Abstract
Introduction
In animal models, maternal obesity (OB) leads to augmented risk of offspring OB. While placental function is influenced by maternal habitus, the effect of maternal obesity on the interacting zones of the placenta [the labyrinth (LZ), junctional (JZ) and metrial gland (MG)] remains unknown.
Methods
Using a rat maternal obesity model, we conducted transcriptomic profiling of the utero-placental compartments and fetal liver (FL) at dpc 18.5, in conjunction with analyses of mRNA expression of key thyroid hormone (TH) signaling genes in the placenta, fetus and weanling offspring.
Results and Discussion
Gene expression analysis of placenta and offspring revealed that each utero-placental compartment responds distinctly to maternal OB with changes in inflammatory signaling, lipid metabolism and hormone stimulus being the predominant effects. OB-induced alterations in 17 genes were confirmed by qPCR, including reductions in thyrotropin-releasing hormone (Trh) in JZ. We further characterized mRNA and protein expression of TH signaling regulators including deiodinases (Dio), TH receptors (Tr), and downstream targets (uncoupling proteins (Ucp)). A concerted down-regulation of multiple facets of thyroid hormone signaling in the JZ and FL was observed. JZ expression of thyroid hormone signaling components Trh, Dio2, Trα, and Ucp2 were negatively associated with maternal leptin. mRNA expression of TRH, TRβ and UCP1 were also decreased in term placenta from OB women. Finally, our studies identified persistent impairments in expression of TH related genes in tissues from offspring of obese dams.
Conclusions
The role of lower placental thyroid expression is worthy of further study as a possible pathway that leads to low energy metabolism and obesity in animals born to obese mothers.
Keywords: Metabolism, Obesity, Placenta, RNA-seq, Thyroid hormone, Uncoupling proteins
INTRODUCTION
Maternal body composition profoundly influences offspring metabolism and obesity risk. Using a rat model, we have shown that gestational exposure to maternal obesity (OB) increases the risk of OB in the offspring (1–4). Offspring born to OB dams gain greater body weight, fat mass, and develop hyperinsulinemia when challenged with a high-fat diet (HFD) post-weaning (1;2;4;5). This hyper-responsive phenotype in the offspring is associated with decreased energy expenditure and impaired metabolic flexibility, prior to HFD exposure (5;6). While many of the metabolic, endocrine and gene expression changes precede the development of obesity in the OB-dam offspring (1;2;4–6), the precise mechanisms in utero leading to alterations in energy metabolism in the offspring remain to be elucidated.
As the sole interface between maternal and fetal environments, the placenta is pivotal in relaying metabolic information about the maternal habitus to the developing offspring (7;8). The rat placentation site is distinctly organized into interacting zones, the metrial gland (MG), junctional zone (JZ) and labyrinth zone (LZ) compartments (9;10), each with unique cell populations and functions. The MG is a chimeric region of uterine stroma and invasive trophoblasts and is the site for vascular remodeling. At the maternal-placental interface, spongiotrophoblasts, trophoblast giant cells and glycogen cells make up the JZ and secrete a variety of hormones, signaling proteins, and tissue remodeling factors. The LZ is composed of multinucleated syncytiotrophoblasts that separate the maternal blood spaces from fetal vasculature and carry out exchange of nutrients, gases, and waste. While the importance of placental changes in response to maternal diet and adiposity has been appreciated, the impact of maternal OB on the specific functional components on the placentation site remains largely unknown. Furthermore, the nature of specific signals associated with maternal OB that mediate changes in offspring metabolism also remains elusive.
Here we examined the hypothesis that maternal OB influences gene expression profiles in the placentation site and the developing offspring. Using high-throughput sequencing of mRNA-derived cDNA libraries (RNA-seq), we catalogued differential gene expression associated with maternal OB within each functional compartment of the placenta and the fetal liver (FL). Based on findings from global gene expression, we further assessed key components of the placental-fetal thyroid axis in both lean and OB dams. We next examined the expression of thyroid signaling components [deiodinases (Dio) and thyroid hormone receptors (Tr)] and downstream metabolic effectors relevant to basal metabolism (uncoupling proteins, Ucp) in liver, skeletal muscle and brown adipose tissues (BAT) of offspring at weaning. Finally, to assess the potential translation of our findings to humans, we measured gene expression of similar targets in term placenta from lean and overweight/obese women. Our results demonstrate that maternal OB not only has distinct effects in individual compartments of the placenta, but also potentially impacts placental and offspring thyroid hormone signaling.
