction of bone-specific agents, it has become possible to test the value of bone-targeted therapy for prostate cancer. Previously, we had demonstrated increased overall survival time in a select group of castrate-resistant prostate cancer patients who had received induction chemotherapy followed by bone-targeted therapy containing one dose of Sr-89.9 Recently, Parker et al. confirmed that bone-targeted therapy using repeated single-agent radium-223 improved overall survival time in patients with metastatic castrate-resistant prostate cancer.10 It remains unknown, however, whether bone-targeted therapy that includes a radiopharmaceutical will also benefit patients with metastatic castrate-sensitive prostate cancer.
We designed a randomized phase II study to determine the clinical efficacy, as measured by progression-free survival (PFS) time, of a bone-targeted strategy using chemohormonal therapy and zoledronic acid with or without a dose of Sr-89 for patients with advanced castrate-sensitive prostate cancer and bone metastases. The experience gained and the data collected in this trial could have value for the advancement of bone-targeted therapy using novel and improved radiopharmaceuticals, including Ra-223, for prostate cancer and other malignancies.
MATERIALS AND METHODS
Patients
This multicenter study was conducted through the MD Anderson Community Clinical Oncology Program and also at MD Anderson Cancer Center. Patients eligibility criteria for study enrollment were: castrate-sensitive prostate cancer metastatic to bone; ECOG performance status ≤ 3 (Karnofsky ≥40%); initiation of hormonal ablative therapy within 3 months of registration; any previous neoadjuvant, concurrent, or intermittent hormonal ablative therapy to have been less than 3 years’ duration and completed at least 3 years prior to entry into this study; normal organ and marrow function as defined by laboratory values of leukocyte ≥3,000/μL, absolute neutrophil count ≥1,500/μL, platelet count ≥100,000/μL, total bilirubin concentration within normal institutional limits, AST(SGOT)/ALT(SGPT) of ≤2.5 × institutional upper limit of normal, serum creatinine concentration ≤3.0, and left ventricular ejection fraction ≥45%. Patient exclusion criteria were: more than one prior chemotherapy regimen, prior radioisotope treatment with Sr-89 or samarium-153, zoledronic acid treatment of more than 3 months’ duration prior to registration, corrected serum calcium levels <8 mg/dL, receiving any other investigational agents at the time of enrollment, known brain metastases prior to registration, predominantly visceral metastasis or small-cell carcinoma, serious illnesses or major organ dysfunction, and HIV-positive patients receiving combination anti-retroviral therapy (because patients with immune deficiency are at increased risk of lethal infections when treated with marrow-suppressive therapy). All patients gave written informed consent, and the study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center.
Treatment Plan
All patients received hormonal-ablative therapy of either continuous treatment with a luteinizing hormone-releasing hormone (LHRH) agonist (e.g., leuprolide or goserelin) or bilateral orchiectomy. This treatment selection was based on the managing physician’s preference, and hormonal ablative therapy was expected to be taken indefinitely. All patients also received chemotherapy with doxorubicin 20 mg/m2 intravenously on days 1, 8, and 15 every 28 days for 2 cycles and biphosphonate therapy with zoledronic acid 4 mg intravenously over 15 minutes every 28 days for a total of 6 doses. Patients were required to have ANC >1,000 K/mL and platelets >70,000 K/mL to receive doxorubicin; otherwise, doxorubicin was withheld for one week and counts rechecked. If counts recovered to ANC >1,000 K/mL and platelets >70,000 K/mL, then doxorubicin was resumed. If longer than 1 week was required for count recovery, treatment was resumed with doxorubicin dose reduced to 15 mg/m2. In patients required to have a second delay of doxorubicin treatment, the drug’s toxicity was considered unacceptable, and no further treatment with doxorubicin was given.
Study-eligible patients were randomized immediately upon entry to the trial to receive either 1 dose of Sr-89 (4 mCi total dose) administered intravenously on the first day of treatment or to receive no Sr-89. Patients were allowed to take anti-emetics, anxiolytics, and other supportive measures freely while in the study.
