Abstract
Direct actions of nicotine in the CNS appear to be essential for its reinforcing properties. However, activation of nicotinic acetylcholine receptors (nAChRs) on afferent sensory nerve fibers are important components of addiction to, and withdrawal from, cigarette smoking. The present study was to identify the neuroanatomical substrates activated by the peripheral actions of nicotine and to determine whether these sites overlap brain structures stimulated by direct actions of nicotine. Mouse brains were examined by immunohistochemistry for c-Fos protein after intraperitoneal injection of either nicotine (NIC, 30 and 40 µg/kg) and/or nicotine pyrrolidine methiodide (NIC-PM, 20 and 30 µg/kg). NIC-PM induced c-Fos immunoreactivity (IR) at multiple brain sites. In the brainstem, c-Fos IR was detected in locus coeruleus, laterodorsal tegmental nucleus and pedunculotegmental nucleus. In the midbrain, c-Fos IR was observed in areas overlapping the ventral tegmental area (VTA) which includes paranigral nucleus, parainterfascicular nucleus, parabrachial pigmental area and rostral VTA. Other structures of the nicotine brain-reward circuitry activated by NIC-PM included hypothalamus, paraventricular thalamic nucleus, lateral habenular nucleus, hippocampus, amygdala, accumbens nucleus, piriform cortex, angular insular cortex, anterior olfactory nucleus, lateral septal nucleus, bed nucleus of stria terminalis, cingulate and medial prefrontal cortex, olfactory tubercle, medial and lateral orbital cortex. Nicotine, acting through central and peripheral nAChRs, produced c-Fos IR in areas that overlapped NIC-PM induced c-Fos expressing sites. These neuroanatomical data are the first to demonstrate that the CNS structures which are the direct targets of nicotine are also anatomical substrates for the peripheral sensory impact of nicotine.
Keywords: Nicotine, Nicotine pyrrolidine methiodide, Addiction, Sensory nerve fibers
INTRODUCTION
The prevalence of tobacco smoking has not changed significantly over the past several years. In the United States alone, about 45 million people smoke tobacco (Joel et al., 2012). According to the Centers for Disease Control and Prevention (CDC), 75% of smokers would like to quit because of the known health risks associated with nicotine. Despite the availability of numerous nicotine addiction treatment strategies, less than 5% of individuals who try to quit remain smoke-free after one year (Yilmazel Ucar et al., 2014). Upon inhalation, nicotine, the major addictive component of tobacco smoke, passes into the bloodstream and, within seconds, crosses the blood-brain barrier to enter the brain parenchyma (Berridge et al., 2010; Rose et al., 2010). Once in the brain, nicotine activates various nicotinic acetylcholine receptors (nAChRs) located throughout the central nervous system (CNS) and modulates the activity of virtually all the major neurotransmitters via pre and/or postsynaptic mechanisms (Wonnacott et al., 2006; Dani and Bertrand, 2007; Albuquerque et al., 2009). Most studies evaluating the neurobiological mechanisms of nicotine addiction have focused on the direct effects of nicotine on the CNS and on the mesocorticolimbic pathways which include dopaminergic neurons of the ventral tegmental area (VTA) and its projections to nucleus accumbens (Acb) and medial prefrontal cortex (MPFC) (Schultz et al., 1997; Wise, 2009; Schultz, 2010; Ikemoto, 2010; De Biasi and Dani, 2011). However, activation of various CNS sites could result from interactions of nicotine with nAChRs abundantly expressed on epithelial cells of airways and afferent sensory nerve fibers (Gu et al., 2008; Rose et al., 1999; Alimohamadi and Silver, 2000; Dehkordi et al., 2009, 2010). Nicotine stimulation of peripheral sensory nerve fibers is important for mediating the sensory impact of nicotine such as taste, aroma and respiratory tract sensation and the associated perceptions are thought to be critically important for smoking satisfaction (Rose et al., 1984, 1985, 1999, 2006; Westman et al., 1996; Palmatier et al., 2006; Yerger and McCandless, 2011). Other drug-related sensory modalities associated with cigarette smoking such as visual, auditory and tactile or haptic sensations are also thought to be relevant in drug addiction (Schneider et al., 2001; Filbery et al., 2008; 2009; Janes et al., 2010; Seo et al., 2011; Claus et al., 2011; Yalachkov et al., 2013). Consistent with this idea, we have previously demonstrated that blockade of the sensory afferents in the upper respiratory tract by local anesthetics makes human smoking much less rewarding (Rose et al., 1985). Nicotine associated with cigarette smoking has a direct effect on the nAChRs located at the central nervous system (CNS), but it also stimulates peripheral nAChRs. Thus, the objectives of the present study were twofold: (1) To explore the brain regions which are activated by intraperitoneal (i.p.) injection of a peripherally-acting nicotine analog, nicotine pyrrolidine methiodide (NIC-PM) that does not cross the blood-brain barrier (Gillis and Lewis, 1956; Aceto et al., 1983; Lenoir et al., 2013) and (2) To determine whether the brain sites activated by NIC-PM overlap those activated by intraperitoneal (i.p.) administration of nicotine hydrogen tartrate, a form of nicotine that does cross the blood-brain barrier.
MATERIALS AND METHODS
Adult (2–3 month-old) CD-1 mice weighing 20–25 g were used. All procedures including the anesthesia and surgery were approved by the Institutional Animal Care and Use Committee (IACUC) of Howard University. All efforts were made to minimize the number of animals used and their suffering. Animals (N=20) were housed at a room temperature 22–24°C with water and food freely available. To reduce the nonspecific effects of handling and experimental environment, animals were handled daily and exposed to the same conditions as during the actual experiments. Following an adaptation period of 3–4 d, the mice were treated by i.p. injection of saline (control), nicotine hydrogen tartrate salt (NIC, Sigma–Aldrich, Saint Louis, MO) and/or nicotine pyrrolidine methiodide (NIC-PM, Toronto Research Chemicals Company), the latter a quaternary nicotine analog which does not penetrate the blood-brain barrier (Gillis and Lewis, 1956; Aceto et al., 1983; Lenoir et al., 2013). The NIC dose (30 and 40 µg/kg) used in the present study is within the range reported to be optimal for maintaining intravenous self-administration of nicotine in rats (Cox et al., 1984; Donny et al., 1995) and comparable to the dose delivered during the smoking of one or two cigarettes in humans (Rose and Corrigall, 1977). NIC–PM (20 and 30 µg/kg) was given at a dose that is equimolar to nicotine. The 20 µg/kg of NIC-PM produced very little c-Fos activation in the brain. Thus, only the data obtained with 30 µg/kg NIC-PM were reported herein. Both forms of nicotine were dissolved in saline and injected i.p. Two h after i.p. injection of the saline (control), NIC and/or the NIC-PM, the mice were anesthetized with 5% isoflurane and were perfused transcardially with saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB) at pH 7.4. After perfusion, the brains were postfixed in 4% paraformaldehyde for one h and then cryoprotected in a 30% sucrose solution for a minimum of 2 d. Transverse sections of the brain were cut at 40 µm using a Bright OTF Cryostat (Hacker Instruments and Industries) and were stored in 0.5% sodium azide in 0.1 M PB (pH 7.4). Immunohistochemical procedures were performed using free floating sections as follows: Briefly, 1-in-5 series of brain sections extending from bregma −5.41 mm to bregma 2.33 mm (Paxinos and Franklin 2013) were rinsed three times in 0.1 M phosphate buffered saline (PBS) at pH 7.4. Nonspecific binding was blocked by incubating the tissues overnight in loading buffer containing 2% normal donkey serum (NDS, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and 0.3% Triton X-100. Tissues were then washed and incubated with rabbit anti- c-Fos antibody (1:5000; Cat # PC38, Millipore Corporation Temecula, CA) in 2% NDS and 0.3% Triton X-100 in PBS at 4°C for 48 h. The sections were then incubated in Alexa Fluor 594 donkey anti-rabbit secondary antibody (1:100; Jackson ImmunoResearch Laboratories Inc) in 0.1 M PBS for 2½ h. After washing in PBS, sections were rinsed in PBS, mounted and cover-slipped using Vecta Shield (Vector Laboratories Inc., CA) antifade mounting media.
Controls for each experiment were performed to determine whether the primary or the secondary antibodies produced false-positive results. The controls involved omission of the primary and/or secondary antisera to eliminate the corresponding specific labeling. Nonspecific activation of c-Fos was assessed by evaluating the CNS expression of c-Fos in animals receiving i.p. injection of normal physiological saline (NS).
