Abstract
Objective
The protein degrading activity of Cathepsin C, combined with its role in leukocyte granule activation, suggests a contribution of this cystein protease in atherosclerosis. However, no experimental data are available to validate this concept.
Approach and results
CatC gene and protein expression were increased in ruptured versus advanced stable human carotid artery lesions. To assess causal involvement of CatC in plaque progression and stability, we generated LDLr−/−//CatC−/− chimeras by bone marrow transplantation. CatC−/− chimeras presented attenuated plaque burden in carotids, descending aorta, aortic arch and root, at both the early and advanced plaque stage. CatC was abundantly expressed by plaque macrophages and foam cells. CatC expression and activity was dramatically downregulated in plaques of CatC−/− chimeras, supporting a hematopoietic origin of plaque CatC. Our studies unveiled an unexpected feedback of CatC deficiency on macrophage activation programs and T helper cell differentiation in as much as that CatC expression was upregulated in M1 macrophages, whereas its deficiency led to combined M2 (in vitro) and Th2 polarization (in vivo).
Conclusions
Our data implicate CatC has a role in the selective tuning of innate and adaptive immune responses, relevant to a chronic immune disease such as atherosclerosis.
Introduction
Cathepsin C (CatC), also known as dipeptidyl peptidase I, is a lysosomal cystein protease that belongs to the papain super family 1. Unlike cathepsins S and K, it is expressed in many tissues, but highest in lymphoid organs such as spleen 2 and homologues have been identified in a variety of species, suggesting an important and widespread role 2–6. In mice, CatC is most abundantly expressed in lung, liver, spleen, and small and large intestines; intermediately expressed in bone marrow, thymus, and stomach, and low expression in kidney, heart and brain 7. CatC has a unique aminodipeptidyl peptidase activity 2 and can progressively remove N-terminal dipeptides from various protein substrates and as such participates in post-translational processing. Indeed, studies in CatC knock-out mice have revealed a central function in the activation of granule serine proteases in cytotoxic T lymphocytes and natural killer cells (granzymes A and B), mast cells (chymase and tryptase) and neutrophils (cathepsin G, proteinase 3 and elastase) 2, 8–11. Furthermore, alveolar macrophage and mast cell derived CatC were seen to cleave extracellular matrix proteins such as fibronectin and collagen types I, III and IV, suggestive of a role of CatC in airway remodeling of chronic airway diseases such as asthma 12. Finally, a contribution of CatC in coagulation as plasminogen 13 and thrombin regulator 14 and in angiogenesis have been documented 15.
CatC deficient mice appear healthy but have defects in serine protease activities in multiple hematopoietic lineages 9, and show unexpected resistance to sepsis as compared to their wild type littermates, possibly by attenuated tryptase dependent IL-6 cleavage 16. Likewise, CatC−/− mice are protected against acute arthritis by reducing neutrophil recruitment to the joints, as well as by modulating the neutrophil production of cytokines and possibly chemokines 8, 17.
Its immunomodulatory effects on mast cells, macrophages and neutrophils, next to its intrinsic proteolytic capacity points to a role of CatC in inflammatory vascular remodeling processes. Indeed CatC was seen to regulate neutrophil recruitment and CXCL12 production in elastase-induced abdominal aortic aneurysm formation 18. While several cathepsins, such as cathepsin S 19, cathepsin K 20 and cathepsin L 21, have already been implicated in atherosclerosis, the impact of CatC in its pathophysiology remains elusive, apart from its identification as a sensitive “vascular injury marker” in rabbits with experimental hypertension and cholesterol fed mini-pigs 22, 23.
Here, we show increased CatC gene and protein expression in advanced compared with ruptured carotid human atherosclerotic plaques, mainly localizing in macrophages. Furthermore, we provide evidence for an attenuated atherogenic response in LDLr deficient mice with hematopoietic deficiency of CatC, via a selective tuning of innate and adaptive immune responses.
Materials and Methods
Materials and Methods are available in the online-only Supplement
Results
CatC is differentially expressed in ruptured human atherosclerotic plaques
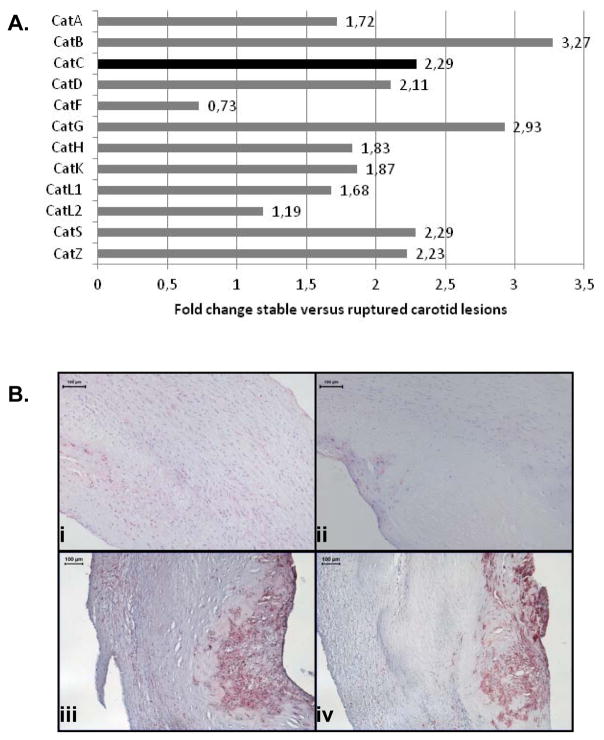
In a candidate approach using microarray analysis, the Cathepsin family was identified as differentially expressed between stable and ruptured segments of the same plaque (all p<0.001) (Fig. 1A). Giving its immunomodulatory effects, proteolytic capacity and unknown role in atherosclerosis, we focused our follow-up research on CatC. Protein expression was validated on a series of early, stable and ruptured carotid plaques (Fig. 1B). CatC expression localized to the same areas with abundant CD68+ macrophages presence (Fig. 1B, panel iii and iv). CatC was expressed significantly higher in ruptured plaques compared with both stable (P<0.05) and early plaques (P<0.05) (Supplement Fig. I).