Patients were evaluated every 4 weeks with a medical history, physical examination, and serum chemistry and prostate-specific antigen (PSA) measurements for the first 21 weeks during treatment. CBC with differential was checked every week during treatment. Once the doxorubicin and zoledronic therapy was completed, patients had follow-ups every 3 months with a history, physical examination, and measurement of CBC with differential and PSA until disease progression. Measurable disease was evaluated by computed tomography every 3 months until progression. A bone scan was obtained at week 13 if the baseline scan was positive for metastases. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria version 3.0.
For all patients, a PSA response was defined as a decline in PSA level by ≥50% without normalization and sustained for at least 8 weeks. PSA progression was defined as a 25% increase over the baseline or the nadir PSA level provided that the increase was a minimum of 1 ng/ml. Measurable disease response and progression were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST). The study’s primary endpoint was PFS duration, which was defined as the time from the date of randomization to the date of first evidence of disease progression or patient death. Secondary endpoints included overall survival (OS) time, which was calculated from date of randomization until the date of the patient’s death from any cause or until the date of last follow-up if the patient was alive.
Statistical methods
The aim of this randomized phase II trial was to estimate the PFS advantage of Sr-89, for patients with castrate-sensitive prostate cancer and bone metastases. However, with a minimum of 12 months’ follow-up, a sample of 80 patients provided 80% power to detect a doubling of the median PFS to 16 months assuming an exponential distribution of PFS and a one-sided type I error of 5%.
Kaplan-Meier methodology was used to estimate both PFS and OS. For PFS estimation, data for patients who who were still alive and did not have disease progression were censored at the last follow-up date. Similarly, for OS estimation, data for patients who were still alive were censored at the last follow-up date. Multivariate Cox proportional hazards regression was used to analyze the effects of Sr-89 after controlling for the number of bone metastases on the pre-treatment scan. All statistical analyses were based on the intention-to-treat principle. All statistical tests were performed at a significance level of 5%.
RESULTS
We enrolled 80 male patients on the study between July 28, 2004, and July 2, 2007. One patient withdrew consent to participate after the screening procedures, and 79 patients were randomized; 40 to the control arm and 39 to the Sr-89 arm (Figure 1). Table 1 shows the demographics and baseline characteristics for the 79 patients.
Figure 1.
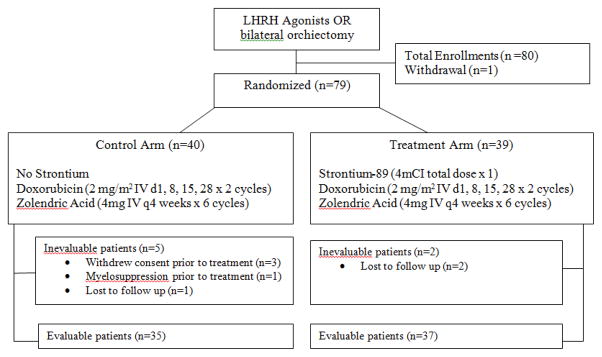
Consort diagram.
Table 1.
