Abstract
The 13C labeling patterns in glutamate and glutamine from brain tissue are quite different after infusion of a mixture of 13C-enriched glucose and acetate. Two processes contribute to this observation, oxidation of acetate by astrocytes but not neurons, and preferential incorporation of α-ketoglutarate into glutamate in neurons, and incorporation of α-ketoglutarate into glutamine in astrocytes. The acetate:glucose ratio, introduced previously for analysis of a single 13C NMR spectrum, provides a useful index of acetate and glucose oxidation in the brain tissue. However, quantitation of relative substrate oxidation at the cell compartment level has not been reported. A simple mathematical method is presented to quantify the ratio of acetate to glucose oxidation in astrocytes, based on the standard assumption that neurons do not oxidize acetate. Mice were infused with [1,2-13C]acetate and [1,6-13C]glucose, and proton decoupled 13C NMR spectra of cortex extracts were acquired. A fit of those spectra to the model indicated that 13C-labeled acetate and glucose contributed approximately equally to acetyl-CoA (0.96) in astrocytes. Since this method relies on a single 13C NMR spectrum, it can be readily applied to multiple physiologic and pathologic conditions.
INTRODUCTION
Energy-providing substrates are oxidized in the brain at varying rates depending on concentration, nutritional state, age and disease (Schurr 2002, Rothman et al. 1999, Medina & Tabernero 2005, Nehlig & Pereira de Vasconcelos 1993). The biochemical pathways involved in metabolism of each substrate also differ in the two major metabolic compartments. Energy production in neurons is derived primarily from oxidation of glucose (van den Berg & Garfinkel 1971, Gruetter et al. 2001, Oz et al. 2004), whereas oxidation of acetate and glucose as well as net synthesis of glutamate and glutamine occur in astrocytes (Martinez-Hernandez et al. 1977, Waniewski & Martin 1998, Mason et al. 1995). Since astrocytes support neuronal energy production and play an essential role in metabolism of glutamate released from neurons, a major goal has been to quantify biochemical processes in astrocytes separate from neurons. 13C NMR spectra acquired over time have been analyzed to measure the rates of oxidation of various 13C-labeled energy substrates in both astrocytes and neurons in cerebral cortex (Rothman et al. 1999, Gruetter et al. 2001, Shen 2013, Sibson et al. 2001, Jeffrey et al. 2013, Lanz et al. 2014). Although highly informative, kinetic data are difficult to acquire. A popular alternative has been the analysis of a single high-resolution 13C NMR spectrum obtained from tissue extracts where it is a simple matter to analyze spin-coupled multiplets.
One consistent finding has been the differences in 13C labeling of glutamate and glutamine at carbon 4 (C4) in the presence of acetate. Cerdan and colleagues attributed these differences to preferential oxidation of acetate in astrocytes, qualitatively illustrated in Figure 1 (Cerdan et al. 1990). Differences in 13C labeling of glutamate and glutamine have been confirmed in cell preparations, isolated tissues, and in vivo (Deelchand et al. 2009, Hassel et al. 1997, Marin-Valencia et al. 2012, Morris & Bachelard 2003, Haberg et al. 1998, Taylor et al. 1996). By presenting a mixture of [1,2-13C]acetate and [1-13C]glucose, Bachelard, Morris and colleagues (Taylor et al. 1996, Morris & Bachelard 2003) defined the acetate:glucose ratio as ([3,4,5-13C]glutamate + [4,5-13C]glutamate) / ([3,4-13C]glutamate + [4-13C]glutamate). In this analysis, labeling in positions 1 and 2 does not significantly influence the 13C NMR spectrum at the carbon 4 position of either glutamate (~34.2 ppm) or glutamine (~31.5 ppm). If 13C enrichment in position 3 is low, the ratio [4,5-13C]glutamate/[4-13C]glutamate is also used to assess the acetate:glucose ratio (Haberg et al. 1998, Kondziella et al. 2003). The approach is logical because carbon 2 of acetyl-CoA becomes carbon 4 of α-ketoglutarate in the parent compartment. Consequently, the C4 signal in downstream glutamate or glutamine encodes compartment-specific information about 13C labeling in acetyl-CoA. The “acetate:glucose ratio” is a convenient description of the 13C spectrum since it is simply the ratio of multiplets in the C4 signal. Although detection of these multiplets is consistent with current knowledge of brain metabolism, it does not provide quantitative information about oxidation of either substrate in either cell compartment.
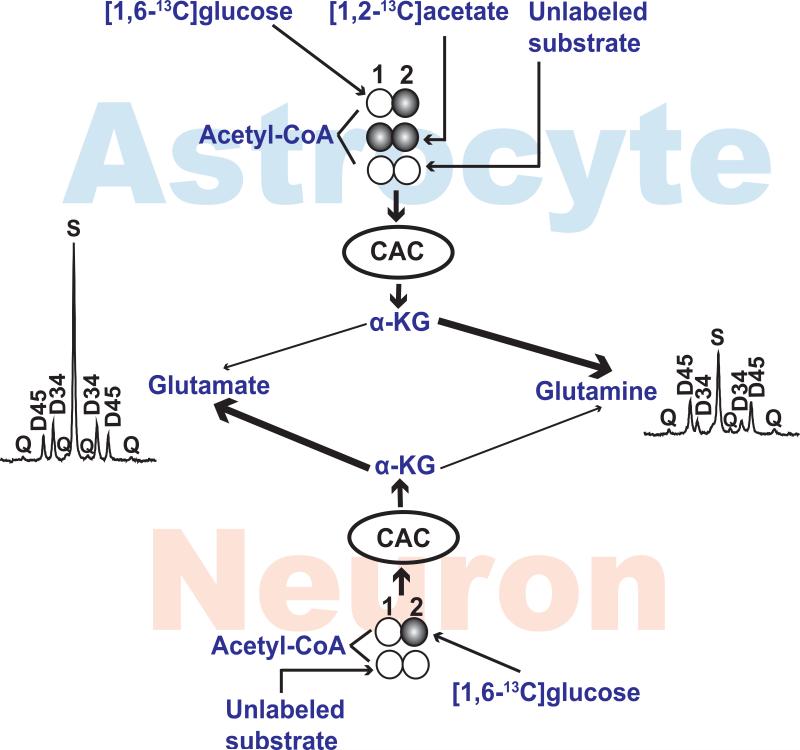
Figure 1. Metabolic Model.
The labeling patterns in acetyl-CoA of astrocyte and neurons are illustrated. Acetyl-CoA will be labeled in carbons 1 and 2 from [1,2-13C]acetate, in carbon 2 from [1,6-13C]glucose and it will be unlabeled if derived from unlabeled substrates such as ketones. Acetyl-CoA is oxidized in the citric acid cycle (CAC) to generate α-ketoglutarate. The connecting arrows do not represent detailed metabolic pathways but rather exchange of carbon backbones between α-ketoglutarate and either glutamate or glutamine. The α-ketoglutarate pool in astrocytes can contribute to both glutamate and glutamine; in this example a preferential exchange between α-ketoglutarate in astrocytes with glutamine is illustrated by the heavy line. Similarly, the α-ketoglutarate pool from neurons can exchange with both glutamate and glutamine; a dominant exchange with glutamate is shown here. Examples of 13C NMR spectra from normal mouse cortex illustrate the differences in 13C labeling in position 4. Abbreviations: CAC, citric acid cycle; α-KG, α-ketoglutarate; S, singlet; D34, doublet due to J34; D45, doublet due to J45; Q, doublet of doublets or quartet due to J34 and J45. 13C-labeled and unlabeled carbons are illustrated with filled circles and unfilled circles, respectively.