Data Analysis
High resolution fluorescent images were acquired using Nikon (Nikon Instruments, Melville, NY) and Olympus (Olympus AX70, Olympus America) microscopes equipped with the adequate filter systems to observe the red fluorescence. 1-in-5 series of brain sections extending from bregma −5.41 mm to bregma 2.33 mm (Paxinos and Franklin, 2013) were identified. NIC and NIC-PM induced c-Fos-expressing structures in representative brain regions were identified. The sections exhibiting the exact same anatomical structures for both the NIC and the NIC-PM treated groups were compared and analyzed. Images from all the brain regions of interest were captured at 4×, 10× and 20× magnification and minor adjustments of brightness and contrast were made using Adobe Photoshop CS3
Cell counting
Semi-quantitative estimate of the numbers of NS-, NIC-, NIC-PM-, c-Fos activated cells in VTA, Acb and PFC was performed using a NIC dose (40 µg/kg) that was approximately equimolar to NIC-PM (30 µg/kg). The counting of c-Fos immunoreactive cells was performed by an individual blinded to the treatment. Four 40 µm sections through each of the aforementioned structures were selected for each group (N=4) and well-defined areas based on anatomical landmarks were chosen for analysis. In VTA, the number of c-Fos IR cells in the sections selected between bregma −3.15 mm and bregma −2.79 mm were counted. In Acb, the number of c-Fos IR cells in the sections selected between bregma 0.61 mm and bregma 1.41 mm were counted. In PFC, the number of c-Fos IR cells in the sections selected between bregma 0.85 mm and bregma 1.21 mm were counted. The c-fos IR cells were counted in a single plane and overlapping cells were counted if they were distinguishable by a staining intensity greater than that of the background. The data were expressed as mean ± standard error. Statistical analysis was performed using one-way analysis of variance (ANOVA) to compare the effects of NS, NIC and NIC-PM in the aforementioned areas. The significance level was set at P≤0.05.
RESULTS
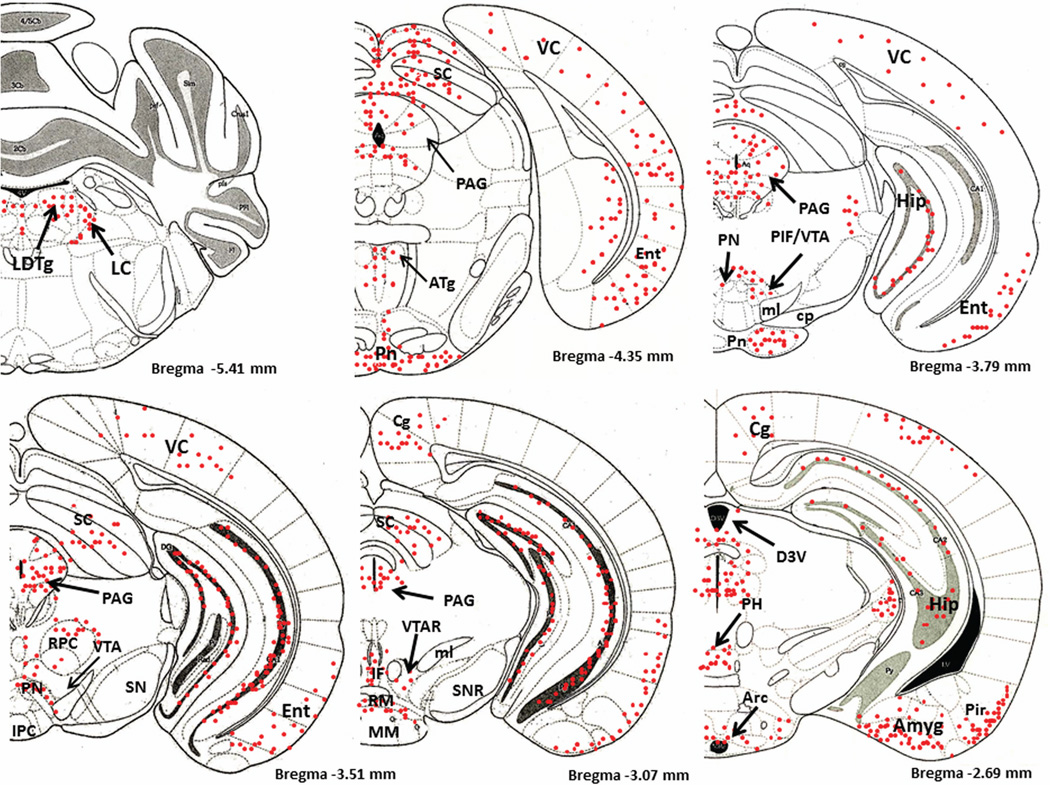
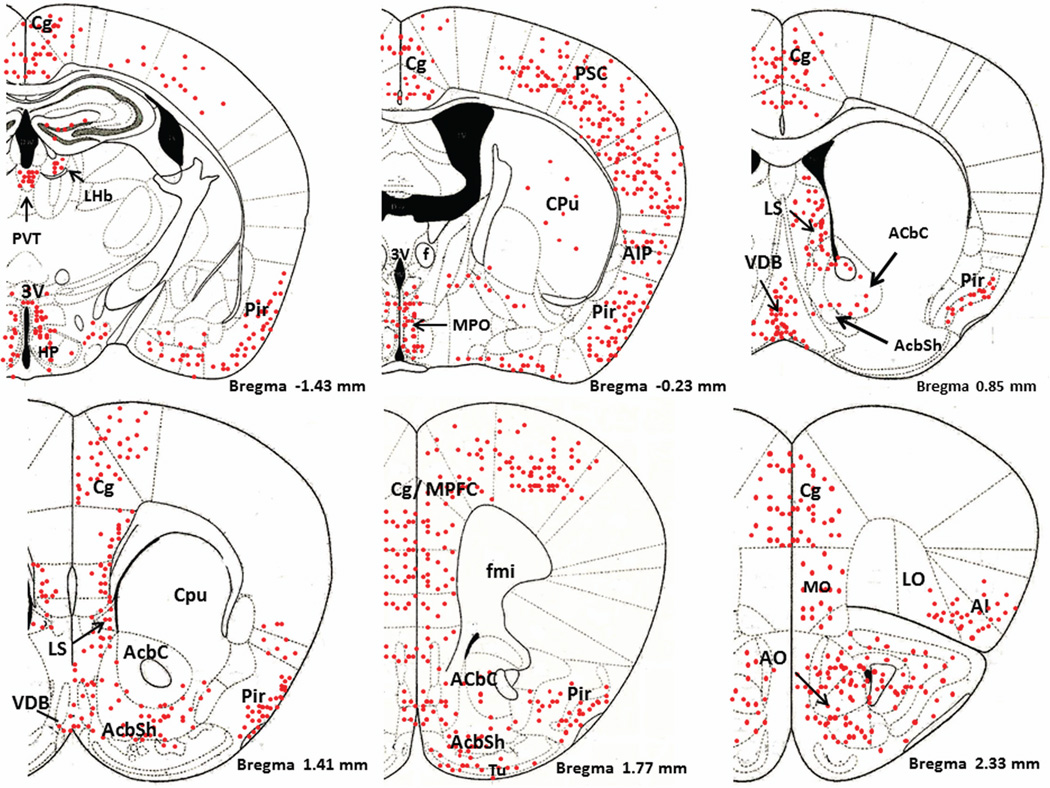
Figure 1 is a schematic diagram of representative brain regions showing NIC-PM induced c-Fos expression at multiple brain sites. The pattern of NIC–PM induced c-Fos expression was similar to that of NIC induced c-Fos expression and areas activated by the peripherally-acting analog of nicotine, often overlapped those which were stimulated by NIC (Figures 2 and 3). Overlap between the central and peripheral effects of i.p. NIC was a limiting factor which did not permit identification of the specific cells activated by the direct effects of nicotine on the CNS. Consequently, these data represent site-specific activations by NIC and NIC-PM and do not represent the specific cell groups activated by these compounds.
Figure 1.