Figure 1.
A: Cathepsin family members were differently expressed in human ruptured carotid endarterectomies, as determined by microarray analysis. Fold changes stable versus ruptured lesion, all p<0.0001. B: Immunohistochemical staining of human carotid atherosclerotic lesions using CatC and CD68 antibodies. Panel (i): representing CatC expression in an early lesion, panel (ii): CatC expression in a stable lesion, panel (iii): CatC expression in a ruptured lesion, panel (iv): parallel section of panel (iii) showing that CatC expression localizes to the same areas of intense CD68-positive staining cells.
LDLr−/− mice with hematopoietic CatC deficiency display reduced atherosclerosis
To address the causal involvement of CatC in atherogenesis, we generated LDLr−/− chimeras with hematopoietic CatC deficiency by a bone marrow transplantation strategy. After recovery mice were put on a high fat diet (HFD) for 13 weeks and equipped with semiconstrictive carotid artery collars after 6 weeks of HFD to induce lesion formation.
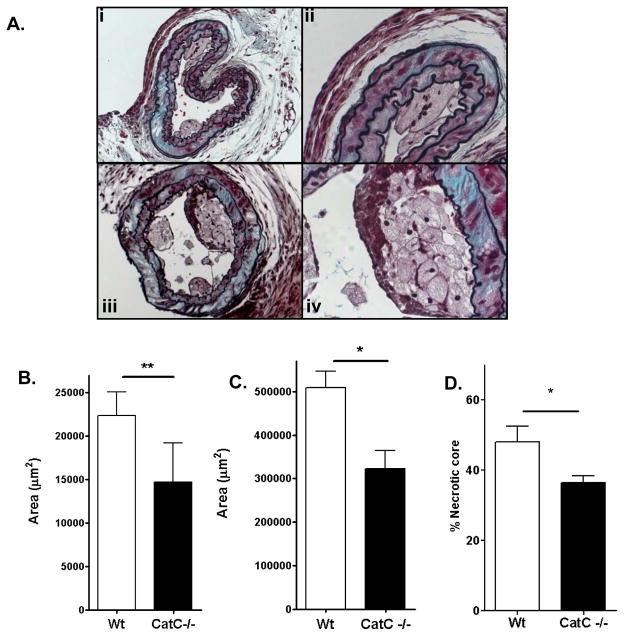
Body weight and plasma total cholesterol levels did not differ between groups throughout the experiment (Supplement Fig. II). At point of sacrifice, lesion formation was analyzed at four sites: the common carotid artery, the aortic arch, the aortic root (all cross sectional), and the descending aorta (en face). Carotid artery plaques were 55% smaller in CatC−/− chimeras compared to wildtype (WT) controls (P<0.05) (Fig. 2B), and had smaller lipid core areas (Supplement Fig. III-B). A representative picture of the carotid lesions is shown in Fig. 2A.
Figure 2.
Plaque analysis of mice lesions. A : Movat’s staining of CatC−/− (i) and WT (iv) lesions. Panel (ii) shows the presence of multinucleated cells in a lesion from a CatC−/− chimeric mouse, panel (iii) the presence of a fibrous cap in a lesion from a WT mouse. Further analysis is shown in B: plaque area of collar-induced atherosclerotic lesions in the carotid artery, C: plaque area of advanced lesions in the aortic root. D:necrotic core area of advanced lesions in the aortic root * = p<0.05, ** p=<0.01.
The aortic arch showed a very similar pattern with reduced plaque size in CatC−/− chimeras. Plaque area of the early lesions was decreased in the CatC−/− chimeras (p=0.009 Supplement Fig. IV-A), whereas advanced lesion size did not differ between groups (Supplement Fig. IV-B). Consequently, the early and total plaque burden (mean number of aortic arch lesions per mouse) was reduced in CatC−/− chimeras, but advanced lesion burden was unaltered (Supplement Fig. IV-C). Given the observations in the advanced plaques of the aortic arch, an additional location was investigated: the lesions of the aortic root. In the advanced lesions of the aortic root, both lesion size (P <0.05, Fig. 2C) and necrotic core content (P<0.05, Fig. 2D) were smaller in the Cat C−/− chimeras). En face analysis of the thoracic and abdominal aorta showed the scanty presence of lipid deposits that had not progressed beyond the early stage. Consistent with the carotid and aortic arch data, the WT group presented a larger area of initial ‘fatty streak’ lesions compared to the CatC−/− chimeras (p=0.009, Supplement Fig. IV-D).