The purpose of this study was to explore whether information about sources of energy in astrocytes and neurons can be determined from a more detailed analysis of the C4 signal from glutamate and glutamine. A previous mathematical model (Malloy et al. 1987, Sherry et al. 1992, Malloyet al. 1990b, Malloy et al. 1988) was extended to accommodate two compartments based on the widely-accepted assumption that neurons do not oxidize acetate. A sensitivity analysis found that the model accurately returned the ratio of acetate oxidation relative to glucose oxidation in astrocytes. This analysis was tested in studies of normal mouse brain acquired during co-infusion of [1,2-13C]acetate plus [1,6-13C]glucose. The model provides an approach to determining the ratio of acetate to glucose oxidation in a specific cell compartment from a single 13C NMR spectrum.
DESCRIPTION OF THE MODEL
Background
Astrocytes are assumed to oxidize acetate, glucose and other substrates. Neurons are assumed to oxidize glucose and other substrates, but not acetate. In this study, [1,6-13C]glucose and [1,2-13C]acetate were available. With this combination of 13C labeling conditions, the possible 13C patterns in acetyl-CoA in astrocytes are [12C]-, [2-13C]- and [1,2-13C]acetyl-CoA. Consequently, α-ketoglutarate synthesized in astrocytes has only 3 possible labeling patterns in positions 4 and 5: unlabeled, [4-13C]α-ketoglutarate, or [4,5-13C]α-ketoglutarate. By definition, any α-ketoglutarate labeled in position 4 and 5 must have been synthesized in astrocytes. Similarly, α-ketoglutarate synthesized in neurons has only 2 possible labeling patterns in positions 4 and 5: unlabeled or [4-13C]α-ketoglutarate. [4,5-13C]α-ketoglutarate is not generated in neurons.
The covalent bond between position 3 and 4 in α-ketoglutarate is preserved regardless of any subsequent exchanges into glutamate or glutamine. Consequently, for example, if [4,5-13C]α-ketoglutarate or [3,4,5-13C]α-ketoglutarate is generated in astrocytes, the carbon skeleton may exchange into other pools of glutamate and glutamine, as well as the α-ketoglutarate pool of neurons. Regardless of the cell compartment, the labeling pattern in carbons 3, 4 and 5 is preserved and the carbon backbone of [3,4,5-13C]glutamate or [4,5-13C]glutamate arose from condensation of acetyl-CoA with oxaloacetate in astrocytes. The same statement applies to 13C labeling in glutamine.
The word “exchange” or a similar term will be used to describe the transfer of a 13C labeling pattern from α-ketoglutarate into glutamate or glutamine. The term “acetate:glucose ratio” will refer to the ratio of multiplets observed in the 13C NMR spectrum of glutamate (or glutamine) C4 obtained from brain tissue containing both neurons and astrocytes: ([3,4,5-13C]glutamate + [4,5-13C]glutamate) / ([3,4-13C]glutamate + [4-13C]glutamate) as defined earlier (Taylor et al. 1996, Morris & Bachelard 2003) (Haberg et al. 1998, Kondziella et al. 2003).
Description of 13C Labeling in Acetyl-CoA and in α-ketoglutarate
Quantitative analysis of the 13C NMR spectrum of glutamate and glutamine in terms of metabolic pathways requires a description of the 13C distribution in acetyl-CoA and in α-ketoglutarate. These variables arise simply from the possible combinations of 12C and 13C in the acetyl moiety of acetyl-CoA and in α-ketoglutarate, and are independent of any specific model of brain metabolism.
As noted above, three labeling patterns of acetyl-CoA occur in this study: unlabeled acetyl-CoA derived from unlabeled sources, [2-13C]acetyl-CoA derived from [1,6-13C]glucose, or [1,2-13C]acetyl-CoA derived from [1,2-13C]acetate. The following variables describe the 13C-labeling pattern in acetyl-CoA in astrocytes:
and in neurons:
By definition, fc0a + fc2a + fc3a = 1, and fc0n + fc2n = 1.
A description of 13C labeling in α-ketoglutarate in astrocytes and in neurons is also needed. Although there are 32 13C isotopomers of α-ketoglutarate, there are only 16 isotopomers of α-ketoglutarate in astrocytes that have 13C in position 4. The variables a – d describe the relative concentrations of these isotopomers of α-ketoglutarate (αKG) in astrocytes, normalized to a sum of 1:
Assuming that neurons do not oxidize [1,2-13C]acetate, only 8 isotopomers of αKG with 13C in carbon 4 are possible in neurons, normalized to a sum of 1:
By definition, a + b + c + d = 1 and e + f = 1.
A Model Relating Metabolic Variables to 13C Isotopomers of α-Ketoglutarate
Models of varying sophistication can be used to understand the relationship between 13C labeling in acetyl-CoA, metabolic pathways, and the observed 13C NMR spectrum of brain tissue. For the purpose of illustrating this analysis, we chose a simple model of brain metabolism, but other models could be used. Anaplerosis was defined as ya and yn for astrocytes and neurons, respectively, following earlier conventions (Malloy et al. 1988, Weinman et al. 1957, Strisower et al. 1952, Malloy et al. 1990a). The fraction of anaplerotic substrate in astrocytes that was 13C-labeled in position 3 was defined as fa3a and the fraction of anaplerotic substrate in astrocytes that was unlabeled was defined as fa0a; fa0a + fa3a = 1. This variable was provided because under these conditions, [1,6-13C]glucose may be metabolized to [3-13C]pyruvate and subsequently undergo carboxylation to oxaloacetate, an anaplerotic pathway. Since pyruvate carboxylation is considered inactive in neurons, it was assumed that all substrate for anaplerotic reactions in neurons was unenriched and therefore fa0n = 1. With this assumption, the expressions describing 13C enrichment in neurons are relatively simple (Equations 5 and 6). If flux through pyruvate carboxylase in neurons is thought to be significant, then somewhat more complex expressions should be used, analogous to equations 3 and 4. All 6 mathematically – independent metabolic variables of interest (fc3a, fc2a, fa3a, ya, fc2n, yn) should be determined to fully interpret the spectra and describe relative fluxes in both compartments.
The metabolic conditions for this experiment include multiple possible labeling patterns in acetyl-CoA, anaplerosis and metabolism of [3-13C]pyruvate (derived from [1,6-13C]glucose) via pyruvate carboxylation in astrocytes. The relations between the groups of isotopomers, a – f, and the metabolic variables were derived previously (Malloy et al. 1988, Malloy et al. 1990a). For α-ketoglutarate in astrocytes:
| [1] |
| [2] |
| [3] |
| [4] |
For α-ketoglutarate in neurons:
| [5] |
| [6] |
From these relations, it is possible to show that:
| [7] |
Relation of α-Ketoglutarate Isotopomers from Astrocytes and Neurons to the 13C NMR Spectrum
The proposed analysis assumes that 13C distribution in glutamate may be sensitive to the 13C distribution in α-ketoglutarate in either compartment. Consequently, the observed 13C isotopomers of glutamate are the sum of glutamate isotopomers arising from exchange with α-ketoglutarate in each compartment. A weighting factor, “g”, is defined as the ratio of the 13C isotopomers in glutamate from neurons relative to the 13C isotopomers in glutamate from astrocytes. The weighting factor, “h”, is defined as the ratio of the 13C isotopomers in glutamine from neurons relative to astrocytes. These variables, a – h, are lower case to emphasize that they cannot be observed directly. The next step is to show the relationship of a – h to the experimentally-observed 13C NMR spectrum.