METHODS
Experimental Design
All experiments were conducted in accordance with the guidelines established by the IACUC at UAMS (protocol# 2971). Female Sprague-Dawley rats (150–175 g) were intragastrically cannulated and fed liquid diets at either 155 Kcal/kg3/4/d (lean, N=8) or 220 Kcal/kg3/4/d (obese, N=7; 40% excess calories) for 3 wk as previously described (1–3;5). Diets were 20% protein, 75% carbohydrate and 5% fat as percentage of total calories. Caloric intake for lean rats mimicked body weight gains of rats consuming standard diets ad libitum (1;2;4;11). Following 3 wk of diets, female rats were bred with lean males and successful mating was confirmed by the presence of sperm in the vaginal lavage the next morning [dpc 0.5]. Placenta were collected on dpc 18.5 and weights of each litter, fetus, and placenta were noted. From each placenta the junctional and labyrinth-enriched zones were separated by dissection (10;12). MG was dissected from the uterus. Corresponding fetal livers (FL) were also collected and frozen in liquid nitrogen. Sex of the fetus was determined via amplification of the Sry gene using hepatic DNA (3). Only tissues from male fetuses were utilized in this study.
In a separate experiment, lean and OB rat dams were allowed to carry pregnancies to term and give birth naturally (N=8 per group). On PND2, four males and four females from each litter were cross-fostered to surrogate dams that had been previously time-impregnated to give birth on the same day as the dams receiving infusion diets (1;2;6). Surrogate dams were not cannulated and had ad libitum access to AIN-93G diets throughout. On PND21 (weaning), liver, gastrocnemius muscle, and brown adipose tissue (BAT) were collected from male offspring in the fed state and frozen in liquid nitrogen.
Human Subjects
Term placenta were collected from lean (BMI < 25) and overweight/obese (BMI ≥25–35) subjects (N=32 per group) participating in an ongoing longitudinal study (ClinicalTrials.gov ID: NCT01131117). The study protocol was approved by the IRB at UAMS. Written informed consent was obtained from all participants. All subjects were recruited <10 wk of pregnancy and were second parity, singleton pregnancies conceived without fertility treatments. Other exclusion criteria and methods to collect and process placenta are provided in supplementary material.
RNA-seq Analysis
RNA-seq libraries were prepared for each placental zone using two biologically separate pools containing equal amounts of RNA from 6–9 individual placenta from at least 3–4 distinct litters. Thus N=15 (lean) and N=13 (obese) placenta samples from N=8 and 7 dams, respectively, were represented over two pools. Methods for RNA isolation, library preparation and data analysis are described in supplementary materials (10;13;14). Differentially expressed genes between lean and OB groups were identified based on P-value ≤ 0.05 (Audic-Claverie test) and ±1.4-fold change (15) and utilized for gene ontology (GO) biological and molecular function enrichment using GoRilla (16). Confirmation of gene expression was performed using real-time RT-PCR on individual placenta (representing N = 7–8 dams per group) using SYBR-green reagents (2;4;10;13;14;17). Relative amounts of mRNA were quantitated using a standard curve and normalized to Srp14 (for rat) or B2M (for human) mRNA (18). Primer sequences are provided in Table S1.
Immunoblotting
Tissue lysates were prepared in RIPA buffer. Immunoblotting was carried out using standard procedures (4;13;17). Details including primary antibodies utilized are included in supplementary materials. Immunoblots were quantified using Quantity One software.
Statistical Analysis
Data are expressed as means ± SEM. Statistical differences between lean and obese groups were determined using a two-tailed Student’s t-test and correlations were determined using the Pearson’s product moment correlation coefficient (r) using SigmaStat 3.5 software. Statistical significance was set at P < 0.05.
RESULTS
Maternal OB alters utero-placental gene expression relative to lean counterparts
Overfed rats were heavier (Figure 1A) and had ~40% greater fat mass and 8% lower lean mass prior to mating (Figure 1B, P<0.05) and at dpc 18.5 (Table S2). Maternal characteristics at dpc 18.5 are included in Table S2. OB rats showed elevated serum leptin and insulin, accompanied with reduced adiponectin (P<0.05) concentrations. Placenta or fetal weights were not affected due to maternal OB (Table S2).
FIG 1. Characteristics of lean and obese female rats.