Schematic diagrams of representative brain regions demonstrating the sites of c-Fos activated cells in the mouse brain following intraperitoneal injection of the peripherally-acting nicotine analog, nicotine pyrrolidine methiodide (NIC-PM, 30 µg/kg). NIC-PM induced c-Fos immunoreactivity at multiple sites extending from bregma −5.41 mm to bregma 2.33 mm. The sites activated by NIC were qualitatively identical to those activated by NIC-PM. The red-colored dots represent the relative intensity of site-specific c-Fos activation and do not represent the exact number of activated cells. For abbreviations, see “abbreviations”
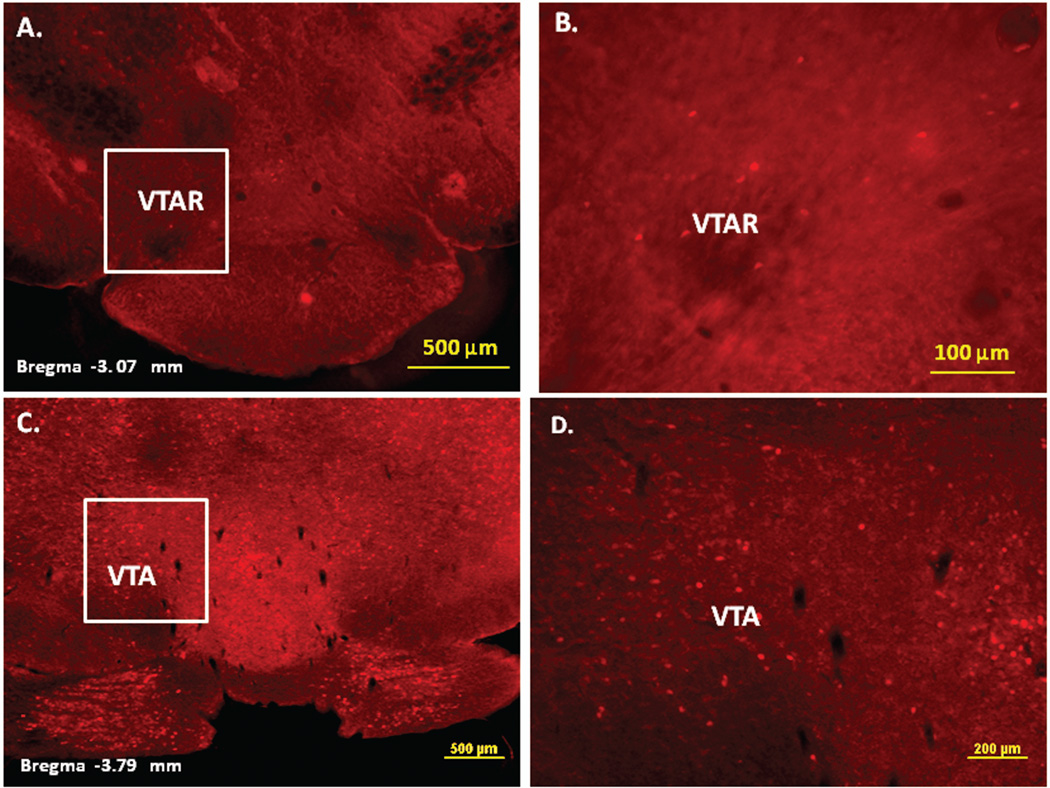
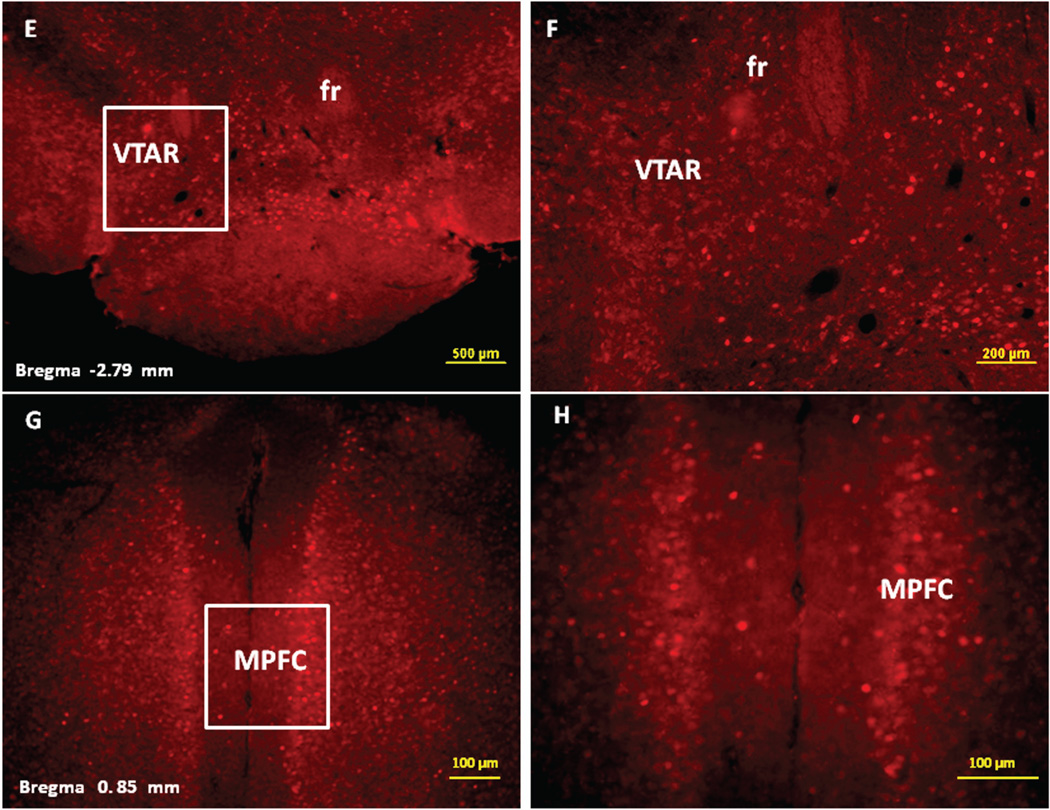
Figure 2.
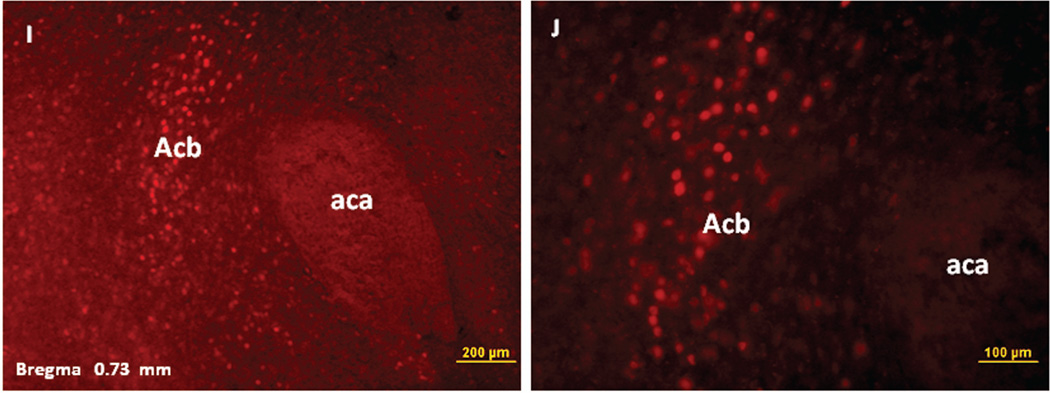
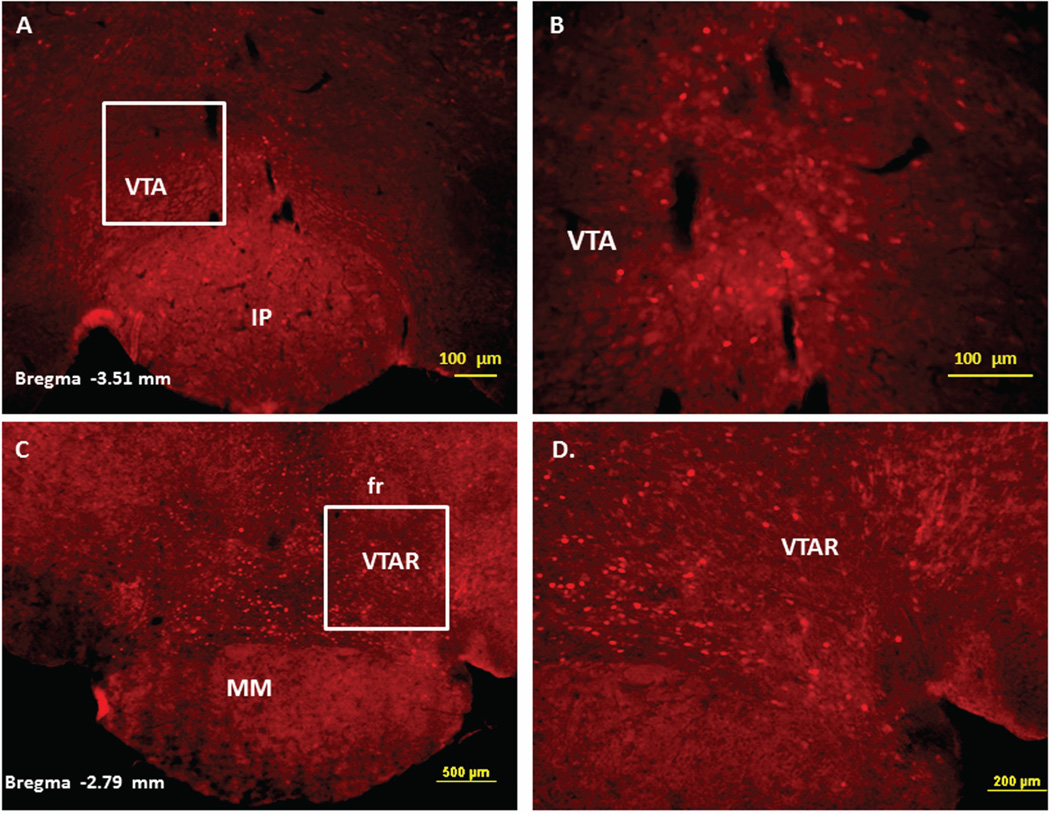
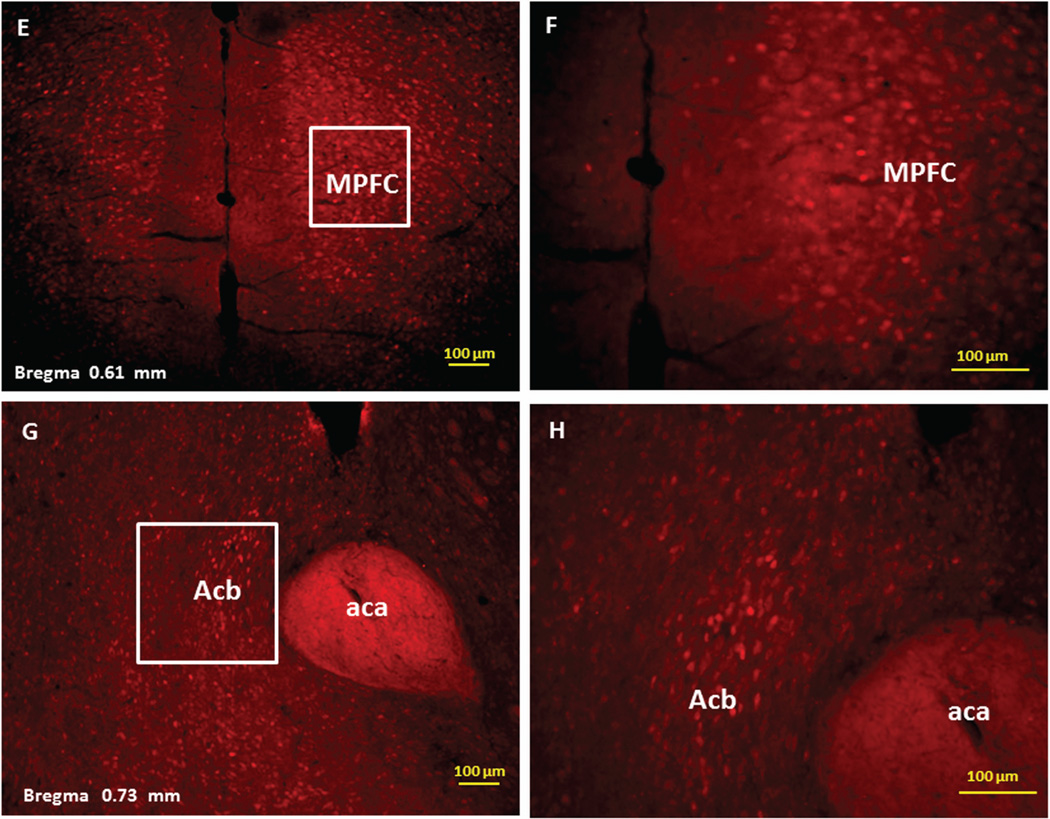
Immunofluorescence staining demonstrating the sites of c-Fos activated cells of the mesocorticolimbic system in the mouse brain following intraperitoneal injection of the peripherally-acting nicotine analog, nicotine pyrrolidine methiodide (NIC-PM, 30 µg/kg). Panels A and B: Control data demonstrating c-Fos immunoreactivity observed following intraperitoneal injection of physiological saline (vehicle) at a representative site. Panels C-F: c-Fos immunoreactive cells in the ventral tegmental area (VTA). Panels G and H: c-Fos immunoreactive cells in medial prefrontal cortex (MPFC). Panels I and J: c-Fos immunoreactive cells in nucleus accumbens (Acb).
Figure 3.
Immunofluorescence staining demonstrating the sites of c-Fos activated cells of the mesocorticolimbic system in the mouse brain following intraperitoneal injection of nicotine hydrogen tartrate (NIC, 30 µg/kg). Panels A–D: c-Fos immunoreactive cells in the ventral tegmental area (VTA). Panels E and F: c-Fos immunoreactive cells in medial prefrontal cortex (MPFC). Panels G and H: c-Fos immunoreactive cells in nucleus accumbens (Acb).
Figures 2 and 3 present the results of representative experiments demonstrating NIC and NIC-PM induced c-Fos activated cells in various structures of the mesocorticolimbic system. In the VTA (Figures 2 and 3), c-Fos expressing cells were scattered at sites medial to substantia nigra (SN) and medial lemniscus (ml) and in regions corresponding to paranigral nucleus (PN), parainterfascicular nucleus (PIF) and interpeduncular nucleus rostral (IPR). More rostrally, c-Fos