The 13C NMR spectrum of glutamate detects glutamate in exchange with α-ketoglutarate from astrocytes plus glutamate in exchange with α-ketoglutarate from neurons, weighted by the relative contribution of each compartment. This concept is illustrated in Figure 2. Similarly, the 13C NMR spectrum of glutamine detects glutamine in exchange with α-ketoglutarate from astrocyte plus glutamine in exchange with α-ketoglutarate from neurons, weighted by the relative contribution of each compartment. The terminology used to describe the carbon-4 resonance was defined previously (Sherry et al. 1992, Malloy et al. 1988, Malloy et al. 1990b). Briefly, the C4 resonance of either metabolite is composed of 4 signals resulting from 13C-13C spin-spin coupling: a singlet (S), a doublet due to J34 (D34), a doublet due to J45 (D45) and a doublet of doublets or quartet due to J34 and J45 (C4Q). The relative areas of these multiplets sum to 1. In this notation, the letter “E” refers to multiplets in glutamate and the letter “Q” refers to multiplets in glutamine. After the letter denoting glutamate or glutamine, the abbreviations S, D34, D45 and Q refer to the singlet, doublet due to J34, doublet due to J45 and quartet or doublet of doublets, respectively. With these definitions, the multiplets in carbon 4 of the 13C NMR spectrum glutamate and glutamine are related to a – h by the following expressions:
| [8] |
| [9] |
| [10] |
| [11] |
| [12] |
| [13] |
| [14] |
| [15] |
The sum of all multiplets in C4 of glutamate or glutamine are normalized to 1 (E4S + E4D34 + E4D45 + E4Q = 1, and Q4S + Q4D34 + Q4D45 + Q4Q = 1). With these definitions, the acetate:glucose ratio in glutamate = (E4D45 + E4Q)/(E4S + E4D34) = (a+b)/(c+d+g), and the acetate:glucose ratio in glutamine = (Q4D45 + Q4Q)/(Q4S + Q4D34) = (a+b)/(c+d+h). Qualitatively these expressions are simple to interpret and consistent with previous interpretations of the acetate:glucose ratio. For example, if most glutamate molecules in the sample are in exchange with α-ketoglutarate in neurons (g >> 1), then acetate:glucose ratio in glutamate is small, meaning that glutamate exchanges with α-ketoglutarate in a compartment that does not oxidize acetate, such as neurons. If the contribution of neurons to glutamine is small (h ~ 0), then the 13C NMR spectrum of glutamine is dominated by glutamine in exchange with α-ketoglutarate in a compartment that oxidizes acetate, such as astrocytes.
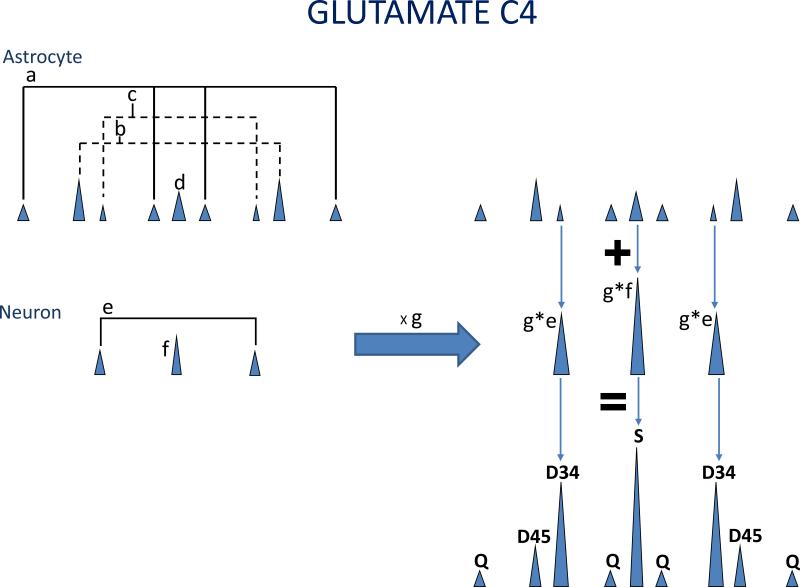
Figure 2. Weighting of the Glutamate Spectrum by Different Contributions from Astrocyte and Neurons.
The variables a – g are illustrated. Glutamate derived from astrocyte may contain up to 9 resonances in the its C4 resonance because both [1,2-13C]acetate and [1,6-13C]glucose may be oxidized in astrocyte. The relative areas of the glutamate labeled in positions 3, 4 and 5 is defined as “a”, glutamate labeled in position 4 and 5 as “b”, glutamate labeled in position 3 and 4 as “c”, and labeled in 4 only as “d”. Similarly, the relative areas of glutamate derived from neurons labeled in position 3 and 4 is defined as “e” and the glutamate labeled in position 4 only as “f”. The observed glutamate spectrum is the sum of glutamate derived from astrocyte plus the glutamate derived from neurons, weighted by the contribution from neurons, termed “g”. A similar analysis applies to interpretation of the glutamine spectrum.
MATERIALS AND METHODS
Animal Preparation, 13C Infusions and 13C NMR Spectroscopy
The spectra from normal cortex analyzed in this work have been published (Marin-Valencia et al. 2012). Briefly, normal female mice (Cre- of CB57BL/6 and FBV/N background, Jackson Laboratory, Bar Harbor) were studied. The right jugular vein was cannulated under general anesthesia. Seven days post-cannulation, mice were loosely confined to a Lucite cage to prevent ambulation. [1,6-13C]-glucose and [1,2-13C]-acetate were co-administered as a bolus of 0.2 mg/g of body weight for each tracer (in 0.2 mL saline) over 1 min, followed by a continuous infusion of 0.006 mg/g body weight/min for each tracer (in 0.375 mL saline) at 150 μL/h for 150 min to assure isotopic steady state. Animals were decapitated and the brains rapidly removed (<15 s), followed by extraction of the cerebral cortex for NMR analysis.
Least Squares Fitting and Simulations
The 13C NMR spectrum provides 6 mathematically independent measurements. From definitions or prior knowledge, 3 additional relations are known: a + b + c + d = 1, e + f = 1, and d/c = b/a. In addition, the constraint that 1 > a, b, c, d, e, f > 0, and g, h > 0, was included so that positivity conditions for all parameters are realized. In order to determine the parameters a - h that best fit the above equations, we applied the method of nonlinear least square procedure described by Nocedal and Wright (Nocedal J 1999). The sum of the squares of the difference between the actual data and their predicted values based on the chosen parameters is minimized. In order to test the proposed numerical method for finding the model parameters, a set of simulated spectra were prepared (Malloy et al. 1990a), assuming that two independent cell compartments contribute to both glutamate and glutamine. The fractional contribution of 13C labeled substrates to acetyl-CoA was allowed to range from 0–1 in each compartment. The fractional contribution of astrocytes to glutamate was allowed to vary from 0-1 with the remaining glutamate provided by neurons. A similar process was applied independently to glutamine. This synthetic data with varying noise was used as input to the computer code to recover the parameters a-h. The influence of natural abundance 13C on this analysis is considered in the discussion. The MATLAB code is provided in the supplementary information.