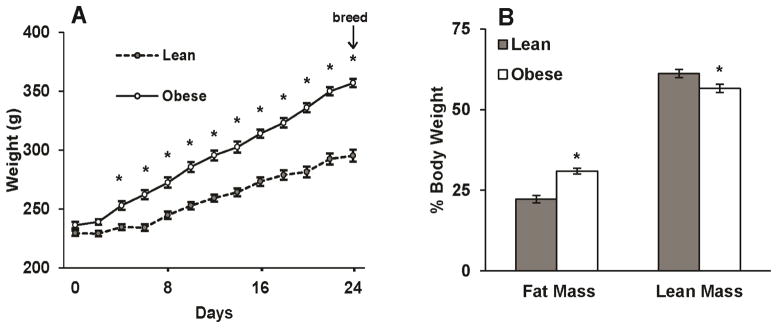
(A) Body weight gains of female rats fed diets via total enteral nutrition (TEN) at 155 Kcal/kg3/4/d (lean) or 220 Kcal/kg3/4/d (obese) for 3 wk prior to mating (N = 7–8 per group). (B) Body composition of lean and obese female rats. Fat mass and lean mass expressed as %body weight was non-invasively estimated via qNMR using EchoMRI prior to mating. Data are expressed as mean ± SEM. * indicates a statistically significant difference between Lean and Obese (P < 0.05).
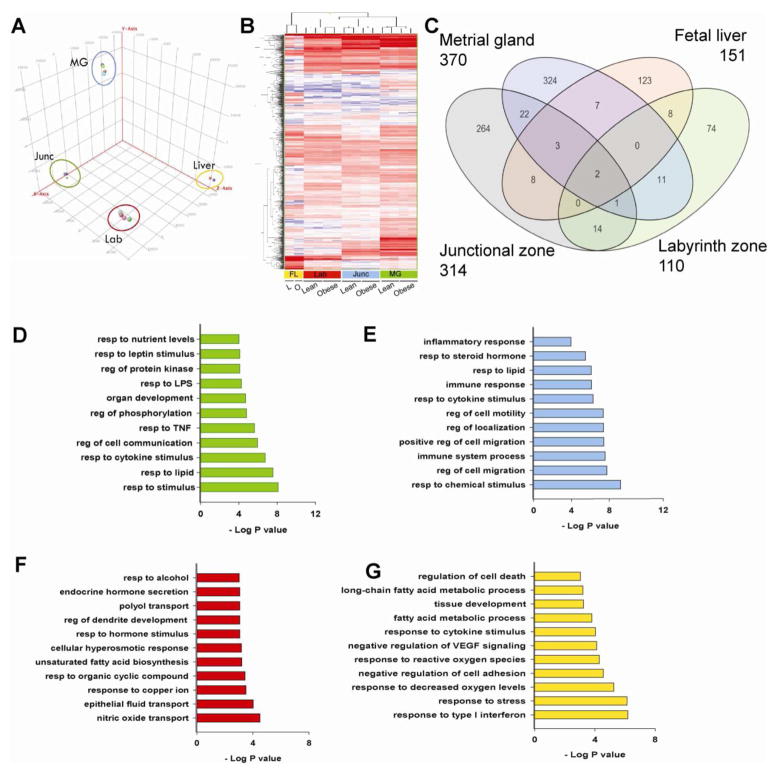
Principal component analysis (Figure 2A) and unsupervised clustering (Figure 2B) of RPKM values revealed clustering of expression profiles based on utero-placental compartment of origin and by maternal phenotype, suggesting that each compartment displayed a unique gene expression signature. Maternal OB significantly altered expression of 370, 314, 110, and 151 transcripts (±1.4-fold, P<0.05, RPKM>1) in the MG, JZ, LZ, and FL, respectively (Figure 2C). Analysis of these differentially expressed transcripts revealed that a majority of altered genes (>70%) were unique to each comparison. Furthermore, the effects of maternal OB were most predominant in the maternal compartment. GO biological processes analysis revealed that response to pro-inflammatory signals, lipids, lipopolysaccharide and nutrients were significantly affected between lean and OB groups in MG (Figure 2D). Within the JZ, maternal OB predominantly affected genes involved with the inflammatory response, steroid hormone stimulus, and cell migration (Figure 2E). Biological processes including response to hormone stimulus, fatty acid biosynthesis, epithelial fluid transport were significantly altered by maternal OB in the LZ (Figure 2F). Alterations in the FL also reflected changes observed in the placentation site as shown by enrichment of immune response, oxidative stress, fatty acid metabolism, and cell death-related genes due to maternal OB (Figure 2G).