IR cells were detected in rostral VTA (VTAR) and parabrachial pigmental area (PBP), as well as in areas that overlap the retromammillary nucleus (RM), interfascicular nucleus (IF) and rostral linear nucleus (RLi) (Figures 2 and 3). NIC and NIC-PM also produced intense activation of cells in medial prefrontal cortex (MPFC). Activated cells were also observed in the core and shell components of nucleus accumbens (AcbC, AcbSh). In addition to the mesocorticolimbic pathways, multiple other CNS sites were activated by both NIC and NIC-PM.
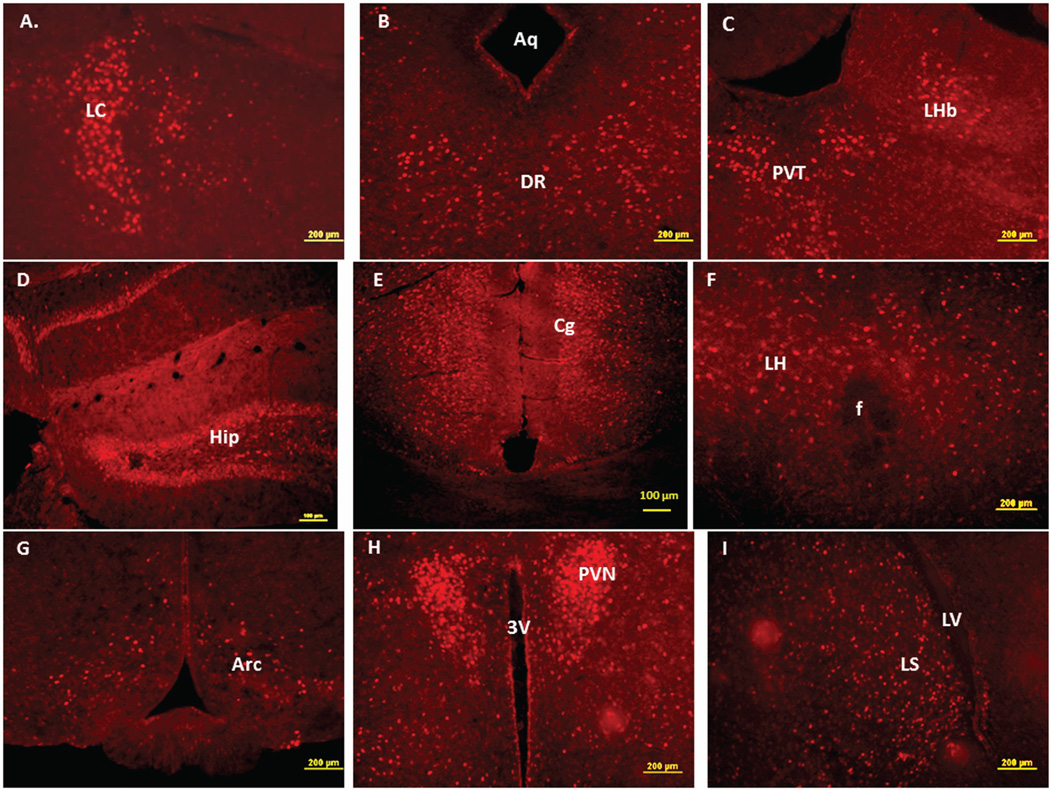
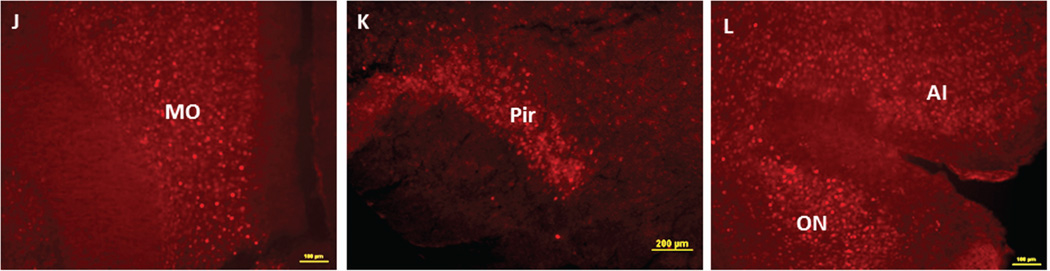
Figure 4 demonstrates NIC-PM induced c-Fos immunoreactivity in representative brain regions. At the pontine medullary junction, c-Fos IR cells were detected at areas corresponding to locus coeruleus (LC), laterodorsal tegmental nucleus (LDTg) and pedunculotegmental nucleus (PTg). More rostrally, intense c-Fos expression was seen in pontine nucleus (Pn), periaqueductal gray (PAG), dorsal raphe nucleus (DRN), superior colliculus (SC) and entorhinal cortex (Ent) (Figures 1 and 4). Scattered c-Fos expression was also observed in areas that overlap the anterior and vental tegmental nucleus (ATg, VTg). In addition to the VTA, other midbrain regions exhibiting c-Fos immunoreactivity were the dentate gyrus (DG), C1, C2, and C3 areas of hippocampus, amygdala (Amyg) and red nucleus (RN) (Figures 1 and 4). NIC-PM also produced intense stimulation of visual and somatosensory cortex (VC, SC) as well as various areas of cingulate cortex including medial prefrontal areas; i.e., prelimbic cortex (A32) and infralimbic cortex (A25). Activated cells were also observed in areas overlapping different nuclei of hypothalamus (HP) including arcuate hypothalamic nucleus (Arc), dorsomedial hypothalamic nucleus (DMN), anterior hypothalamic nucleus (AHN), paraventricular hypothalamic nucleus (PVN), medial preoptic nucleus (MPO), as well as lateral and posterior hypothalamic areas (LH, PH). Other areas strongly stimulated by NIC-PM included paraventricular thalamic nuclei (PVT), lateral habenular nucleus (LHb), lateral septal nucleus (LS), bed nucleus of stria terminalis (BST), nucleus of ventral limb of diagonal band (VDB), piroform cortex (Pir), angular insular cortex (AI), medial and lateral orbital cortex (MO, LO), anterior olfactory nucleus (AON) and olfactory tubercle (Tu).