An example of generating simulated spectra from equations 1 – 6 is shown in Table 1. The details are described in the legend. Under these conditions, the observed 13C NMR spectrum in carbon 4 of glutamate (E4S, 0.106170; E4D34, 0.780196; E4D45, 0.040584; E4Q, 0.073050) and glutamine (Q4S, 0.127703; Q4D34, 0.362109; Q4D45, 0.182212; Q4Q, 0.327976) are easily calculated. With this set of data from a simulated spectrum, metabolic.m returns a=0.4018, b= 0.2232, c = 0.2411, and d = 0.1339. In this example, a large fraction of glutamate reflects the labeling pattern in α-KG of neurons, and a large fraction of glutamine reflects the labeling pattern in α-KG of astrocytes. The ratio of [1,2-13C]acetyl-CoA / [2-13C]acetyl-CoA in astrocytes is (a+b)/(c+d) = 1.67 = fc3a/fc2a. The program accurately recovered the 13C acetyl-CoA labeling pattern in astrocytes.
Table 1.
Example. To illustrate the model and test the fitting algorithm, it was assumed that acetyl-CoA in neurons was derived from [1,6-13C]glucose (90%) and unlabeled sources (10%); acetyl-CoA in astrocytes was derived from [1,2-13C]acetate (50%), [1,6-13C]glucose (30%) and unlabeled sources (20%); flux through pyruvate carboxylase in astrocytes was 20% of flux through citrate synthase; and 50% of the pyruvate undergoing carboxylation was derived from [1,6-13C]glucose. These assumptions correspond to fc2n = 0.9, fc3a = 0.5, fc2a = 0.3, ya = 0.2, fa3 = 0.5, and yn = 0 in equations 1 – 6. All 32 isotopomers of α-ketoglutarate in neurons or astrocytes were calculated; α-ketoglutarate with 13C in position 5 but not 4 cannot occur. The remaining possible isotopomers are shown in 6 groups depending on their appearance in the 13C NMR spectrum of C4. The sum of all possible α-ketoglutarate labeling patterns in each compartment is 1. A multiplication factor (“Factor”) was used to simulate the concentration in arbitrary units of glutamate or glutamine in exchange with each α-ketoglutarate isotopomer. For example, in neurons α-ketoglutarate labeled in position 4 and 5 is 0.81 of the total α-ketoglutarate synthesized in neurons. The total amount of glutamate in exchange with α-ketoglutarate in neurons is 0.81 × 8 = 6.48. The sum of each isotopomer group of glutamate or glutamine is shown (“Total”). In this example the amount of glutamate isotopomers labeled in position 4 but not 3 or 5 in the brain is 0.93430. This is the sum of glutamate exchanging in astrocytes (0.21430) plus glutamate exchanging in neurons (0.72). The 13C NMR spectrum that would occur in position 4 of glutamate or glutamine was calculated.
| α-Kg | glutamate | glutamine | |
|---|---|---|---|
| Neurons | Factor: 8 | Factor: 1 | |
| [12C], [1-13C], [2-13C], [1,2-13C] | 0.01000 | 0.08000 | 0.01000 |
| [3-13C], [1,3-13C], [2,3-13C], [1,2,3-13C] | 0.09000 | 0.72000 | 0.09000 |
| [4-13C], [1,4-13C], [2,4-13C], [1,2,4-13C] | 0.09000 | 0.72000 | 0.09000 |
| [3,4-13C], [1,3,4-13C], [2,3,4-13C], [1,2,3,4-13C] | 0.81000 | 6.48000 | 0.81000 |
| [4,5-13C], [1,4,5-13C], [2,4,5-13C], [1,2,4,5-13C] | 0 | 0 | 0 |
| [3,4,5-13C],[1,3,4,5-13C],[2,3,4,5-13C], [1,2,3,4,5-13C] | 0 | 0 | 0 |
| Astrocytes | Factor: 2 | Factor: 5 | |
| [12C], [1-13C], [2-13C], [1,2-13C] | 0.07142 | 0.14284 | 0.35710 |
| [3-13C], [1,3-13C], [2,3-13C], [1,2,3-13C] | 0.12858 | 0.25716 | 0.64290 |
| [4-13C], [1,4-13C], [2,4-13C], [1,2,4-13C] | 0.10715 | 0.21430 | 0.53575 |
| [3,4-13C], [1,3,4-13C], [2,3,4-13C], [1,2,3,4-13C] | 0.19287 | 0.38574 | 0.96435 |
| [4,5-13C], [1,4,5-13C], [2,4,5-13C], [1,2,4,5-13C] | 0.17857 | 0.35714 | 0.89285 |
| [3,4,5-13C],[1,3,4,5-13C],[2,3,4,5-13C], [1,2,3,4,5-13C] | 0.32142 | 0.64284 | 1.60710 |
| Total | |||
| [12C], [1-13C], [2-13C], [1,2-13C] | 0.22284 | 0.36710 | |
| [3-13C], [1,3-13C], [2,3-13C], [1,2,3-13C] | 0.97716 | 0.73290 | |
| [4-13C], [1,4-13C], [2,4-13C], [1,2,4-13C] | 0.93430 | 0.62575 | |
| [3,4-13C], [1,3,4-13C], [2,3,4-13C], [1,2,3,4-13C] | 6.86574 | 1.77435 | |
| [4,5-13C], [1,4,5-13C], [2,4,5-13C], [1,2,4,5-13C] | 0.35714 | 0.89285 | |
| [3,4,5-13C],[1,3,4,5-13C],[2,3,4,5-13C], [1,2,3,4,5-13C] | 0.64284 | 1.60710 | |
| 13C NMR spectrum | |||
| C4S | 0.106170 | 0.127703 | |
| C4D34 | 0.780196 | 0.362109 | |
| C4D45 | 0.040584 | 0.182212 | |
| C4Q | 0.073050 | 0.327976 | |
Statistical Analysis
Goodness-of-fit for the fitting program was calculated by finding sum of squares of the residuals. It was less than 10-4 for all considered data. Descriptive statistics in Table 2 include mean, standard deviation and % standard deviation calculated in Excel.
Table 2.