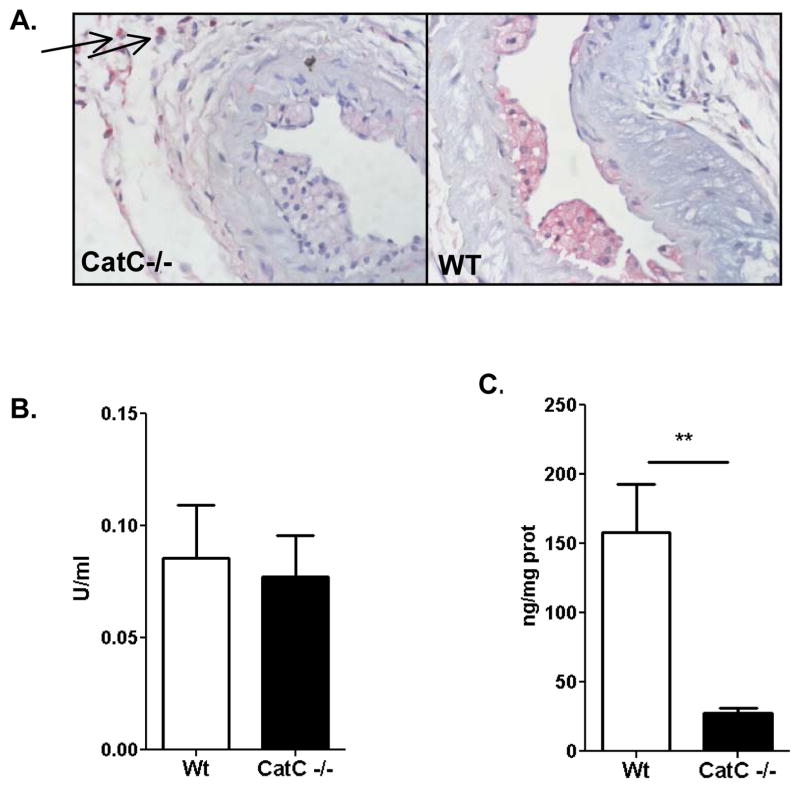
Further morphometric analysis of the carotid lesions did not show any effects of hematopoietic CatC deficiency on lumen area, media area, and total vessel area (data not shown). Zooming in on plaque composition, we first quantified the contribution of hematopoietic CatC to total plaque CatC. Clearly almost all plaque CatC was derived from leukocytes, as CatC expression was almost abrogated in CatC−/− chimera plaques, whereas it was predominant in foam cells and macrophages of WT plaques (Fig. 3A, Supplement Fig. III-A). In fact while 93% of WT plaques showed robust CatC staining, only 18% of that of CatC−/− chimeras did (P<0.01). Of interest, we did observe scanty CatC expression in the carotid adventitia, co-localizing with granular cells possibly mast cells, and resident macrophages (see arrows) (Fig. 3A). In concert with the low plaque CatC expression in CatC−/− chimeras, CatC activity was extremely low in aortic arch tissue homogenates of CatC−/− chimeras, while elastolytic activity was comparable to WT (Fig. 3B–C).
Figure 3.
Further analysis of the collar-induced atherosclerotic lesions in the carotid artery, A: Expression of CatC, as determined by immunohistochemistry in CatC−/− and WT lesions, arrows point to positive cells in the adventitia in CatC −/− lesions, B: Elastolytic activity, C: CatC activity in vivo in lysate of the aortic arch. **= p<0.01.
Compatible with the reduced plaque progression, CatC−/− chimera carotid lesions were almost devoid of lipid cores that were prominent in plaques of WT controls (P<0.01). In fact 10/14 WT mice had lipid cores compared to only 1/11 CatC−/− chimeras (p=0.002, Supplement Fig. III-B). Lesions from CatC−/− chimeras tended to have lower collagen content (p=0.165, Supplement Fig. III-C), but did not differ in CD45+ leukocyte content (not shown), Mac3+ macrophage (Supplement Fig. III-D), and CD3+ T cell content (Supplement Fig. III-E). Mast cells were present in the plaque adventitia, at numbers that did not differ between the groups. Plaque granulocytes tended to be increased in WT mice (8/14 plaques) versus CatC−/− chimeras (3/11 plaques), but this effect did not reach statistical significance (p=0.22). Essentially similar findings were obtained for aortic arch plaques with borderline statistical significance in plaque granulocyte content (number of granulocytes in WT aortic arch lesions 0.53 ± 0.22, in CatC−/− aortic arch lesions 0.00 ± 0.00, NS (P=0.053)).
Effects of CatC deficiency on monocyte/macrophage function
The profound reduction in plaque size, the rather subtle effects on plaque composition and the abundant expression of CatC by plaque macrophages, foam cells and granulocytes, prompted further study of monocyte/macrophage function in CatC deficiency. We found that CD11b+Ly6G− blood monocyte levels tended to be increased in CatC−/− mice, albeit not significantly (Supplement Fig. V-A).