Demographics and baseline characteristics of the study patients
| Characteristic | No Strontium-89 (n=40) | Strontium-89 (n=39) | Total (n=79)* | |||
|---|---|---|---|---|---|---|
|
| ||||||
| n | % | n | % | n | % | |
|
| ||||||
| Extent of Disease (Stratification) | ||||||
|
| ||||||
| 1: ≤ 6 Bone Mets | 18 | 45.0% | 19 | 48.7% | 37 | 47% |
|
| ||||||
| 2: > 6 Bone Mets | 22 | 55.0% | 20 | 51.3% | 42 | 53% |
|
| ||||||
| Unknown | 0 | 0.0% | ||||
|
| ||||||
| Demographics | ||||||
|
| ||||||
| Age (years): | ||||||
|
| ||||||
| Range | 40 | 46–82 | 39 | 46–77 | 79 | 46–82 |
| Median | 63 | 62 | 63 | |||
|
| ||||||
| Race and Ethnicity: | ||||||
|
| ||||||
| White | 34 | 85.00% | 37 | 94.87% | 71 | 90% |
|
| ||||||
| Black | 3 | 7.50% | 2 | 5.13% | 5 | 6.25% |
|
| ||||||
| Not Reported | 3 | 7.50% | 0 | 0.00% | 3 | 3.75% |
|
| ||||||
| PSA (ng/mL): | 40 | 39 | 79 | |||
| Range | 0.1–124.6 | 0.1–995.6 | 0.1–995.6 | |||
| Median | 2.5 | 5.4 | 3.7 | |||
|
| ||||||
| LDH (IU/L): | 40 | 39 | 79 | |||
| Range | 152–1021 | 124–886 | 124–1021 | |||
| Median | 470 | 431 | 459 | |||
|
| ||||||
| Alkaline Phosphatase (IU/L): | 40 | 39 | 79 | |||
| Range | 31–2679 | 48–1212 | 31–2679 | |||
| Median | 127 | 99 | 127 | |||
|
| ||||||
| ECOG: | ||||||
|
| ||||||
| 0 | 22 | 55% | 29 | 74% | 51 | 65% |
|
| ||||||
| 1 | 16 | 40% | 9 | 23% | 25 | 31% |
|
| ||||||
| 2 | 0 | 0% | 0 | 0% | 0 | 0% |
|
| ||||||
| 3 | 2 | 5% | 1 | 3% | 3 | 4% |
|
| ||||||
| Gleason Score: | ||||||
|
| ||||||
| 10, 9, 8 | 24 | 60% | 26 | 67% | 50 | 63.3% |
|
| ||||||
| 7 | 11 | 27.5% | 9 | 23% | 20 | 25.3% |
|
| ||||||
| <7 | 0 | 0% | 1 | 2.3% | 1 | 1.3% |
|
| ||||||
| Not reported | 5 | 12.5% | 3 | 7.7% | 8 | 10.1% |
|
| ||||||
| Previous treatments: | ||||||
|
| ||||||
| Surgery only | 27 | 68% | 23 | 59% | 50 | 63% |
|
| ||||||
| Chemotherapy only | 1 | 3% | 1 | 1% | ||
|
| ||||||
| Combination therapy including surgery, chemotherapy and/or XRT | 7 | 17% | 7 | 18% | 14 | 18% |
|
| ||||||
| No prior therapy | 6 | 15% | 8 | 20% | 14 | 18% |
|
| ||||||
| Other features: | ||||||
|
| ||||||
| Lymph Node involvement | 6 | 15% | 8 | 20% | 14 | 18% |
|
| ||||||
| Visceral Metastasis | 0 | 0% | 0 | 0% | 0 | 0% |
One patient withdrew consent after the screening procedures. (Mets: Metastasis, PSA: Prostate specific antigen, LDH: Lactate dehydrogenase, XRT: Radiation theraphy)
In addition to the patient who was not randomized, four patients in the control arm also were not treated. Three withdrew consent to participate after being randomized to the control arm, and the other patient had persistent myelosuppression, which precluded him from remaining on protocol. Moreover, one patient in the control arm and two in the Sr-89 arm did not complete therapy because they were lost to follow-up (Figure 1).
Of the 79 patients randomized to study arm, the median follow-up time for the 29 who were alive at the last follow-up was 76.9 (range: 0.07 – 103) months. In the control arm, the median follow-up time for the 16 (of 40) patients alive at the last follow-up was 72.6 (range: 0.07 – 103) months. In the Sr-89 arm, the median follow-up time for the 13 (of 39) patients alive at the last follow-up was 78.7 (range: 2.14 – 99.0) months.