Results of Fitting Multiplets from C4 of Glutamate and Glutamine. The variables a – h are reported for cortex from 3 animals. The mean, standard deviation (SD) and % standard deviation (%DEV) are included. The ratio of 13C-labeled acetate:glucose oxidation in astrocyte, (a+b)/(c+d), is shown in the final column.
| Tissue | a | b | c | d | e | f | g | h | (a+b)/(c+d) |
|---|---|---|---|---|---|---|---|---|---|
| Cortex_#1 | 0.039 | 0.478 | 0.036 | 0.447 | 0.164 | 0.836 | 2.826 | 0.205 | 1.070 |
| Cortex_#2 | 0.068 | 0.456 | 0.062 | 0.414 | 0.243 | 0.756 | 2.523 | 0.233 | 1.101 |
| Cortex_#3 | 0.055 | 0.361 | 0.077 | 0.507 | 0.195 | 0.805 | 2.032 | 0.151 | 0.712 |
| Mean | 0.054 | 0.432 | 0.058 | 0.456 | 0.201 | 0.799 | 2.460 | 0.196 | 0.961 |
| SD | 0.014 | 0.062 | 0.020 | 0.047 | 0.040 | 0.040 | 0.400 | 0.042 | 0.216 |
| %DEV | 27 | 14 | 35 | 10 | 20 | 5. | 16. | 21. | 22. |
RESULTS
13C-NMR Spectra from Normal Cortex
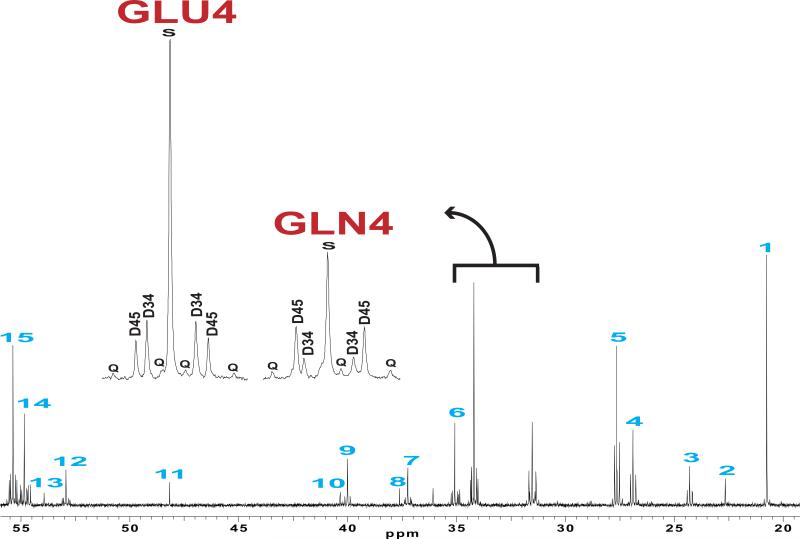
The multiplets in the carbon 4 signal of glutamate and glutamine from normal cortex were quite different, as reported earlier (Figure 3) (Cerdan et al. 1990, Deelchand et al. 2009, Hassel et al. 1997, Marin-Valencia et al. 2012, Morris & Bachelard 2003, Haberg et al. 1998, Taylor et al. 1996). Glutamine C4 exhibited a large doublet D45, indicating that a significant fraction of glutamine exchanged with α-ketoglutarate in a compartment capable of oxidizing [1,2-13C]acetate. In contrast, glutamate C4 was dominated by the singlet and the doublet D34, suggesting that most of the glutamate exchanged with α-ketoglutarate in a compartment oxidizing [1,6-13C]glucose, and that the compartment oxidizing [1,2-13C] acetate contributed relatively little to glutamate.
Figure 3. 13C NMR spectrum of an Extract from Normal Cortex.
The mouse was infused with [1,6-13C]glucose and [1,2-13C]acetate. The insets display the labeling patterns of glutamate and glutamine in carbon 4. Assignments are: 1, Lactate C3; 2, N-acetylaspartate C6; 3, GABA C3; 4, Glutamine C3; 5, Glutamate C3; 6, GABA C2; 7, aspartate C3; 8, creatine C2; 9, GABA C4; 10, N-acetylaspartate C3; 11, taurine; 12, aspartate 2; 13, N-acetylaspartate C2; 14, Glutamine C2; 15, Glutamate C2; S, singlet; D, doublet; Q, quartet or doublet of doublets; GLN4, glutamine C4; GLU4, glutamate C4.
Previously-Defined Acetate:Glucose Ratio Does Not Indicate Unique Metabolic Conditions
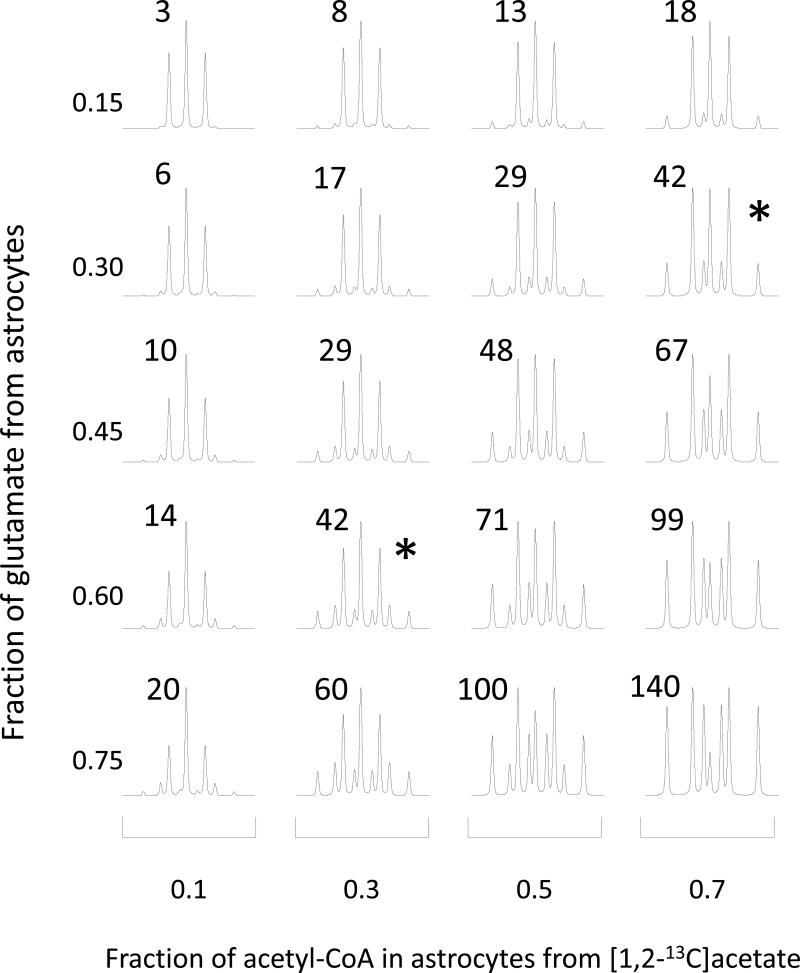
To explore the relationship between the 13C NMR spectrum and values returned for a – h from fitting the model, spectra were constructed for a wide range of metabolic conditions in both compartments using equations 1 – 6. The contribution of neurons to glutamate and glutamine (g and h) was allowed to vary for each set of generated synthetic data. From these values of a - h, 13C NMR spectra of glutamate and glutamine were generated. A subset of results from these simulations is shown in Figure 4. In this set, the 13C NMR spectra of glutamate C4 are shown assuming that the fraction of acetyl-CoA derived from glucose is 60% in neurons (the remainder provided by unlabeled substrates such as ketones) and 30% in astrocytes. The fraction of glutamate derived from astrocytes and the fraction of acetyl-CoA derived from acetate in astrocyte were allowed to vary. The previously –defined acetate:glucose ratio was calculated for all conditions illustrated in Figure 4 (Morris & Bachelard 2003). An identical acetate:glucose ratio, 0.42, was observed for the combination of conditions marked with an asterisk in Figure 4. These simulations illustrate that acetate:glucose ratio described earlier does not reflect a unique set of metabolic conditions when two distinct compartments influence the spectrum.