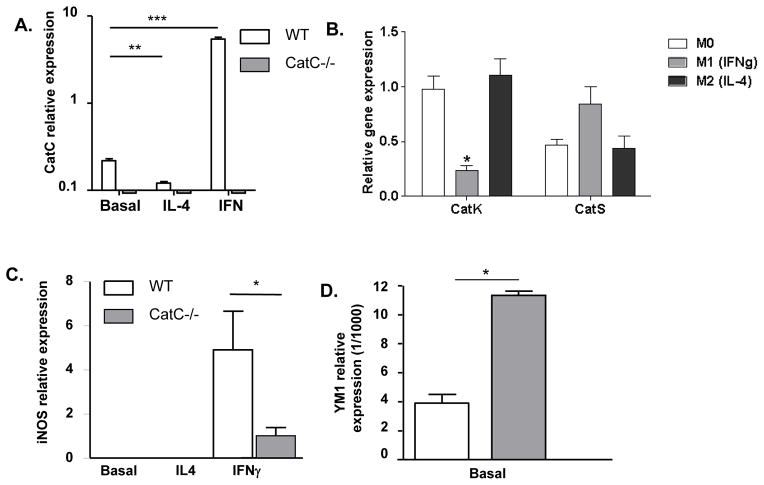
As the number of macrophages in plaques did not differ significantly between WT and CatC−/− chimeras, we next examined whether CatC expression affects or is affected by macrophage polarization. As expected, CatC−/− bone marrow-derived macrophages (BMDM), whether at baseline (M0) or IL-4 (M2) or IFN-gamma primed (M1), did not express CatC (Fig. 4A). However, CatC expression is upregulated >20-fold in WT M1 macrophages (P<0.001), and, conversely, downregulated by >70% in WT M2 (P<0.01) (Fig. 4A). Thus CatC appears to be a selective M1 marker.
Figure 4.
A: Relative mRNA expression of CatC in bone-marrow- derived macrophages (BMDM) of donor WT and CatC−/− mice (n=4) in basal conditions and after stimulation with IL-4 and IFNγ in vitro. B: Relative mRNA expression (Q-PCR) of CatK and CatS after M1 and M2 macrophage polarization. Relative mRNA expression of iNOS (C), in BMDM in basal conditions and after stimulation with IL-4 and IFNγ in vitro, YM-1 expression is shown in basal conditions only (D), donor WT and CatC−/− mice (n=4). * p<0.05, **= p<0.01, ***p<0.001
Cathepsin K (CatK) and Cathepsin S (CatS) were next measured to verify if any of these proteases are overexpressed to compensate for CatC deficiency, and potentially contribute to the CatC phenotype. The results obtained show that CatS but not CatK expression is slightly but significantly increased in CatC−/− chimeras (P<0.001) (Supplement Fig. V-B and V-C). CatK and CatS gene expression was also assessed in WT versus CatC deficient macrophages, polarized to M0, M1 and M2. CatS expression was not affected by M1 or M2 polarization, while CatK was downregulated in M1 macrophages (Fig. 4B). However, CatC deficiency did not impact macrophage CatK expression at activity level, as macrophage CatK activity was similar in M1 and M2 macrophages of both Wt and CatC−/− BMDM (Supplement Fig. V-D). This suggests that a differential regulation at the posttranscriptional level resulted in comparable CatK activity levels in M1 and M2 macrophages in vitro.
The observation that CatC is selectively expressed in M1 macrophages raised the question whether CatC may have a regulatory role in macrophage polarization and in this way could impact atherosclerosis. CatC−/− BMDM showed greatly reduced induction of the M1 markers iNOS (P<0.05) (Fig. 4C), IL-18 (Supplement Fig. V-E, in IL4 and IFN-gamma stimulated BMDM; P<0.05), and TNF-alpha (Supplemental Fig. V-F, P<0.05). In contrast, we observed increased expression of the M2 marker YM1 (baseline) in CatC−/− BMDM (Fig. 4D, P<0.05), supporting the hypothesis that M2 polarization in vitro is favored in the absence of CatC. To further investigate this phenomenon, immunohistochemical stainings for a M1 marker (iNOS) and a M2 marker (Arg-1) were performed on the initial collar-induced carotid lesions and advanced aortic root lesions. M1 macrophages were more dominant both in early and advanced lesions, this in contrast to a report of Khallou-Laschet et al, that showed a pre-dominance of M2 macrophages in early lesions 24. An increased expression of the M2 marker was evident in the advanced aortic root lesions (Supplement Fig. VI). There was no difference in M1 and M2 presence in the carotid and aortic root lesions of the WT and CatC−/− chimeras. Thus, despite the clear in vitro M2 phenotype of CatC−/− BMDM, this did not lead to a higher presence of M2 macrophages in the lesions of the CatC−/− chimeras, albeit that we used a limited panel of M1/M2 markers and assessed only 1 (end) timepoint.
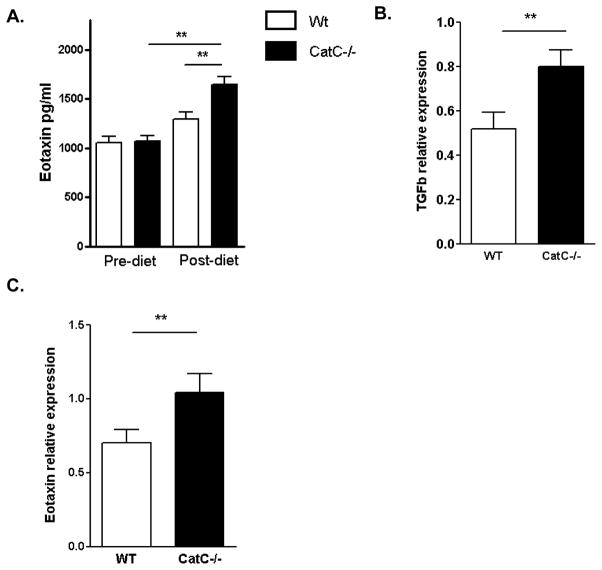
Congruent with the M2 skewed macrophage polarization observed in CatC deficiency, of the 7 cytokines detectable in plasma of WT and CatC−/− chimeras, on chow and HFD, only eotaxin levels differed between both groups (P<0.01). HFD upregulated plasma eotaxin in CatC−/− (P<0.01), but not WT chimeras (Fig. 5A). Moreover, TGF-beta as well as eotaxin expression by spleen was increased in CatC−/− chimeras (P<0.01; Fig. 5B–C).