Of the 79 randomized patients, 56 (70.9%) had disease progression: 28 (70.0%) of 40 patients in the control arm and 28 (71.8%) of 39 patients in the Sr-89 treatment arm. Median PFS was 18.5 months (95% CI: [9.7, 49.4]) in the control arm compared with 12.9 months (95% CI: (8.9, 72.5)) in the Sr-89 arm. PFS did not differ statistically between the two arms (Figure 2A, p = 0.86). Table 2 presents the corresponding hazard ratios for PFS after controlling for the number of bony metastases. Unplanned subgroup analysis suggested that the efficacy of bone-targeted therapy may be more efficacious for patients with greater extent of bone involvement (i.e., >6 vs ≤6 bone metastases identified on the bone scan). Table 3 presents corresponding hazard ratios for PFS for the study patients by treatment and number of bone metastases at baseline. The test of equality over stratum of EOD showed that it was heterogeneous (p=0.02). The median nadir PSA levels for patients with ≤6 bone metastases who received and did not receive Sr-89 were 0.1 [range: <0.1–30] and 0.1 [range: <0.1–23.8], respectively, and for those patients with >6 bone metastases, 1.0 [range: <0.1–29] and 0.9 [range: <0.1–87.5], respectively.11
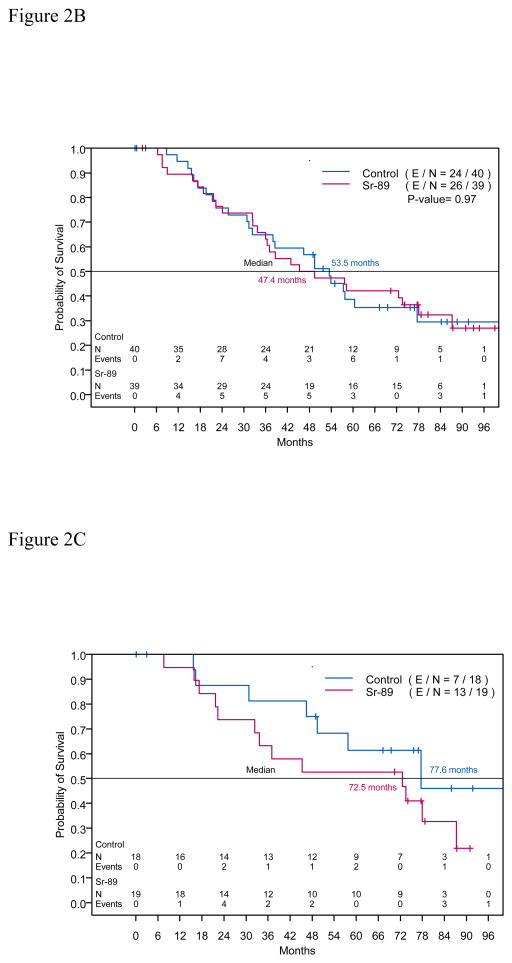
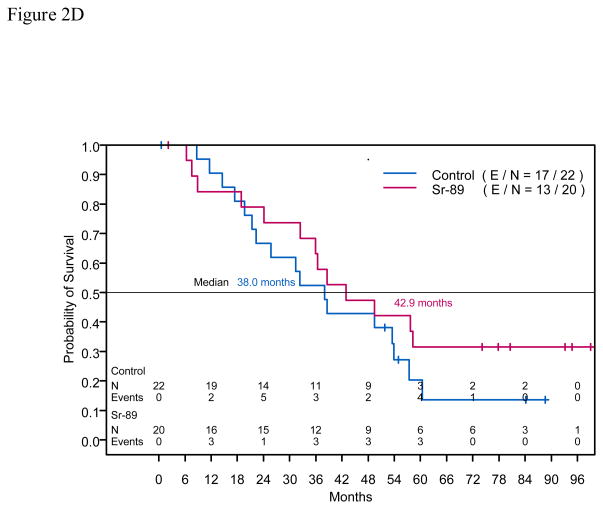
Figure 2.