Figure 4. Simulated 13C NMR Spectra of Carbon 4 of Glutamate.
These spectra were generated assuming that 60% of acetyl-CoA in neurons is derived from [1,6-13C]glucose and that 30% of acetyl-CoA in astrocyte is derived from [1,6-13C]glucose. Two parameters were allowed to vary: the fraction of glutamate derived from astrocyte (range, 0.15 – 0.75) and the fraction of acetyl-CoA in astrocyte derived from [1,2-13C]acetate (range, 0.1 – 0.7). When astrocytes contribute substantially to glutamate (bottom row) the 13C NMR spectrum is very sensitive to changes in metabolism in astrocytes. When astrocytes contribute little to the 13C NMR spectrum of glutamate (top row), the spectrum is insensitive to changes in metabolism in astrocyte. The two spectra marked with “*” had essentially identical acetate:glucose ratios, 42%. The acetate:glucose ratio (in %) for each spectrum is shown in the inset.
Data from these simulations were also provided to the MATLAB program. As illustrated in Figure 5, the agreement between the actual and recovered fc3a/fc2a is optimal as long as astrocyte and neurons contribute somewhat differently to glutamate and glutamine. The percentage error in fc3a/fc2a becomes large if g and h are close, within 20%. This result is not surprising. If g and h are nearly equal, then by definition the multiplets in the 13C-NMR spectra of glutamate and glutamine are indistinguishable and the NMR evidence for compartmentation vanishes, exactly as indicated by equations 8 - 15. However, for the case when h differs substantially from g, the simulated result indicates the recovered parameters are reliable and report fc3a/fc2a.
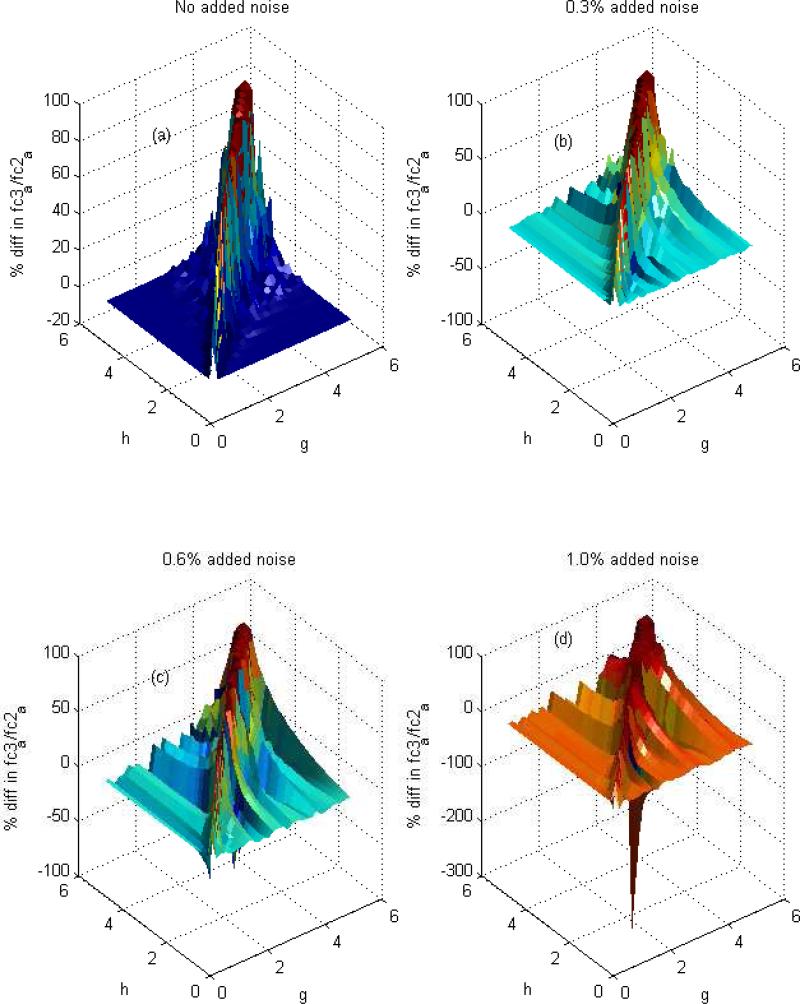
Figure 5. Fitting Simulated Data.
Illustrated are the results from analysis of simulated data under different conditions using the program “metabolic.m” in the supplementary material. The procedure is capable of computing a-h most accurately when exchange of glutamate and glutamine with α-ketoglutarate of astrocytes and neurons is quite different. This is demonstrated by finding the difference between the actual ratio of fc2a (acetyl-CoA labeled in positions 2) and fc3a (acetyl-CoA labeled in position 1 and 2) for a test model and the estimated fc3a/fc2a, using the synthetic data associated with this test model, as the input for the proposed procedure. Figure 5A shows that the error is small if g and h are considerably different from each other, but the procedure becomes less accurate in estimating a-h if g and h are nearly equal (error is highest on the diagonal). The noise plays a significant role in the precision of the computation (Figures 5B, 5C and 5D). With increasing the noise from 0 to 1 %, the procedure is less accurate in estimating a-h as represented by significantly worsening error.
The signal-to-noise ratio of the 13C-NMR spectrum is limited, so it is also important to determine the effect of noise in the spectrum on the variables returned from the least-squares fitting routine. A simple sensitivity analysis was performed to estimate the effects of noise on the accuracy of the parameters returned from the routine. This was accomplished by generating random numbers between -1 and 1, multiplying them by 0.3% of each area of E4S, E4D34, E4D45, E4Q, Q4S, Q4D34, Q4D45 and Q4Q (for Figure 5B) and adding the result to the synthetic data (area for E4S, E4D34, E4D45, E4Q, Q4S, Q4D34, Q4D45 and Q4Q) as input data for calculation of fc3a / fc2a with 0.3% noisy data. The percentage difference between these values and exact values are shown in Figure 5B. Similar procedures were used for finding the effect of 0.6% and 1% noise, shown in Figure 5C and 5D, respectively. Not surprisingly, the analysis is most accurate if signal-to-noise is high. With increased noise, the accuracy of returned values is more limited as illustrated by the scattered distribution of the results. We found with noise of greater than 1%, the recovered fc3a/fc2a is not good even when g and h are quite different.
Relative Rates of Oxidation of 13C-Labeled Glucose and Acetate
The data from normal cortex were fit using the MATLAB program in the supplemental information and the results for a – h are summarized in Table 2. The ratio of acetate vs. glucose oxidation in astrocyte was estimated from the fitting results for a – d. The ratio (a+b)/(c+d) (see equation 7) is the ratio of oxidation of [1,2-13C]acetate relative to the oxidation of [1,6-13C]glucose, or 0.96 ± 0.22 (Table 2). These results demonstrate that during infusion of both acetate and glucose, both 13C-labeled substrates are oxidized at approximately equal rates in astrocytes.