Figure 5.
A: Eotaxin cytokine levels in serum pre- and post-diet (sacrifice) were determined by LUMINEX (pg/ml) in WT (n=15) and CatC−/− chimeric mice (n=11), B–C: relative mRNA expression of TGF-beta (B) and Eotaxin (C) in spleen of WT (n=15) and CatC−/− chimeric mice (n=11). ** p<0.01
Macrophage polarization is a major determinant of the cell’s phagocytotic capacity. However, the CatC dependent shift in macrophage polarity was not accompanied by an altered ability to ingest or accumulate DiI labeled LDL or oxLDL (Supplement Fig. VII-A), excluding that CatC deficiency affects macrophage lipid uptake. In contrast, CatC−/− BMDM showed a marginal decreased uptake of apoptotic Jurkat cells at baseline compared to WT mice (P=0.053, Supplement Fig. VII-B); intriguingly phagocytosis by CatC−/− BMDM was largely un-responsive to LPS challenge: whereas LPS increased efferocytosis of WT BMDM by 30.4% (P<0.003), it had little effect on CatC−/− BMDM (6.8%, P=0.38, Supplement Fig. VII-B). Consequently, a significantly higher phagocytosis index was observed in WT compared with CatC−/− BMDM (P<0.05, Supplement Fig. VII-B).
In addition, when testing cell migration/tissue repair in BMDM, we found no difference between the groups in any of the conditions tested (no stimulation, LPS stimulation, data not shown).
Altogether, the above data point to autocrine positive feedback of CatC in macrophage polarization towards M1.
Peripheral effects on T-cell function
The increased eotaxin and TGF-beta expression in CatC deficient mice might also be reflective of or affect Th2 skewed T-cell responses. We did observe increased CD4 and lower CD8 numbers in lymph node and blood with CatC deficiency. Hence CD4/CD8 ratios were elevated in both tissues of CatC−/− chimeras (P<0.001 and P<0.05, respectively) (Supplement. Fig. VIII-A-C). Moreover, relative expression of GATA3 (Th2 marker) was 2-fold increased (P<0.01), whereas that of TBX21 (Th1 marker) was unchanged (Supplement Fig. IX-A and IX-D). Of note, Foxp3 expression was also increased, albeit weakly (Supplement Fig. IX-B), which is in accordance with the flow cytometry data showing enrichment in spleen of CatC−/− chimeras of CD4+CD25+Foxp3+ cells (P<0.05), as well as CD4+CD25+Foxp3− activated T-cells (Supplement Fig. XIII-D and XIII-E).
Discussion
CatC is reported to exert immunomodulatory effects on mast cells, macrophages and neutrophils, which next to its intrinsic proteolytic capacity points to a potential role of CatC in inflammatory vascular remodeling processes such as atherosclerosis. We show here that CatC is not only upregulated with lesion progression in human atherosclerotic lesions, but may also be causally involved in the pathogenesis of this disease. Hematopoietic CatC deficiency led to attenuated lesion formation, in the carotid artery, the aortic arch and the descending aorta in our mouse model. While CatC expression appeared to be reflective of macrophage M1 polarization, it surprisingly fostered an autocrine positive feedback loop for M1 polarization in vitro. Combined with the CatC deficiency associated Th2 skewing, this effect may well be responsible for the observed phenotype.
In our study, the results pointed to a T cell dependent effect of leukocyte CatC deficiency in lesion development. This is in contrast to the effect of Cat C deficiency in a model of collagen-induced arthritis17. Here, CatC deficiency reduced the development of CIA, independently of T cell and B cell function. Specific measures of humoral immunity (IgG1 and IgG2a anticollagen antibodies) were comparable in WT and CatC−/− mice as was ConA induced T cell proliferation. These are specific parameters of T and B cell function, which do not exclude difference in the parameters that were assessed in our atherosclerosis study. In addition, in our model, T cell proliferation was comparable in the WT and CatC−/− chimeric mice (data not shown). Thus, the mechanism via which CatC modulate disease outcome may differ in distinctive pathologies.
The majority of cathepsin family members showed upregulation in an advanced yet stable segment compared to a ruptured segment of a carotid lesion of the same patient. CatC was among the top three of upregulated cathepsins (Fig. 1A). CatC was also significantly upregulated on the protein level in ruptured lesions and was largely confined to inflammatory foci within the plaque. To study the role of CatC, a mouse model of atherogenesis was used. In the collar-induced atherosclerotic lesion model, a semi-constrictive but non-stenotic collar is placed around the carotid artery in a hyperlipidemic background. The lesions are the result of perturbed hemodynamics, and not of restenosis. Several papers have shown that the processes and mechanisms that are involved in collar-induced atherosclerosis are also involved in longitudinal, age-induced models of atherosclerosis; i.e. inflammation, lipid metabolism, and intraplaque hemorrhage 25–29. Mouse models ‘model’ human atherogenesis, but show inherent differences with the human pathophysiology 30, the most striking of which being the virtual absence of plaque rupture, therefore this study focused on the role of CatC in early and more advanced stages of disease progression. Our experimental mice study suggested that CatC deficiency (or therapeutic intervention in its activity) during atherogenesis may be beneficial. Given limitations intrinsic to the animal model, extrapolating these findings to the human situation should be done cautiously, especially as CatC proteolysis could contribute to processes that are relevant to vulnerable plaque formation and rupture (e.g. hemorrhage, coagulation, cap erosion, fibrinolysis). Also, human ruptured plaques likely have entered an “active wound-healing” phase, and the overexpressed CatC might well be involved in the healing process after plaque rupture. Importantly, in the mouse model, the effect of CatC deficiency on disease progression was not only based on collar-induced atherosclerotic lesions, but also on other locations that were investigated (aortic arch and descending aorta, aortic root). Collectively these data suggested that CatC deficiency affected lesion progression, with some site specificity, as the effect in the advanced lesions of the aortic arch was less evident. CatC was abundantly expressed in mouse plaques, where it co-localized mainly with foam cells and plaque macrophages. As intimal CatC expression and CatC activity were almost absent in chimeric mice with hematopoietic CatC deficiency, we may infer that plaque CatC is of leukocyte origin.