FIG 2. Placental gene expression changes with maternal obesity.
Gene expression in the placental compartments junctional zone, labyrinth zone and metrial gland, and fetal liver were assessed using RNA-seq. Reads Per Kilobase per Million mapped reads (RPKM) values for all Refseq genes were calculated using Avadis-NGS. (A) Principal Component Analysis of all genes showing expression profiles of individual samples belonging to the same compartment are highly related and group together. (B) Hierarchical clustering the union of genes altered due to maternal obesity in each zone are presented. Differentially expressed genes between lean and OB groups were identified based on P value ≤ 0.05 using Audic-Claverie test and ±1.4- fold change. A complete list is included in supplementary material. Heat-map colors red, white, and blue represent up-regulation, no relative effect, and down-regulation of transcripts, respectively. (C) Venn diagram of genes altered with maternal obesity in each zone. Enrichment of GO biological processes terms within differentially expressed genes in (D) metrial gland (E) junctional zone (F) labyrinth zone and (G) fetal liver. Gene ontology enrichment was carried out using GoRilla.
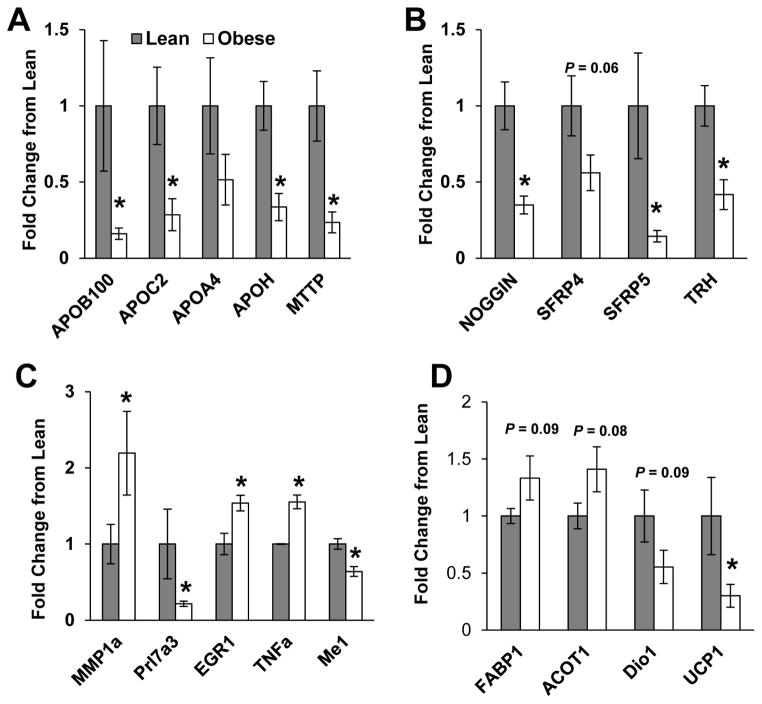
Confirmation using qPCR revealed that maternal OB was associated with lower expression of apolipoproteins; ApoB100, ApoC2, and ApoH (P<0.05) and of Mttp, (P<0.01) in the MG compared to lean controls (Figure 3A). Likewise, consistent with effects on hormone stimulus and cell migration in the JZ, mRNA expression of Trh (P<0.01) and that of Wnt inhibitors Sfrp4 (P=0.06) and Sfrp5 (P<0.05) and BMP-4 inhibitor Noggin (P<0.01) (Figure 3B) were significantly reduced in the JZ of obese rats. PCR also confirmed that maternal OB was associated with a significant increase in pro-inflammatory genes; matrix metalloproteinase-1a (Mmp-1a), early growth response protein-1 (Egr1) and Tnfα, and a significant decrease in the growth-associated prolactin gene, Prl7a3 (P < 0.05) in the LZ (Figure 3C). Additionally, gene expression of thyroid hormone regulated NADP-dependent malic enzyme (Me1) was significantly reduced (P < 0.05) in LZ of OB dams (Figure 3C). In FL, mRNA expression of fatty acid binding protein-1 (Fabp1, P=0.09) and acyl-CoA thioesterase-1 (Acot1, P=0.08) was increased and Dio1 (P=0.09) was decreased in offspring from OB dams, although not significantly (Figure 3D). We also observed a significant decrease in uncoupling protein-1 (Ucp1, P<0.05) in FL from OB verses lean dams.