Figure 2A. Kaplan-Meier estimates of progression free survival time for intent to treat population
Figure 2B. Kaplan-Meier estimates of overall survival time for intent to treat population
Figure 2C. Kaplan-Meier estimates of overall survival time for intent to treat population with 6 bone metastasis at study enrollment
Figure 2D. Kaplan-Meier estimates of overall survival time for intent to treat population with >6 bone metastasis at study enrollment
Table 2.
Hazard ratios for progression-free survival time for intent to treat population
| Factor | Multivariate Model | ||||
|---|---|---|---|---|---|
| n | HR | 95% CI | p-value | ||
| Treatment | No Sr-89 | 40 | 1.00 | ||
| Sr-89 | 39 | 0.93 | (0.55, 1.58) | 0.80 | |
| Bone Metastases | ≤ 6 | 37 | 1.00 | ||
| > 6 | 42 | 1.85 | (1.08, 3.16) | 0.03 | |
HR = hazard ratio; CI=confidence interval
Table 3.
Hazard ratios for progression-free survival time by Number of Bone Metastasis and Treatment
| Number of Bone Mets at Study Enrollment | Treatment | Number of Patients | HR | 95% CI |
|---|---|---|---|---|
| ≤ 6 | No Strontium | 18 | 1.00 | |
| Strontium | 19 | 1.54 | (0.66, 3.56) | |
| > 6 | No Strontium | 22 | 1.00 | |
| Strontium | 20 | 0.68 | (0.34, 1.35) |
HR = hazard ratio; CI=confidence interval
Of 79 randomized patients, 50 (63.3%) died: 24 (60.0%) of 40 patients in the control arm and 26 (66.7%) of 39 patients in the Sr-89 treatment arm. Median Ross were 53.5 months (95% CI: 38.0, not attained) in the control arm and 47.4 months (95% CI: 35.9, 87.3) in the treatment arm. OS did not differ statistically between the arms (Figure 2B, p = 0.97). Table 4 presents the corresponding hazard ratios for OS time adjusted for the number of bone metastases. Figure 2C shows OS for patients who had ≤6 bone metastases identified on the bone scan, and Figure 2D shows OS for patients who had >6 bone metastases.
Table 4.
Hazard ratios for overall survival time for intent to treat population
| Factor | Multivariate Model | ||||
|---|---|---|---|---|---|
| n | HR | 95% CI | p-value | ||
| Treatment | No Sr-89 | 40 | 1.00 | ||
| Sr-89 | 39 | 0.97 | (0.56, 1.70) | 0.92 | |
| Bone Metastases | ≤ 6 | 37 | 1.00 | ||
| > 6 | 42 | 1.73 | (0.98, 3.06) | 0.06 | |
HR = hazard ratio; CI=confidence interval
Table 5 presents the grade 3 and grade 4 adverse events that occurred in each treatment arm. Most common grade 3 and 4 adverse events were constitutional symptoms. Bone marrow-related adverse events were seen in 1 patient in the control arm (grade 3) and in 2 patients in the Sr-89 arm (grade 3). No patient developed myelodysplastic syndrome or hematologic malignancy during the study.
Table 5.