The reduced plaque formation during CatC deficiency was similar to murine intervention studies with other cathepsin family members such as CatS and CatK 19, 20. Similar to leukocyte CatS deficiency, CatC deficiency appeared to prevent plaque progression beyond the early macrophage-rich stage. CatC deficiency did not affect CatK expression but slightly induced CatS expression. As the chromosomal location of both genes (chr.3, 3 F2.1) differs from that of CatC (chr.7), CatS upregulation may be compensatory, rather than a transgenesis artifact.
The lack of effect of CatC deficiency on plaque collagen content led us to investigate peripheral effects of CatC on leukocyte function. Since no overt differences were seen in granulocyte and monocyte counts, we focused on effects of CatC on monocyte/macrophage differentiation. CatC expression was downregulated in IL-4 primed macrophages and upregulated in IFN-gamma primed macrophages. In this regard the CatC expression profile mimics that of established M1 markers such as IL-19 and iNOS, and the proinflammatory proteases MMP9 and MMP12. CatC expression was previously reported to be responsive to IRF8 and PU.1, suggesting a link to macrophage differentiation and interferon signaling 31. The most intriguing finding however was that CatC deficiency dampened M1 but not M2 polarization in vitro, the net effect being macrophage skewing towards M2. Although macrophage motility on a gelatin matrix was not affected during CatC inhibition, efferocytosis of apoptotic cells by CatC deficient macrophages was impaired during pro-inflammatory conditions. The CatC deficiency-associated reduction in necrotic core expansion seems to confirm that apoptotic cell handling is not notably defective in CatC−/− plaque macrophages. The blunted LPS response on efferocytosis in CatC deficiency is interesting, and may suggest interference with TLR signaling, in analogy to the previously reported inhibition of TLR9 signaling by CatK 32 of for protein kinases 33. Direct evidence for CatC for such interaction is however lacking. Despite the clear in vitro polarization phenotype, a concomitant shift in local plaque macrophage polarization was not observed, although this was assessed using a single M1 and M2 marker and only at 1 (end) timepoint. This is possibly related to the overt dominance of M1 macrophages in early and more advanced stages of plaque development, as also reported by Stöger et al. 34. Of note, this does not exclude that CatC deficiency could be associated with a dampened M1 polarization stage in plaques.
In relation to the M2 shift in vitro, increased eotaxin plasma levels and a concomitant increase in spleen eotaxin and TGF-beta expression were noted. Eotaxin is generally viewed as prototypical Th2 cytokine 35, and compatible with this notion CatC deficiency led to reduced CD4/CD8 ratios in blood and spleen and elevated GATA3, but not TBX21, expression in spleen T cells. Whether Th2-favored immune responses ameliorate or worsen atherosclerosis is still subject to controversy 36, thus consequently whether the CatC deficiency-associated Th2 shift affords atheroprotection remains to be determined.
Of note, as shown by Devadas et al., a critical step in Th2/Th1 cell differentiation involves selective granzyme B mediated apoptosis of Th2 cells 37. Hence, leukocyte deficiency in CatC, a key enzyme in granzyme activation, could well favor survival of Th2 cells. While conceivable, further studies will be required to establish this pathway. The CD4+ T cell pool was enriched in CD25+Foxp3+ Treg numbers and in agreement, FoxP3 expression by spleen T cells was elevated as well. As demonstrated in numerous studies, Tregs display atheroprotective activity (for a review see 38). Whether or not this effect is directly attributable to CatC deficiency remains to be elucidated.
In conclusion, we are the first to show that CatC gene and protein expression is upregulated in atherosclerotic lesions of both mice and man. Its expression appears confined to inflammatory foci within the plaque, and in particular involve intimal foam cells and macrophages. CatC deficiency led to an impaired atherogenic response. While effects of CatC deficiency on plaque matrix composition seemed subtle, our studies unveiled an unexpected feedback effect on macrophage activation programs in vitro, as well as on T helper cell differentiation. In that regard, our findings could open the way for selective tuning of innate and adaptive immune responses, relevant to chronic immune diseases such as atherosclerosis.
Supplementary Material
Significance section.