Sources of Glutamate and Glutamine
The scaling factor, g, represents the contribution of 13C – labeled α-ketoglutarate from neurons to total brain glutamate. Similarly, h represents the contribution of 13C – labeled α-ketoglutarate from neurons to total brain glutamine. It should be emphasized that these variables do not directly represent the fraction of glutamate or glutamine derived from neurons because the fraction of α-ketoglutarate that is 13C labeled cannot be determined from this analysis. Nevertheless, the values of g and h in Table 2 indicate that much of the 13C labeling in glutamate was derived from of α-ketoglutarate in neurons, and that relatively little of the 13C labeling in glutamine was derived from of α-ketoglutarate in neurons. These results are consistent with the standard notion that in brain, the labeling pattern in glutamate largely reflects the α-ketoglutarate pool of neurons and that glutamine originates mainly from astrocytes (Martinez-Hernandez et al. 1977, Waniewski & Martin 1998, Mason et al. 1995).
DISCUSSION
The differences in 13C labeling in glutamate and glutamine carbon 4 that arise when brain tissue is provided 13C-labeled acetate or mixtures of 13C-labeled acetate and 13C-labeled glucose have been emphasized previously (Cerdan et al. 1990, Deelchand et al. 2009, Hassel et al. 1997, Marin-Valencia et al. 2012, Morris & Bachelard 2003, Haberg et al. 1998, Taylor et al. 1996). Simultaneous infusion of both 13C-labeled glucose and 13C-labeled acetate is now a popular experimental approach for studies of neurological diseases such as stroke (Pascual et al. 1998, Haberg et al. 2009), epilepsy (Eloqayli et al. 2003, Eloqayli et al. 2004), hydrocephalus (Kondziella et al. 2003), diabetic encephalopathy (Garcia-Espinosa et al. 2003) and metabolic disorders such as succinic semialdehyde dehydrogenase deficiency (Chowdhury et al. 2007). Although there is general agreement that the differences in 13C labeling in glutamate and glutamine are due to metabolic compartmentation in brain, there has been no method for compartment-specific quantitation of relative substrate oxidation from a single 13C NMR spectrum.
This approach illustrates a number of points. First, although the acetate:glucose ratio defined earlier demonstrates that both acetate and glucose can be oxidized in the brain ADDIN EN.CITE (, there is no direct relation between the previously-defined acetate:glucose ratio and substrate oxidation in a particular cell compartment. As illustrated in Figure 4, dramatically different metabolic conditions may yield the same acetate:glucose ratio. Second, evidence for differences in sources of acetyl-CoA in astrocyte and neurons arises from differences in the 13C NMR spectrum of glutamate vs. glutamine. The 13C NMR spectrum of glutamate and glutamine will be identical when the fractional contribution of astrocytes and neurons to glutamate and glutamine do not differ significantly, meaning that g ~ h in the current terminology. Consequently, the 13C NMR spectrum of glutamate and glutamine may show identical 13C-13C coupling patterns even if the oxidized substrates in astrocytes and neurons are quite different. Third, the sensitivity of this experimental approach will be greatest if the contribution of the two compartments to glutamate and glutamine differ significantly.
The model also demonstrates the limits on metabolic information that can be derived from the 13C NMR spectrum of carbon 4. Even in the very simple model used to test this analysis, there are 6 independent mathematical variables (fc3a, fc2a, fa3a, ya, fc2n, yn) that determine the relative concentration of 13C isotopomers in the α-ketoglutarate pools of astrocyte and neurons. However, the 13C NMR spectrum of glutamate and glutamine C4 provides only 3 mathematically-independent measures of these isotopomers (a, b, and f, since c, d and e are determined by difference from 1 and equation d/c= b/a). Consequently, none of the metabolic parameters specific to each compartment can be determined independently without additional information. However, the ratio of acetate to glucose oxidation can be determined in astrocytes because this ratio is insensitive to the effects of pyruvate carboxylation and 13C labeling in pyruvate used in pyruvate carboxylase as shown in equation 7.
Several limitations should be noted. First, if the relative contribution of neurons and astrocyte to 13C glutamate and glutamine are the same (equivalent to g = h), then metabolism in astrocyte and neurons could be quite different, but the difference would be undetectable based on examination of the glutamate and glutamine spectra. Second, signal-to-noise must be high, so this analysis is well-suited to analysis of tissue extracts, but likely difficult to apply in vivo. Third, in these studies the fractional enrichment of acetate was not determined. Therefore, all results are reported as metabolism of 13C-labeled acetate and 13C labeled glucose. In the future, these results should be corrected for the measured fractional enrichment of the substrates. This analysis also does not correct for natural abundance 13C. Any natural abundance signal will be detected as an excess singlet and hence as a slight over-estimation of the glucose contribution to acetyl-CoA. The analysis assumes that any [1,2-13C]acetyl-CoA in brain tissue is derived exclusively from [1,2-13C]acetate. Consequently, the analysis does not account for the possibility of pyruvate recycling in neurons under these conditions, which implies significant conversion of oxaloacetate → pyruvate → acetyl-CoA → citrate. Finally, this analysis assumes that steady-state metabolic conditions have been achieved.
In summary, the ratio of acetate to glucose oxidation in astrocytes may be measured from a single 13C NMR spectrum. The analysis is optimal for conditions in which the fraction of glutamate and glutamine in exchange with each compartment is substantially different. Current results in normal cortex are consistent with substantial oxidation of acetate relative to glucose in astrocytes, and a dominant contribution of astrocytes to glutamine labeling. The method is applicable to a wide range of conditions.
Supplementary Material
ACKNOWLEDGEMENTS
This project was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health through P41EB015908 and through R37HL034557.