Figure 4.
Immunofluorescence staining in the mouse brain demonstrating representative c-Fos activated regions following intraperitoneal injection of the peripherally-acting nicotine analog, nicotine pyrrolidine methiodide (NIC-PM, 30 µg/kg). A: Locus coeruleus (LC). B: dorsal raphe nucleus (DRN). C: Paraventricular thalamic nucleus (PVT) and lateral habenular nucleus (LHb). D: Hippocampus (Hip). E: Cingulate cortex (Cg). F: Lateral hypothalamus (LH). G: Arcuate hypothalamic nucleus (Arc). H: Paraventricular hypothalamic nucleus (PVN). I: Lateral septal nucleus (LS). J: Medial orbital cortex (MO). K: Piriform cortex (Pir). L: Angular insular cortex (AI) and olfactory nucleus (ON). The data obtained for the NIC treatment at the above representative sites were qualitatively identical to those obtained for the NIC-PM treatment.
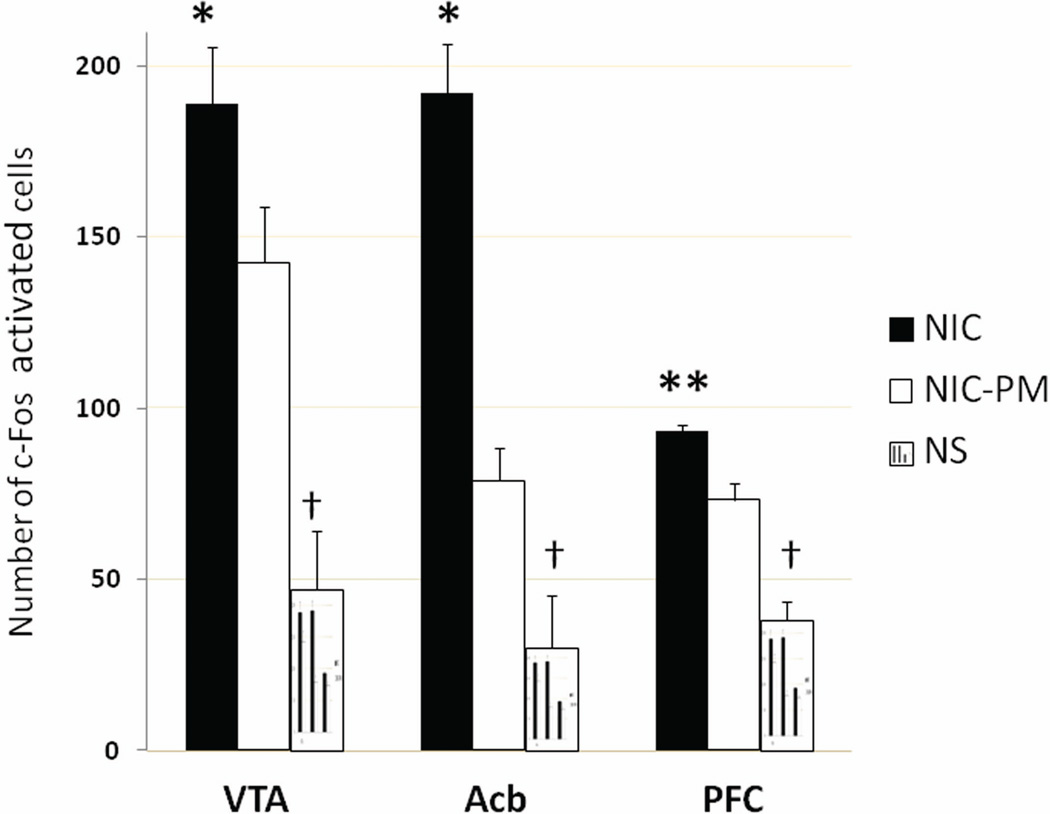
Semi-quantitative estimation of the number of c-Fos activated cells in VTA, Acb and PFC demonstrated significantly greater activation by NIC than by NIC-PM (Figure 5). The number of c-Fos activated cells was also significantly greater for the NIC and NIC-PM treatments compared to the NS control ((P<0.001). At VTA, the number of NIC activated cells was 189.0 ± 16.4 vs. 142.5 ± 16.2 for NIC-PM (P=0.05). At Acb, the number of NIC activated cells was 192.3 ± 14.4 vs. 78.8 ± 9.5 for NIC-PM (P=0.03) and at PFC, the number of NIC activated cells was 93.3 ± 1.9 vs. 73.3 ± 4.6 for NIC-PM (P<0.001).
Figure 5.
Nicotine-induced activation of c-Fos at three representative sites in the mouse brain. Bars represent the mean (± standard error) number of c-Fos activated cells counted in ventral tegmental area (VTA), nucleus accumbens (Acb) and prefrontal cortex (PFC). c-Fos immunoreactivity was assessed following intraperitoneal administration of normal physiological saline (NS, control), nicotine hydrogen tartrate salt (NIC) or its peripherally-acting analog nicotine pyrrolidine methiodide (NIC-PM). Differences between the NS control and NIC or NIC-PM treatments were significant at †P<0.001 and between NIC and NIC-PM were significant at *P≤0.05, **P<0.001.
DISCUSSION
The present study demonstrates that NIC-PM, a peripherally-acting nicotine analog, acts through nAChRs present on afferents of somatic and visceral sensory nerve fibers, to stimulate cells at multiple sites throughout the brain. These include cells in brain regions overlapping the mesocorticolimbic reward pathways, and in areas implicated in tobacco-related arousal, cognition, memory, stress, and interoceptive awareness (Panagis et al., 2000; Kirouac et al., 2005; Parsons et al., 2006; Levin et al., 2006; Guillem et al., 2011; Verdejo-Garciaa et al., 2012; Flawin and Winder, 2013; Ramirez et al., 2014). The findings that virtually all the brain areas stimulated by nicotine actions on peripherally- and centrally-located nAChRs, were also activated by NIC-PM, suggests that the sensory impact of nicotine may be critical in mediating its direct CNS effects. These anatomical data are supported by previous studies demonstrating that hexamethonium, a peripherally-acting nicotine antagonist, drastically decreased EEG and EMG responses to nicotine (Lenoir and Kiyatkin, 2011; Lenoir et al., 2013). The immediate EEG responses to nicotine were also strongly inhibited during urethane anesthesia (Lenoir and Kiyatkin, 2011). Importance of the sensory impact of nicotine has also been demonstrated in our previous human studies showing that blockade of sensory afferents in the upper respiratory tract by local anesthetics and by peripheral nicotinic receptor antagonists makes human smoking much less rewarding (Rose et al., 1985, 1999).