Treatment-related toxicities
| Adverse Events | Control Arm | Sr-89 Arm | ||||
|---|---|---|---|---|---|---|
| Grade | Grade | |||||
| 3 | 4 | Total | 3 | 4 | Total | |
| Constitutional Symptoms | 8 | 8 | 7 | 7 | ||
| Pain | 2 | 1 | 3 | 3 | 3 | |
| Infection | 3 | 3 | ||||
| Blood/Bone Marrow | 1 | 1 | 2 | 2 | ||
| Gastrointestinal | 1 | 1 | ||||
| Pulmonary/Upper Respiratory | 1 | 1 | ||||
| Renal/Genitourinary | 1 | 1 | ||||
| Surgery/Intra-operative Injury | 1 | 1 | ||||
| Vascular | 1 | 1 | ||||
| Grand Total | 12 | 1 | 13 | 19 | 19 | |
DISCUSSION
To our knowledge this is the first study that combines ADT with a radiopharmaceutical agent for the treatment of patients with advanced castrate-sensitive prostate cancer. In this randomized phase II trial, we did not detect any difference in the PFS time between patients who received one dose of Sr-89 as part of the therapy and patients who did not receive it. Importantly, we did not observe any serious long-term adverse events, such as myelodysplastic syndrome or acute myelogenous leukemia, with the combination treatment. Interestingly, several patients with multiple bone lesions (>6 on the initial bone scan) and high PSA levels (>1,000 ng/mL) who had received Sr-89 experienced prolonged remission (>7 years) that at the time of this report required either no additional or minimal treatments (i.e., no chemotherapy). Whether the combined regimen containing Sr-89 affected the bone microenvironment and kept the bone metastases in a dormant state in those individual cases needs further investigation.
Our study did not demonstrate statistically significant differences in PFS or OS between patients treated with Sr-89 and controls. A possible explanation for this finding is that because ADT is so effective, additional benefit from a single dose of Sr-89 may not be apparent in a small study. That is, since the control arm treatment already provides such good results, detecting a modest benefit of Sr-89 is more difficult, i.e., more patients are needed to demonstrate a statistical difference in this patient population unless we know how to better select the appropriate subgroup to study. To design a more feasible study (e.g., using radium-22310), we may need to focus on patients with a higher burden of bone metastasis,(e.g., > 6 bone metastases) or progressive bone metastasis (e.g., 2 new lesions on a bone scan over 6 months) to detect a treatment benefit. Otherwise, results for patients who do well regardless (because they have fewer bone metastases) will dilute or mitigate any potential therapeutic benefits from this study.
Combined therapy may be advantageous for patients with more aggressive prostate cancer such as progressive bone metastasis or new lesions on bone scans. Soloway et al. showed a direct relationship between extent of bone metastasis (extent of disease [EOD]) and patient survival rate.4 In that study, the 2-year survival rates for patients receiving ADT with EOD I (<6 bone lesions), EOD II (6–20 lesions), EOD III (>20 lesions), and EOD IV (superscan) were 94%, 74%, 68%, and 40%, respectively.4 Pollen et al. reported that patients with castrate-resistant prostate cancer with rapidly progressive prostate cancer with multiple new lesions on the bone scan had a median OS time of 4 months and a 1-year survival rate of 7%.12 In our study, patients with >6 bone metastases on pretreatment bone scan who received treatment with Sr-89 had longer PFSs than did patients treated without Sr-89. However, patients with ≤6 bone metastasis on pretreatment bone scan who were treated with Sr-89 had shorter PFSs than patients treated without Sr-89 (Table 3). These data suggest that patients with high-volume bone disease experience greater clinical benefit from bone-targeted radiopharmaceuticals than do patients with low-volume bone disease.
Because patients with castrate-sensitive prostate cancer in particular and patients with castrate-resistant prostate cancer in general are anticipated to have a longer life expectancy with the advent of more effective treatments, the long-term effects of radiopharmaceuticals have important clinical implications. There has been concern that the use of radiopharmaceuticals could potentially damage the bone marrow, leading to myelodysplastic syndrome or hematologic malignancy.13, 14 Fortunately, we did not observe any such events in this study after a median follow-up of 78.7 months. It is hoped that the risk of such complications will be further reduced using shorter-acting and less-penetrant radiopharmaceuticals, such as Ra-223.