Abbreviations
- aKG
alpha-ketoglutarate
- CAC
citric acid cycle
- GLN
glutamine
- NMR
nuclear magnetic resonance
REFERENCES
- Cerdan S, Kunnecke B, Seelig J. Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro 13C NMR. The Journal of biological chemistry. 1990;265:12916–12926. [PubMed] [Google Scholar]
- Chowdhury GM, Gupta M, Gibson KM, Patel AB, Behar KL. Altered cerebral glucose and acetate metabolism in succinic semialdehyde dehydrogenase-deficient mice: evidence for glial dysfunction and reduced glutamate/glutamine cycling. Journal of neurochemistry. 2007;103:2077–2091. doi: 10.1111/j.1471-4159.2007.04887.x. [DOI] [PubMed] [Google Scholar]
- Deelchand DK, Nelson C, Shestov AA, Ugurbil K, Henry PG. Simultaneous measurement of neuronal and glial metabolism in rat brain in vivo using co-infusion of [1,6-13C2]glucose and [1,2-13C2]acetate. J Magn Reson. 2009;196:157–163. doi: 10.1016/j.jmr.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloqayli H, Dahl CB, Gotestam KG, Unsgard G, Hadidi H, Sonnewald U. Pentylenetetrazole decreases metabolic glutamate turnover in rat brain. Journal of neurochemistry. 2003;85:1200–1207. doi: 10.1046/j.1471-4159.2003.01781.x. [DOI] [PubMed] [Google Scholar]
- Eloqayli H, Dahl CB, Gotestam KG, Unsgard G, Sonnewald U. Changes of glial-neuronal interaction and metabolism after a subconvulsive dose of pentylenetetrazole. Neurochemistry international. 2004;45:739–745. doi: 10.1016/j.neuint.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Garcia-Espinosa MA, Garcia-Martin ML, Cerdan S. Role of glial metabolism in diabetic encephalopathy as detected by high resolution 13C NMR. NMR in biomedicine. 2003;16:440–449. doi: 10.1002/nbm.843. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Seaquist ER, Ugurbil K. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. American journal of physiology. Endocrinology and metabolism. 2001;281:E100–112. doi: 10.1152/ajpendo.2001.281.1.E100. [DOI] [PubMed] [Google Scholar]
- Haberg A, Qu H, Haraldseth O, Unsgard G, Sonnewald U. In vivo injection of [1-13C]glucose and [1,2-13C]acetate combined with ex vivo 13C nuclear magnetic resonance spectroscopy: a novel approach to the study of middle cerebral artery occlusion in the rat. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1998;18:1223–1232. doi: 10.1097/00004647-199811000-00008. [DOI] [PubMed] [Google Scholar]
- Haberg AK, Qu H, Sonnewald U. Acute changes in intermediary metabolism in cerebellum and contralateral hemisphere following middle cerebral artery occlusion in rat. Journal of neurochemistry. 2009;1(109 Suppl):174–181. doi: 10.1111/j.1471-4159.2009.05940.x. [DOI] [PubMed] [Google Scholar]
- Hassel B, Bachelard H, Jones P, Fonnum F, Sonnewald U. Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1997;17:1230–1238. doi: 10.1097/00004647-199711000-00012. [DOI] [PubMed] [Google Scholar]
- Jeffrey FM, Marin-Valencia I, Good LB, Shestov AA, Henry PG, Pascual JM, Malloy CR. Modeling of brain metabolism and pyruvate compartmentation using (13)C NMR in vivo: caution required. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1160–1167. doi: 10.1038/jcbfm.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziella D, Qu H, Ludemann W, Brinker T, Sletvold O, Sonnewald U. Astrocyte metabolism is disturbed in the early development of experimental hydrocephalus. Journal of neurochemistry. 2003;85:274–281. doi: 10.1046/j.1471-4159.2003.01656.x. [DOI] [PubMed] [Google Scholar]
- Lanz B, Xin L, Millet P, Gruetter R. In vivo quantification of neuro-glial metabolism and glial glutamate concentration using 1H-[13C] MRS at 14.1T. Journal of neurochemistry. 2014;128:125–139. doi: 10.1111/jnc.12479. [DOI] [PubMed] [Google Scholar]
- Malloy CR, Sherry AD, Jeffrey FM. Carbon flux through citric acid cycle pathways in perfused heart by 13C NMR spectroscopy. FEBS letters. 1987;212:58–62. doi: 10.1016/0014-5793(87)81556-9. [DOI] [PubMed] [Google Scholar]
- Malloy CR, Sherry AD, Jeffrey FM. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy. The Journal of biological chemistry. 1988;263:6964–6971. [PubMed] [Google Scholar]
- Malloy CR, Sherry AD, Jeffrey FM. Analysis of tricarboxylic acid cycle of the heart using 13C isotope isomers. The American journal of physiology. 1990a;259:H987–995. doi: 10.1152/ajpheart.1990.259.3.H987. [DOI] [PubMed] [Google Scholar]
- Malloy CR, Thompson JR, Jeffrey FM, Sherry AD. Contribution of exogenous substrates to acetyl coenzyme A: measurement by 13C NMR under non-steady-state conditions. Biochemistry. 1990b;29:6756–6761. doi: 10.1021/bi00481a002. [DOI] [PubMed] [Google Scholar]
- Marin-Valencia I, Good LB, Ma Q, Malloy CR, Patel MS, Pascual JM. Cortical metabolism in pyruvate dehydrogenase deficiency revealed by ex vivo multiplet (13)C NMR of the adult mouse brain. Neurochemistry international. 2012;61:1036–1043. doi: 10.1016/j.neuint.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Mason GF, Gruetter R, Rothman DL, Behar KL, Shulman RG, Novotny EJ. Simultaneous determination of the rates of the TCA cycle, glucose utilization, alpha-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1995;15:12–25. doi: 10.1038/jcbfm.1995.2. [DOI] [PubMed] [Google Scholar]
- Medina JM, Tabernero A. Lactate utilization by brain cells and its role in CNS development. Journal of neuroscience research. 2005;79:2–10. doi: 10.1002/jnr.20336. [DOI] [PubMed] [Google Scholar]
- Morris P, Bachelard H. Reflections on the application of 13C-MRS to research on brain metabolism. NMR in biomedicine. 2003;16:303–312. doi: 10.1002/nbm.844. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Pereira de Vasconcelos A. Glucose and ketone body utilization by the brain of neonatal rats. Progress in neurobiology. 1993;40:163–221. doi: 10.1016/0301-0082(93)90022-k. [DOI] [PubMed] [Google Scholar]
- Nocedal J,WS. Numerical Optimization. Springer; New York: 1999. [Google Scholar]
- Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual JM, Carceller F, Roda JM, Cerdan S. Glutamate, glutamine, and GABA as substrates for the neuronal and glial compartments after focal cerebral ischemia in rats. Stroke; a journal of cerebral circulation. 1998;29:1048–1056. doi: 10.1161/01.str.29.5.1048. discussion 1056-1047. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Sibson NR, Hyder F, Shen J, Behar KL, Shulman RG. In vivo nuclear magnetic resonance spectroscopy studies of the relationship between the glutamate-glutamine neurotransmitter cycle and functional neuroenergetics. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1999;354:1165–1177. doi: 10.1098/rstb.1999.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr A. In: Neuronal energy requirements. In: The neuronal environment: brain homeostasis in health and disease. Walz W, editor. Humana Press; New Jersey: 2002. pp. 25–54. [Google Scholar]
- Shen J. Modeling the glutamate-glutamine neurotransmitter cycle. Frontiers in neuroenergetics. 2013;5:1. doi: 10.3389/fnene.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry AD, Malloy CR, Zhao P, Thompson JR. Alterations in substrate utilization in the reperfused myocardium: a direct analysis by 13C NMR. Biochemistry. 1992;31:4833–4837. doi: 10.1021/bi00135a014. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL, Shulman RG. In vivo (13)C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during. Journal of neurochemistry. 2001;76:975–989. doi: 10.1046/j.1471-4159.2001.00074.x. [DOI] [PubMed] [Google Scholar]
- Strisower EH, Kohler GD, Chaikoff IL. Incorporation of acetate carbon into glucose by liver slices from normal and alloxan-diabetic rats. The Journal of biological chemistry. 1952;198:115–126. [PubMed] [Google Scholar]
- Taylor A, McLean M, Morris P, Bachelard H. Approaches to studies on neuronal/glial relationships by 13C-MRS analysis. Developmental neuroscience. 1996;18:434–442. doi: 10.1159/000111438. [DOI] [PubMed] [Google Scholar]
- van den Berg CJ, Garfinkel D. A stimulation study of brain compartments. Metabolism of glutamate and related substances in mouse brain. The Biochemical journal. 1971;123:211–218. doi: 10.1042/bj1230211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman EO, Strisower EH, Chaikoff IL. Conversion of fatty acids to carbohydrate; application of isotopes to this problem and role of the Krebs cycle as a synthetic pathway. Physiological reviews. 1957;37:252–272. doi: 10.1152/physrev.1957.37.2.252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.