Numerous other studies have used methiodides of nicotine as probes to distinguish the central and peripheral actions of nicotine (Domino, 1965; Geller et al., 1971; Shechter and Rosecrans, 1972; Thompson et al., 1972; Tang and Kiyatkin, 2011; Lenoir and Kiyatkin, 2011; Lenoir et al., 2013). The present study using NIC-PM to differentiate the peripheral and central actions of nicotine, is based on the assumption that NIC-PM, which is a highly polar molecule, does not cross the blood-brain barrier and that NIC-PM has nicotine-like properties. This assumption is based on previous studies in mice demonstrating that radiolabeled NIC-PM penetrates the brain poorly (Aceto et al., 1983). The inability of NIC-PM to enter the brain has also been confirmed using highly-sensitive mass spectrometric methods (Lenoir et al., 2013). NIC-PM has also been shown to have potent nicotine-like properties (Gillis and Lewis, 1956). NIC and NIC-PM were both found to be equipotent in their pressor effects in cats (Barlow and Dobson, 1955), dogs (Larson and Haig, 1943) and monkeys (Zuo et al., 2009). Furthermore, CNS administration of NIC-PM in mice and rats is reported to produce antinociceptive effects comparable to those of nicotine (Aceto et al., 1983). Therefore, based on these studies, we assume that NIC-PM mimics the peripheral effects of NIC and that the c-Fos activation following NIC-PM administration, in the present study, is primarily due to interactions of nicotine with peripheral nAChRs.
The peripheral actions of nicotine are also believed to act as interoceptive cues capable of eliciting nicotine-like physiological and neural responses after repeated nicotine exposure. This notion is based on animal studies demonstrating that peripheral sensory stimuli associated with NIC-PM produce weak responses in drug naïve conditions. However, the same stimuli produce strong physiological and neural effects when injected after animals have nicotine experience (Lenoir et al., 2013). Numerous other studies have shown that pairing between exteroceptive environmental and/or peripheral interoceptive stimuli and a natural reinforcer (e.g., nicotine) results in classical conditioning and plays an important role in drug addiction (Razran, 1961; Gauvin et al., 1993; Bernstein and Koh 2007; Verdejo-Garcia et al., 2012). Importance of the sensory impact of nicotine has also been documented by studies demonstrating that damage to the insular cortex, a critical neural hub of interoceptive awareness, can lead to disruption of smoking behavior (Naqvi et al., 2007). In the present study, NIC and NIC-PM activated cells in cingulate, insular, olfactory (Pir AON and Tu) and orbitofrontal cortexes. These areas are implicated in processing conscious emotion via representation of body interoceptive states (Naqvi et al., 2007; Naqvi and Bechara, 2010; Kutlu et al., 2013).
NIC and NIC-PM Induced c-Fos Activation in VTA
In the present study, we observed a mild c-Fos response to NIC and/or NIC-PM in areas overlapping VTA, an important center for reward-seeking behavior (Schultz et al., 1997; Wise, 2009; Schultz, 2010; Ikemoto, 2010; De Biasi and Dani, 2011). However, VTA is known to receive excitatory synaptic inputs from a wide range of structures including various brainstem nuclei (Phillipson, 1979; Mejías-Aponte et al., 2009), PAG (Geisler et al. 2007; Moreira et al., 2009), MPFC (Takahata and Moghaddam, 2003; Kalivas et al., 2009), hypothalamus (Kempadoo et al., 2013), BST (Georges and Aston-Jones, 2001, 2002), LS (Talishinsky et al., 2012), Acb (Usuda et al., 1998) and SC (Comoli et al., 2003). All of these structures were shown to be activated by both NIC and NIC-PM. Intense activation of cells at MFPC by NIC and /or NIC-PM confirms numerous other studies regarding the importance of this cortical control center in induction of burst activity in VTA (Gariano and Groves, 1988; Tong et al., 1996; Nisell et al., 1997; Takahata and Moghaddam, 2003; Kalivas, 2009). Dopaminergic neurons of VTA are known to receive direct and indirect glutamatergic innervations from MPFC and enhancement of this pathway underlies drug addiction (Takahata and Moghaddam, 2003; Kalivas, 2009). PAG, the third largest subcortical source of glutamate input to VTA (Geisler et al. 2007) was also strongly stimulated by NIC and NIC-PM. The importance of this structure in reward and addictive properties of nicotine is not known. However, PAG is thought to supply VTA neurons with information important for processing nociceptive signals, defensive and stress behaviors (Moreira et al., 2009).
NIC and NIC-PM Induced c-Fos Activation of Brainstem Nuclei
The brainstem structures known to project to VTA and also activated by both NIC and NIC-PM were LDTg, PTg, LC and SC (Phillipson, 1979; Mejías-Aponte et al, 2009). Of these structures, LDTg and PTg comprise cholinergic, glutamatergic and GABAergic neurons and these three transmitters are expressed in the projections to VTA (Hallanger and Wainer, 1988; Oakman et al., 1995; Maskos, 2008; Ishibashi et al, 2009). The involvement of LDTg/PTg in nicotine addiction has been demonstrated in previous studies wherein a normally functioning LDTg was reported to be necessary for the burst firing of dopaminergic VTA neurons (Lodge and Grace, 2006) and that PTg lesions reduce nicotine self-administration behaviors (Lanca et al. 2000; Picciotto and Corrigall, 2002). VTA neurons are also shown to be modulated by adrenergic agents and LC, the main source of norepinephrine in the brain, was also found to be activated by NIC and NIC-PM (Phillipson, 1979; Mejías-Aponte et al., 2009). The exact role of LC in the nicotine reward and addiction circuitry is not known. However, based on its known functions, LC may provide VTA with signals related to arousal, cognition and stress associated with nicotine use (Samuels and Szabadi, 2008; Okere and Waterhouse, 2013). SC is another brainstem structure activated by NIC and NIC-PM. SC relays information about visual stimuli to VTA dopaminergic neurons (Comoli et. al., 2003; Overton et. al., 2014). The importance of Pn, another brainstem nucleus with intensive c-Fos IR after administering NIC and NIC-PM, in reward and addiction properties of nicotine is not clear. However, Pn is known to be critical in eyeblink conditioning in an associative learning paradigm (Villarreal and Steinmetz, 2005) and nicotine is widely believed to convey its reinforcing properties upon tobacco-related stimuli through associative learning (Gould and Davis, 2008).
NIC and NIC-PM Induced c-Fos Activation of Hypothalamic and Thalamic Nuclei
Among the hypothalamic and thalamic structures that were activated by NIC and NIC-PM, the LH, PVN, PVT and LHb are particularly important because of their roles in nicotine addiction (Kirouac et al., 2005; Parsons et al., 2006; Balcita-Pedicino, 2011; Martin-Fardon and Boutrel, 2012; Mahler et al., 2012). Orexin/hypocretin neurons of LH project to reward-associated brain regions such as Acb and VTA and these projections are important in relaying interoceptive-related signals to VTA (Peyron et al., 1998; Fadel and Deutch, 2002; Harris et al., 2005). Orexin/hypocretin neurons of PVN also play roles in the reinforcing and aversive properties of nicotine, as well as in the aversive properties of nicotine withdrawal (Maler et al., 2012). Of the thalamic nuclei, PVT contributes to the effects of nicotine on arousal and cognition and receives major orexin/hypocretin projections from LH (Kirouac et al., 2005; Parsons et al., 2006; Martin-Fardon and Boutrel, 2012). LHb, an epithalamic nucleus activated by NIC and NIC-PM, is thought to be a component of an anti-reward circuitry which inhibits VTA neurons via GABAergic projections to rostromedial tegmental nucleus (Balcita-Pedicino, 2011).
NIC and NIC-PM Induced c-Fos Activation of Other Limbic Structures
Other brain structures in the neurocircuitry of addiction which were activated by NIC and NIC-PM include hippocampus, LS and BST (Kenney and Gould, 2008; Luo et al., 2011; Sartor and Aston-Jones, 2012; Falvin and Winder, 2013). Nicotine addiction is considered to be a disorder of learning and memory and the hippocampus is a critical center for learning and memory (Gould, 2006; Gould and Leach, 2014; Ramirez et al., 2014). Nicotine alters various forms of hippocampus-dependent learning and memory through various long-lasting effects on hippocampal synaptic function (Gould, 2006; Gould and Leach, 2014). The hippocampus also receives input from midbrain dopaminergic cells and, in turn, projects to the prefrontal cortex and ventral striatum, areas involved in impulse control, decision-making, and reward evaluation (Kelley, 2004; Jones and Bonci, 2005). Intense c-Fos IR was also seen in BST, a component of the extended amygdala (ExtA). This nucleus plays critical roles in responses to stress, anxiety and reward (Falvin and Winder, 2013). BST projects to, and exerts a strong excitatory influence on the firing of dopaminergic neurons within VTA (Georges and Aston-Jones, 2001, 2002) and these projections appear to be necessary for learning to associate drug rewards with specific environmental cues (Dumont et al., 2005). LS, a nucleus associated with stress and drug addiction, was another structure exhibiting intense c-Fos IR (Olds & Milner, 1954; Sheehan et al., 2004; Luo et al., 2011; Sartor and Aston-Jones, 2012). That LS is part of the reward network is supported by previous studies on selfstimulation (Olds & Milner, 1954). LS has strong projections to LH-orexin neurons of the hypothalamus and to the midbrain regions (Risold and Swanson, 1997; Yoshida et al., 2006; Sartor and Aston-Jones., 2012 Talishinsky el al., 2012). Furthermore, LS has been associated with processing contextual information and sending this information to other brain regions for expression of drug reward-related behaviors (Luo et al., 2011; Sartor and Aston-Jones., 2012).