Cysteine cathepsins are a family of proteases involved in protein turnover and extracellular matrix (ECM) degradation. Given their central role in ECM remodeling, cathepsins have been linked to several diseases such as osteoporosis, cancer and cardiovascular disease. Cathepsin (Cat) C has a unique aminodipeptidyl peptidase activity and has a role in leukocyte granule activity. While there is considerable interest for the role of the Cathepsin family members, like CatS, CatK, CatL in vascular diseases, there is no data yet on the role of CatC in experimental atherosclerosis. In this study, it was shown that CatC deficiency attenuated plaque progression in mice with atherosclerosis. CatC deficiency had unexpected effects on macrophage activation programs and T helper cell differentiation. These data suggest that CatC has a role in the selective tuning of innate and adaptive immune responses, relevant to a chronic immune disease such as atherosclerosis.
Acknowledgments
none
Sources of funding: This work was supported by a grant from the National Institutes of Health (NIH AI049261) to Dr Pham.
Abbreviations
- Cat
Cathepsin
- BMDM
bone marrow-derived macrophages
- HFD
High fat diet
- WT
wildtype
Footnotes
Disclosure: none
References
- 1.Rao NV, Rao GV, Hoidal JR. Human dipeptidyl-peptidase I. Gene characterization, localization, and expression. J Biol Chem. 1997;272:10260–10265. doi: 10.1074/jbc.272.15.10260. [DOI] [PubMed] [Google Scholar]
- 2.McGuire MJ, Lipsky PE, Thiele DL. Purification and characterization of dipeptidyl peptidase I from human spleen. Arch Biochem Biophys. 1992;295:280–288. doi: 10.1016/0003-9861(92)90519-3. [DOI] [PubMed] [Google Scholar]
- 3.Hola-Jamriska L, Tort JF, Dalton JP, Day SR, Fan J, Aaskov J, Brindley PJ. Cathepsin C from Schistosoma japonicum--cDNA encoding the preproenzyme and its phylogenetic relationships. Eur J Biochem. 1998;255:527–534. doi: 10.1046/j.1432-1327.1998.2550527.x. [DOI] [PubMed] [Google Scholar]
- 4.Hutchinson DW, Tunnicliffe A. The preparation and properties of immobilised dipeptidyl-aminopeptidase I (cathepsin C) Biochim Biophys Acta. 1987;916:1–4. doi: 10.1016/0167-4838(87)90203-2. [DOI] [PubMed] [Google Scholar]
- 5.Klemba M, Gluzman I, Goldberg DE. A Plasmodium falciparum dipeptidyl aminopeptidase I participates in vacuolar hemoglobin degradation. J Biol Chem. 2004;279:43000–43007. doi: 10.1074/jbc.M408123200. [DOI] [PubMed] [Google Scholar]
- 6.Molgaard A, Arnau J, Lauritzen C, Larsen S, Petersen G, Pedersen J. The crystal structure of human dipeptidyl peptidase I (cathepsin C) in complex with the inhibitor Gly-Phe-CHN2. Biochem J. 2007;401:645–650. doi: 10.1042/BJ20061389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pham CT, Armstrong RJ, Zimonjic DB, Popescu NC, Payan DG, Ley TJ. Molecular cloning, chromosomal localization, and expression of murine dipeptidyl peptidase I. Journal of Biological Chemistry. 1997;272:10695–10703. doi: 10.1074/jbc.272.16.10695. [DOI] [PubMed] [Google Scholar]
- 8.Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109:363–371. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci U S A. 1999;96:8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolters PJ, Pham CT, Muilenburg DJ, Ley TJ, Caughey GH. Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Biol Chem. 2001;276:18551–18556. doi: 10.1074/jbc.M100223200. [DOI] [PubMed] [Google Scholar]
- 11.Turk D, Janjic V, Stern I, Podobnik M, Lamba D, Dahl SW, Lauritzen C, Pedersen J, Turk V, Turk B. Structure of human dipeptidyl peptidase I (cathepsin C): exclusion domain added to an endopeptidase framework creates the machine for activation of granular serine proteases. Embo J. 2001;20:6570–6582. doi: 10.1093/emboj/20.23.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolters PJ, Laig-Webster M, Caughey GH. Dipeptidyl peptidase I cleaves matrix-associated proteins and is expressed mainly by mast cells in normal dog airways. Am J Respir Cell Mol Biol. 2000;22:183–190. doi: 10.1165/ajrcmb.22.2.3767. [DOI] [PubMed] [Google Scholar]
- 13.Nauland U, Rijken DC. Activation of thrombin-inactivated single-chain urokinase-type plasminogen activator by dipeptidyl peptidase I (cathepsin C) Eur J Biochem. 1994;223:497–501. doi: 10.1111/j.1432-1033.1994.tb19018.x. [DOI] [PubMed] [Google Scholar]
- 14.Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J Exp Med. 2003;198:423–431. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muramatsu M, Katada J, Hayashi I, Majima M. Chymase as a proangiogenic factor. A possible involvement of chymase-angiotensin-dependent pathway in the hamster sponge angiogenesis model. J Biol Chem. 2000;275:5545–5552. doi: 10.1074/jbc.275.8.5545. [DOI] [PubMed] [Google Scholar]
- 16.Mallen-St Clair J, Pham CT, Villalta SA, Caughey GH, Wolters PJ. Mast cell dipeptidyl peptidase I mediates survival from sepsis. J Clin Invest. 2004;113:628–634. doi: 10.1172/JCI19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Y, Pham CT. Dipeptidyl peptidase I regulates the development of collagen-induced arthritis. Arthritis Rheum. 2005;52:2553–2558. doi: 10.1002/art.21192. [DOI] [PubMed] [Google Scholar]