Until now, almost all studies using bone-targeted radiopharmaceutical treatment have focused on castrate-resistant prostate cancer. Of interest is whether adjunct treatments that complement or supplement ADT and are designed to control minimal residual disease and/or the bone microenvironment after ADT will improve clinical outcome. The rationale for this therapeutic approach is the synergistic effect of radiation therapy with ADT, a combination that is well established for the treatment of primary prostate cancer15–17 and that may be just as efficacious for the treatment of prostate cancer bone metastases. After initiation of ADT, a flare reaction occurs in bone metastasis, indicating rapid bone repair and increased osteoblastic activity.18, 19 Importantly, Bushnell et al. showed that an increased uptake of bone-targeting radiopharmaceuticals occurs 4 weeks to 3 months after initiation of ADT.20
A principal determinant of bone metastasis is the bone microenvironment. There is evidence suggesting radiation not only treats prostate cancer metastasis but also affects the bone microenvironment such that the bone become inhospitable to metastasis.15 Hence, Jacobsson et al. showed that previously irradiated bone appeared to be protected from future metastasis.21 We hypothesize that using bone-targeting radiopharmaceuticals may similarly improve control of the bone onco-niche and improve clinical outcome. Controlling the bone microenvironment via radiopharmaceuticals may also keep the bone metastases “dormant” by inhibiting specific bone factors.22–28 Similar studies in the future may provide an unique opportunity to investigate specific bone factors that play a role in the induction of cancer dormancy and, conversely, in the activation of prostate cancer bone metastasis from dormancy.
By using hormonal ablative therapy, a bisphosphonate (zoledronic acid), and a radiopharmaceutical agent (strontium-89) that together target various components of prostate cancer bone metastasis, we hope to substantially lower the markers of prostate cancer cells (prostate-specific antigen, PSA), osteoblasts (bone-specific alkaline phosphotase, BSAP), and osteoclasts (urine n-telopeptide, NTX, and deoxypyridinoline, DPD).29 Although these markers are not ideal (not sensitive or specific enough), they may sufficiently serve our purposes by simply tracking changes in their levels with time. We speculate that when patients develop castrate-resistant prostate cancer and progression of bone metastasis, a resurgence of PSA, BSAP, and NTX/DPD levels in a temporal sequence may reflect potential interactions among the respective epithelial, osteoblast, and osteoclast compartments that need to be further elucidated.
In summary, our data suggest that bone-targeted therapy using one dose of Sr-89 combined with chemohormonal ablative therapy failed to demonstrate PFS benefit in patients with castrate-sensitive prostate cancer and bone metastases. However, the combined treatment was safe and feasible. Whether this bone-targeted therapy could provide a favorable outcome for these patients with a greater tumor burden (i.e., >6 lesions on the bone scan) in the bone requires further investigation.
Acknowledgments
This study was conducted as a collaborative trial of the MD Anderson Cancer Center Community Clinical Oncology Program Research Base and MD Anderson Cancer Center. The study was supported in part by NCI grant U10 CA045809. MD Anderson is supported in part by the National Institutes of Health through Cancer Center Support Grant, CA016672. We thank Diane S. Hackett in MD Anderson’s Department of Scientific Publications for editing the manuscript.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 Sep-Oct;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972 Jul-Aug;22(4):232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 3.Thalmann GN, Anezinis PE, Chang SM, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994 May 15;54(10):2577–2581. [PubMed] [Google Scholar]
- 4.Soloway MS, Hardeman SW, Hickey D, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988 Jan 1;61(1):195–202. doi: 10.1002/1097-0142(19880101)61:1<195::aid-cncr2820610133>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Sabbatini P, Larson SM, Kremer A, et al. Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J Clin Oncol. 1999 Mar;17(3):948–957. doi: 10.1200/JCO.1999.17.3.948. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg SL, Bruchovsky N, Rennie PS, Coppin CM. The combination of cyproterone acetate and low dose diethylstilbestrol in the treatment of advanced prostatic carcinoma. J Urol. 1988 Dec;140(6):1460–1465. doi: 10.1016/s0022-5347(17)42073-8. [DOI] [PubMed] [Google Scholar]