Conclusion
The present study provides the first neuroanatomical data demonstrating that peripherally-mediated sensory effects of nicotine are capable of eliciting neuronal activation at multiple levels of the drug addiction-reward circuitry in the mouse brain. The areas activated by NIC-PM overlapped those stimulated by NIC. Consistent with nicotine’s actions on both central and peripheral nAChRs, semi-quantitative analysis demonstrated that a significantly greater number of cells in VTA, Acb and PFC were activated by nicotine than by its peripherally-acting analog NIC-PM. The widespread and overlapping activation of multiple brain areas by both nicotine and its peripherally-acting analog implies that the neurotransmitters and brain circuitry mediating the effects of nicotine on the addiction-reward pathways are much more diverse and complex than previously thought. Since these peripherally mediated effects always precede the direct central action of nicotine, they may condition (prepare) the brain for yet-to-come important reward signaling. The conjunction of anatomical sites where peripheral actions of nicotine overlap with sites known to be important for reinforcement may encourage conditioned associations between sensory cues and the reinforcing effects of nicotine, thereby promoting addiction.
ACKNOWLEDGMENTS
This work was supported by a grant from Philip Morris USA; the company had no role in the design and execution of the study, data analysis or publication of results. The authors acknowledge use of the resources provided by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number G12MD007597.
Grant Information: Supported by a grant from Philip Morris USA
ABBREVIATIONS
- Aca
Anterior Commissure, Anterior Part
- AcbC
Accumbens Nucleus, Core Region
- AcbSh
Accumbens Nucleus, Shell Region
- AI
Angular Insular Cortex
- AIP
Agranular Insular Cortex, Posterior Part
- Amyg
Amygdala
- AO
Anterior Olfactory Nucleus
- APir
Amygdalopiriform Transition Area
- Arc
Arcuate Nucleus
- ArcM
Arcuate Hypothalamic Nucleus
- ATg
Anterior Tegmental Nucleus
- Cg
Cingulate cortex
- cp
Cerebral Peduncle
- CPu
Caudate Putamen (Striatum)
- DRN
Dorsal raphe nucleus
- D3V
Dorsal 3rd Ventricle
- Ent
Entorhinal Cortex
- f
Fornix
- fmi
Forceps Minor of the Corpus Callosum
- fr
Fasciculus Retroflexus
- Hip
Hippocampus
- HP
Hypothalamus
- IP
Interpeduncular Nucleus
- IPC
Interpeduncular Nucleus
- LC
Locus Coeruleus
- LDTg
Laterodorsal Tegmental Nucleus
- LHb
Lateral Habenular Nucleus
- LS
Lateral Septal Nucleus
- LV
Lateral Ventricle
- ml
Medial Lemniscus
- MM
Medial Mamillary Nucleus
- MO
Medial Orbital Cortex
- MPFC
Medial Prefrontal Cortex
- MPO
Medical Preoptic Nucleus
- NS
Normal physiological saline
- OC
Orbital Cortex
- ON
Olfactory Nucleus
- PAG
Periaqueductal Gray
- PH
Posterior Hypothalamic Nucleus
- PIF
Parainterfascicular Nucleus of the Ventral Tegmental Area
- Pir
Piriform Cortex
- PN
Paranigral Nucleus of the Ventral Tegmental Area
- Pn
Pontine Nuclei
- PSC
Primary Somatosensory Cortex
- PVT
Paraventricular Thalamic Nucleus
- PVN
Paraventricular hypothalamic nucleus
- Py
Pyramidal Cell Layer of the Hippocampus
- RPC
Red Nucleus, Parvicellular Part
- SC
Superior Colliculus
- SNR
Substantia Nigra
- VC
Visual Cortex
- VDB
Nucleus of the Vertical Limb of the Diagonal Band
- VTA
Ventral Tegmental Area
- 3V
3rd Ventricle
Footnotes
The authors declare that there are no conflicts of interest.
REFERENCES
- Aceto MD, Awaya H, Martin BR, May EL. Antinociceptive action of nicotine and its methiodide derivatives in mice and rats. Br J Pharmacol. 1983;79:869–876. doi: 10.1111/j.1476-5381.1983.tb10531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira Edna FR., Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimohammadi H, Silver WL. Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem Senses. 2000;25:61–66. doi: 10.1093/chemse/25.1.61. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RB, Dobson NA. Nicotine monomethiodide. J Pharm Pharmacol. 1955;7:27–34. doi: 10.1111/j.2042-7158.1955.tb12000.x. [DOI] [PubMed] [Google Scholar]
- Bernstein IL, Koh MT. Molecular signaling during taste aversion Learning. Chem. Senses. 2007;32:99–103. doi: 10.1093/chemse/bjj032. [DOI] [PubMed] [Google Scholar]
- Berridge MS, Apana SM, Nagano KK, Berridge CE, Leisure GP, Boswell MV. Smoking produces rapid rise of [11C]nicotine in human brain. Psychopharmacology. 2010;209:383–394. doi: 10.1007/s00213-010-1809-8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Surgeon General’s Report – The health consequences of smoking. 2004 [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, Redgrave P. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci. 2003;6:974–980. doi: 10.1038/nn1113. [DOI] [PubMed] [Google Scholar]
- Cox BM, Goldstein A, Nelson WT. Nicotine self-administration in rats. Br J Pharmacol. 1984;83:49–55. doi: 10.1111/j.1476-5381.1984.tb10118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- De Biasi M, Dani JA. Reward, Addiction, Withdrawal to Nicotine. Annu Rev Neurosci. 2011;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehkordi O, Rose JE, Balan KV, Kc P, Millis RM, Jayam-Trouth A. Neuroanatomical relationships of substance P-immunoreactive intrapulmonary C-fibers and nicotinic cholinergic receptors. J Neurosci Res. 2009;87:1670–1678. doi: 10.1002/jnr.21967. [DOI] [PubMed] [Google Scholar]
- Dehkordi O, Rose JE, Balan KV, Millis RM, Bhatti B, Jayam-Trouth A. Co-expression of nAChRs and molecules of the bitter taste transduction pathway by epithelial cells of intrapulmonary airways. Life Sci. 2010;86:281–288. doi: 10.1016/j.lfs.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Domino EF. Some comparative pharmacological actions of (−)-nicotine, its optical isomer, and related compounds. In: Von Euler US, editor. Tobacco Alkaloids and Related Compounds. London: Pergamon Press; 1965. pp. 303–313. [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology. 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Fadel J, Deutch A. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci USA. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin SA, Winder DG. Noradrenergic control of the bed nucleus of the stria terminalis in stress and reward. Neuropharmacology. 2013;70:324–330. doi: 10.1016/j.neuropharm.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano RF, Groves PM. Burst firing induced in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulate cortices. Brain Res. 1988;462:194–198. doi: 10.1016/0006-8993(88)90606-3. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Carl KL, Goulden KL, Holloway FA. Cross-generalization between drug discriminative stimuli and conditioned safety and danger cues. Exp Clin Psychopharmacol. 1993;1:133–141. [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller I, Hartmann R, Blum K. Effects of nicotine, nicotine monomethiodide, lobeline, chlordiazepoxide, meprobamate and caffeine on a discrimination task in laboratory rats. Psychopharmacol. 1971;20:355–365. doi: 10.1007/BF00403567. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis CN, Lewis JJ. The pharmacology of nicotine monomethiodide. J Pharm Pharmacol. 1956;8:46–54. doi: 10.1111/j.2042-7158.1956.tb12130.x. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Davis JA. Associative learning, the hippocampus, and nicotine addiction. Curr Drug Abuse Rev Pages. 2008;1:9–19. doi: 10.2174/1874473710801010009. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Mol Neurobiol. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Leach PT. Cellular, molecular, and genetic substrates underlying the impact of nicotine on learning. Neurobiol Learn Mem. 2014;107:108–132. doi: 10.1016/j.nlm.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Ni D, Lee LY. Expression of nicotinic acetylcholine receptors in rat pulmonary sensory neurons. Respir Physiol Neurobiol. 2008;161:87–91. doi: 10.1016/j.resp.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, Spijker S, Mansvelder HD. Nicotinic acetylcholine receptor beta2 subunits in the medial prefrontal cortex control attention. Science. 2011;333:888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Wainer BH. Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;274:483–515. doi: 10.1002/cne.902740403. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Leonard CS, Kohlmeier KA. Nicotinic activation of laterodorsal tegmental neurons: implications for addiction to nicotine. Neuropsychopharmacology. 2009;34:2529–2547. doi: 10.1038/npp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, deB Frederick B, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel DL, Denlinger RL, Dermody SS, Hatsukami DK, Benowitz NL, Donny EC. Very low nicotine content cigarettes and potential consequences on cardiovascular disease. Curr Cardiovasc Risk Rep. 2012;6:534–541. doi: 10.1007/s12170-012-0266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;25:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56:169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci. 2013;338:7618–7626. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059:179–188. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Kutlu MG, Burke D, Slade S, Hall BJ, Rose JE, Levin ED. Role of insular cortex D1 and D2 dopamine receptors in nicotine self-administration in rats. Behavioural Brain Research. 2013;256:273–278. doi: 10.1016/j.bbr.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lança AJ, Adamson KL, Coen KM, Chow BL, Corrigall WA. The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience. 2000;96:735–742. doi: 10.1016/s0306-4522(99)00607-7. [DOI] [PubMed] [Google Scholar]