- 18.Pagano MB, Bartoli MA, Ennis TL, Mao D, Simmons PM, Thompson RW, Pham CT. Critical role of dipeptidyl peptidase I in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc Natl Acad Sci U S A. 2007;104:2855–2860. doi: 10.1073/pnas.0606091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Nooijer R, Bot I, von der Thüsen JH, et al. Leukocyte cathepsin S is a potent regulator of both cell and matrix turnover in advanced atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:188–194. doi: 10.1161/ATVBAHA.108.181578. [DOI] [PubMed] [Google Scholar]
- 20.Lutgens E, Lutgens S, Faber B, Heeneman S, Gijbels M, de Winther M, Frederik P, van der Made I, Daugherty A, Sijbers A. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113:98–107. doi: 10.1161/CIRCULATIONAHA.105.561449. [DOI] [PubMed] [Google Scholar]
- 21.Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, Duramad P, Sun C, Zhang Y, Yang X, Peters C, Shi GP. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115:2065–2075. doi: 10.1161/CIRCULATIONAHA.107.688523. [DOI] [PubMed] [Google Scholar]
- 22.Lojda Z. The importance of protease histochemistry in pathology. Histochem J. 1985;17:1063–1089. doi: 10.1007/BF01002534. [DOI] [PubMed] [Google Scholar]
- 23.Lojda Z, Petrasko M, Havrankova E, Lojda L. Early post-natal development of the brush border enzymes of enterocytes in the rat and mini-pig. Histochem J. 1984;16:364–369. doi: 10.1007/BF01002851. [DOI] [PubMed] [Google Scholar]
- 24.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston A-T, Clement M, Dussiot Ml, Levillain O, Graff-Dubois Sp, Nicoletti A. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bot I, Bot M, van Heiningen SH, van Santbrink PJ, Lankhuizen IM, Hartman P, Gruener S, Hilpert H, van Berkel TJ, Fingerle J. Mast cell chymase inhibition reduces atherosclerotic plaque progression and improves plaque stability in −/− mice. Cardiovascular research. 2011;89:244–252. doi: 10.1093/cvr/cvq260. [DOI] [PubMed] [Google Scholar]
- 26.Bot M, Bot I, Lopez-Vales R, van de Lest CH, Saulnier-Blache JS, Helms JB, David S, van Berkel TJ, Biessen EA. Atherosclerotic lesion progression changes lysophosphatidic acid homeostasis to favor its accumulation. The American journal of pathology. 2010;176:3073–3084. doi: 10.2353/ajpath.2010.090009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Nooijer R, Verkleij C, Von der Thüsen J, Jukema J, van der Wall E, van Berkel TJ, Baker A, Biessen E. Lesional overexpression of matrix metalloproteinase-9 promotes intraplaque hemorrhage in advanced lesions but not at earlier stages of atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:340–346. doi: 10.1161/01.ATV.0000197795.56960.64. [DOI] [PubMed] [Google Scholar]
- 28.von der Thüsen JH, van Berkel TJ, Biessen EA. Induction of rapid atherogenesis by perivascular carotid collar placement in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Circulation. 2001;103:1164–1170. doi: 10.1161/01.cir.103.8.1164. [DOI] [PubMed] [Google Scholar]
- 29.Zadelaar ASM, Thüsen JH, Boesten MLS, Hoeben RC, Kockx MM, Versnel MA, van Berkel TJ, Havekes LM, Biessen LEA, van Vlijmen BJ. Increased vulnerability of pre-existing atherosclerosis in ApoE-deficient mice following adenovirus-mediated Fas ligand gene transfer. Atherosclerosis. 2005;183:244–250. doi: 10.1016/j.atherosclerosis.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 30.Bentzon JF, Falk E. Atherosclerotic lesions in mouse and man: is it the same disease? Current opinion in lipidology. 2010;21:434–440. doi: 10.1097/MOL.0b013e32833ded6a. [DOI] [PubMed] [Google Scholar]
- 31.Tamura T, Thotakura P, Tanaka TS, Ko MS, Ozato K. Identification of target genes and a unique cis element regulated by IRF-8 in developing macrophages. Blood. 2005;106:1938–1947. doi: 10.1182/blood-2005-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asagiri M, Hirai T, Kunigami T, et al. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science. 2008;319:624–627. doi: 10.1126/science.1150110. [DOI] [PubMed] [Google Scholar]
- 33.Meng F, Lowell CA. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med. 1997;185:1661–1670. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stöger L, Gijbels M, van der Velden S, Manca M, van der Loos C, Biessen E, Daemen M, Lutgens E, de Winther M. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225:461–468. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Miyagaki T, Sugaya M, Fujita H, Ohmatsu H, Kakinuma T, Kadono T, Tamaki K, Sato S. Eotaxins and CCR3 interaction regulates the Th2 environment of cutaneous T-cell lymphoma. J Invest Dermatol. 2010;130:2304–2311. doi: 10.1038/jid.2010.128. [DOI] [PubMed] [Google Scholar]
- 36.Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol. 2006;26:2421–2432. doi: 10.1161/01.ATV.0000245830.29764.84. [DOI] [PubMed] [Google Scholar]
- 37.Devadas S, Das J, Liu C, Zhang L, Roberts AI, Pan Z, Moore PA, Das G, Shi Y. Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity. 2006;25:237–247. doi: 10.1016/j.immuni.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Mallat Z, Ait-Oufella H, Tedgui A. Regulatory T cell responses: potential role in the control of atherosclerosis. Curr Opin Lipidol. 2005;16:518–524. doi: 10.1097/01.mol.0000182532.11512.90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.