- 7.Millikan RE, Wen S, Pagliaro LC, et al. Phase III trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer. J Clin Oncol. 2008 Dec 20;26(36):5936–5942. doi: 10.1200/JCO.2007.15.9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fizazi K, Lesaunier F, Delva R, et al. A phase III trial of docetaxel-estramustine in high-risk localised prostate cancer: a planned analysis of response, toxicity and quality of life in the GETUG 12 trial. Eur J Cancer. 2012 Jan;48(2):209–217. doi: 10.1016/j.ejca.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Tu SM, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001 Feb 3;357(9253):336–341. doi: 10.1016/S0140-6736(00)03639-4. [DOI] [PubMed] [Google Scholar]
- 10.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013 Jul 18;369(3):213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 11.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006 Aug 20;24(24):3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 12.Pollen JJ, Gerber K, Ashburn WL, Schmidt JD. Nuclear bone imaging in metastatic cancer of the prostate. Cancer. 1981 Jun 1;47(11):2585–2594. doi: 10.1002/1097-0142(19810601)47:11<2585::aid-cncr2820471113>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Kossman SE, Weiss MA. Acute myelogenous leukemia after exposure to strontium-89 for the treatment of adenocarcinoma of the prostate. Cancer. 2000 Feb 1;88(3):620–624. doi: 10.1002/(sici)1097-0142(20000201)88:3<620::aid-cncr19>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Tu SM, Kim J, Pagliaro LC, et al. Therapy tolerance in selected patients with androgen-independent prostate cancer following strontium-89 combined with chemotherapy. J Clin Oncol. 2005 Nov 1;23(31):7904–7910. doi: 10.1200/JCO.2005.01.2310. [DOI] [PubMed] [Google Scholar]
- 15.Tu SM, Lin SH, Podoloff DA, Logothetis CJ. Multimodality therapy: bone-targeted radioisotope therapy of prostate cancer. Clin Adv Hematol Oncol. 2010 May;8(5):341–351. [PMC free article] [PubMed] [Google Scholar]
- 16.D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008 Jan 23;299(3):289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 17.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009 Jun 11;360(24):2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 18.Pollen JJ, Shlaer WJ. Osteoblastic response to successful treatment of metastatic cancer of the prostate. AJR Am J Roentgenol. 1979 Jun;132(6):927–931. doi: 10.2214/ajr.132.6.927. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu N, Masuda H, Yamanaka H, Oriuchi N, Inoue T, Endo K. Fluorodeoxyglucose positron emission tomography scan of prostate cancer bone metastases with flare reaction after endocrine therapy. J Urol. 1999 Feb;161(2):608–609. [PubMed] [Google Scholar]
- 20.Bushnell DL, Madsen M, Kahn D, Nathan M, Williams RD. Enhanced uptake of 99Tcm-MDP in skeletal metastases from prostate cancer following initiation of hormone treatment: potential for increasing delivery of therapeutic agents. Nucl Med Commun. 1999 Oct;20(10):875–881. doi: 10.1097/00006231-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsson H, Naslund I. Reduced incidence of bone metastases in irradiated areas after external radiation therapy of prostatic carcinoma. Int J Radiat Oncol Biol Phys. 1991 Jun;20(6):1297–1303. doi: 10.1016/0360-3016(91)90241-u. [DOI] [PubMed] [Google Scholar]
- 22.McAllister SS, Gifford AM, Greiner AL, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008 Jun 13;133(6):994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu X, Mu E, Wei Y, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell. 2011 Dec 13;20(6):701–714. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calon A, Espinet E, Palomo-Ponce S, et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012 Nov 13;22(5):571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003 Jun;3(6):537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 26.Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011 Feb 15;19(2):192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003 Oct 23;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 28.Peinado H, Aleckovic M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012 Jun;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michaelson MD, Smith MR. Bisphosphonates for treatment and prevention of bone metastases. J Clin Oncol. 2005 Nov 10;23(32):8219–8224. doi: 10.1200/JCO.2005.02.9579. [DOI] [PubMed] [Google Scholar]