- Larson PS, Haig HB. Studies on the fate of nicotine in the body. III. On the pharmacology of some methylated and demethylated derivatives of nicotine. J Pharm Exp Ther. 1943;77:343–349. [Google Scholar]
- Lenoir M, Kiyatkin EA. Critical role of peripheral actions of intravenous nicotine in mediating its central effects. Neuropsychopharmacology. 2011;36:2125–2138. doi: 10.1038/npp.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Tang JS, Woods AS, Kiyatkin EA. Rapid sensitization of physiological, neuronal, and locomotor effects of nicotine: Critical role of peripheral drug actions. J Neurosci. 2013;33:9937–9949. doi: 10.1523/JNEUROSCI.4940-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci USA. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Boutrel B. Orexin/hypocretin (Orx/Hcrt) transmission and drug-seeking behavior: is the paraventricular nucleus of the thalamus (PVT) part of the drug seeking circuitry? Front Behav Neurosci. 2012;6:75. doi: 10.3389/fnbeh.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol. 2008;153:S438–S445. doi: 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejías-Aponte CA, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: Prominent inputs from medullary homeostatic centers. J Neurosci. 2009;29:3613–3626. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Campos AC, Lisboa SF, Terzian AL, Resstel LB, Guimarães FS. Antiaversive effects of cannabinoids: Is the periaqueductal gray involved? Neural Plast. 2009:625469. doi: 10.1155/2009/625469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Chergui K, Grillner P, Svensson TH. Chronic nicotine enhances basal and nicotine-induced Fos immunoreactivity preferentially in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 1997;17:151–161. doi: 10.1016/S0893-133X(97)00040-7. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okere CO, Waterhouse BD. Nicotine withdrawal upregulates nitrergic and galaninergic activity in the rat dorsal raphe nucleus and locus coeruleus. Neurosci Lett. 2013;536:29–34. doi: 10.1016/j.neulet.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Overton PG, Vautrelle N, Redgrave P. Sensory regulation of dopaminergic cell activity: Phenomenology, circuitry and function. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.01.023. pii: S0306-4522(14)00035-9. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology. 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Panagis G, Hildebrand BE, Svensson TH, Nomikos GG. Selective c-fos induction and decreased dopamine release in the central nucleus of amygdala in rats displaying a mecamylamine-precipitated nicotine withdrawal Syndrome. Synapse. 2000;35:15–25. doi: 10.1002/(SICI)1098-2396(200001)35:1<15::AID-SYN3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse. 2006;59:480–490. doi: 10.1002/syn.20264. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 4th Edition. New York: Academic Press; 2013. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Picciotto MR1, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S, Tonegawa S, Liu X. Identification and optogenetic manipulation of memory engrams in the hippocampus. Front Behav Neurosci4. 2014;7:226. doi: 10.3389/fnbeh.2013.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razran G. The observable unconscious and the inferable conscious in current Soviet psychophysiology: interoceptive conditioning, semantic conditioning, and the orienting reflex. Psychological Review. 1961;68:1–147. [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Zinser MC, Tashkin DP, Newcomb R, Ertle A. Subjective response to cigarette smoking following airway anesthetization. Addict Behav. 1984;9:211–215. doi: 10.1016/0306-4603(84)90060-1. [DOI] [PubMed] [Google Scholar]
- Rose JE, Tashkin DP, Ertle A, Zinser MC, Lafer R. Sensory blockade of smoking satisfaction. Pharmacol Biochem Behav. 1985;23:289–293. doi: 10.1016/0091-3057(85)90572-6. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology. 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Rose JE, Westman EC, Behm FM, Johnson MP, Goldberg JS. Blockade of smoking satisfaction using the peripheral nicotinic antagonist trimethaphan. Pharmacol Biochem Behav. 1999;62:165–172. doi: 10.1016/s0091-3057(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology. 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Mukhin AG, Lokitz SJ, Turkington TG, Herskovic J, Behm FM, Garg S, Garg PK. Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarette containing 11C-nicotine. PNAS. 2010;107:5190–5195. doi: 10.1073/pnas.0909184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function. Part II: Physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol. 2008;6:254–285. doi: 10.2174/157015908785777193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci. 2012;32:4623–4631. doi: 10.1523/JNEUROSCI.4561-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD, Rosecrans JA. Nicotine as a discriminative cue in rats: inability of related drugs to produce a nicotine-like cueing effect. Psychopharmacology. 1972;27:379–387. doi: 10.1007/BF00429392. [DOI] [PubMed] [Google Scholar]
- Schneider NG, Olmstead RE, Franzon MA, Lunell E. The nicotine inhaler: clinical pharmacokinetics and comparison with other nicotine treatments. Clin Pharmacokinet. 2001;40(9):661–684. doi: 10.2165/00003088-200140090-00003. Review. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex differences in neural responses to stress and alcohol context cues. Hum Brain Map. 2011;32:1998–2013. doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology. 2003;28:1117–1124. doi: 10.1038/sj.npp.1300127. [DOI] [PubMed] [Google Scholar]
- Talishinsky A, Rosen GD. Systems genetics of the lateral septal nucleus in mouse: heritability, genetic control, and covariation with behavioral and morphological traits. PLoS One. 2012;7:e44236. doi: 10.1371/journal.pone.0044236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Kiyatkin EA. Fluctuations in central and peripheral temperatures induced by intravenous nicotine: central and peripheral contributions. Brain Res. 2011;1383:141–153. doi: 10.1016/j.brainres.2011.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JH, Angulo M, Choi L, Roch M, Jenden DJ. The chronic effects of nicotine monomethiodide on gastric secretion in pylorus-ligated rats. Experientia. 1972;28:1176–1177. doi: 10.1007/BF01946153. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse. 1996;22:195–208. doi: 10.1002/(SICI)1098-2396(199603)22:3<195::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- Yerger VB. Menthol's potential effects on nicotine dependence: a tobacco industry perspective. Tobacco Control. 2011;20:ii29–ii36. doi: 10.1136/tc.2010.041970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garciaa A, Clark BL, Dunnc BD. The role of interoception in addiction: A critical review. Neurosci Biobehav Rev. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Villarreal RP, Steinmetz JE. Neuroscience and learning: Lessons from studying the involvement of a region of cerebellar cortex in eyeblink classical conditioning. J Exp Anal Behav. 2005;84:631–652. doi: 10.1901/jeab.2005.96-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacol Biochem Behav. 1996;53:309–315. doi: 10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S, Barik J, Dickinson J, Jones IW. Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. J Mol Neurosci. 2006;30:137–140. doi: 10.1385/JMN:30:1:137. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Gorres A, Seehaus A, Naumer MJ. Sensory modality of smoking cues modulates neural cue reactivity. Psychopharmacology (Berl) 2013;225:461–471. doi: 10.1007/s00213-012-2830-x. [DOI] [PubMed] [Google Scholar]
- Yerger VB, McCandless PM. Menthol sensory qualities and smoking topography: a review of tobacco industry documents. Tobacco Control. 2011;20:ii37–ii43. doi: 10.1136/tc.2010.041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucar EY, Araz O, Yilmaz N, Akgun M, Meral M, Kaynar H, Saglam L. Effectiveness of pharmacologic therapies on smoking cessation success: three years results of a smoking cessation clinic. Multidisciplinary Respiratory Medicine. 2014;9:9. doi: 10.1186/2049-6958-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;49:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Lu H, Vaupel B, Zhang Y, Yang Y, Stein EA. Neuronal sensitization precedes tolerance to repeated nicotine challenges following overnight withdrawal in chronic nicotine-treated rats. Society of Neuroscience. 2009 Abstracts 447.15. [Google Scholar]