FIG 3. mRNA expression in metrial gland, junctional zone, labyrinth zone and fetal liver.
mRNA expression of select genes at dpc 18.5 in (A) metrial gland, (B) junctional zone, and (C) labyrinth zone and (D) fetal liver (N = 7 –8 dams per group) of lean and obese dams. Gene expression was assessed via real-time RT-PCR and normalized to abundance of Srp14 mRNA. Data are expressed relative to the lean group. Data are expressed as mean ± SEM. * indicates a statistically significant difference between Lean and Obese (P < 0.05).
Maternal OB impairs expression of thyroid hormone signaling components in the JZ
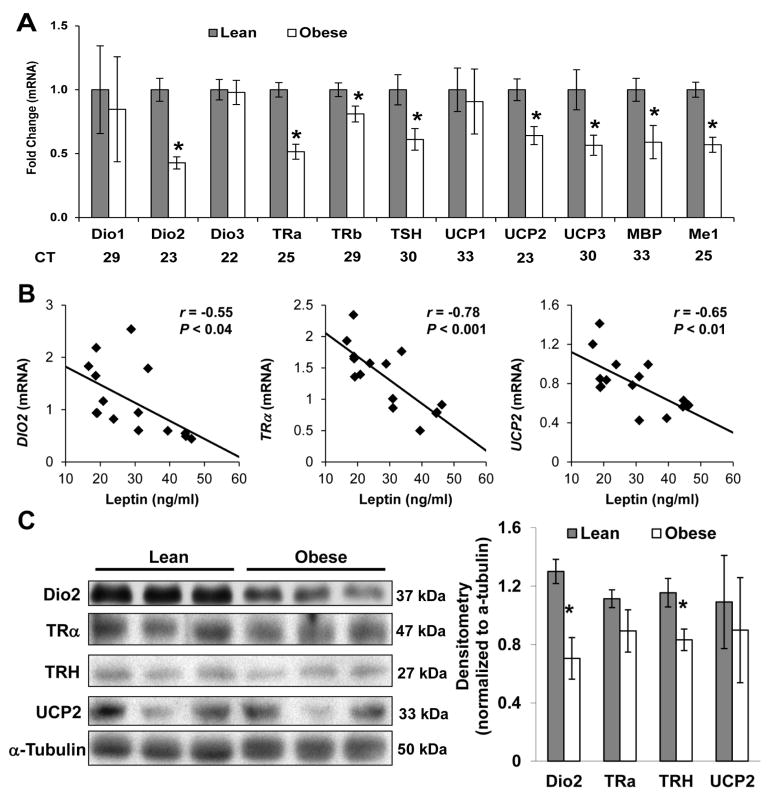
Consistent with lower Trh expression found in the JZ of OB dams, mRNA expression of Dio2 (P<0.001), Trα (P<0.001), Trβ (P<0.05), thyroid-stimulating hormone (Tsh, P<0.05), Ucp2 (P<0.01), Ucp3 (P<0.05), Me1 (P<0.05) and myelin basic protein (Mbp) (P<0.05) was significantly reduced in obese placenta when compared to lean, suggesting that thyroid hormone signaling was impaired by maternal OB (Figure 4A). Importantly, maternal serum leptin concentrations showed a strong and significant inverse correlation between JZ Trh (r = −0.75, P<0.001), Dio2 (r = −0.55, P < 0.05), Trα (r = −0.78, P<0.001), and Ucp2 (r = −0.65, P<0.01) mRNA expression (Figure 4B). Trh could presumably affect fetal metabolism through thyroid signaling in utero. Accordingly, JZ Trh expression positively correlated with FL Ucp1 mRNA (r = 0.56, P<0.03), a transcriptional target of thyroid signaling. There were no significant correlations between maternal leptin and gene expression in other placental zones (data not shown). Finally, we confirmed lower protein levels of DIO2 and TRH (P<0.05) in placenta from OB rats (Figure 4C).
FIG 4. Maternal obesity alters thyroid hormone signaling in the junctional zone.
(A) mRNA expression of Dio1, Dio2, Dio3, TRα, Trβ, Tsh, Ucp1, Ucp2, Ucp3, Mbp and Me1 in the junctional zone of lean and obese rats at dpc 18.5 (N = 7 –8 dams per group). Average Ct values are provided for each gene as an indicator of relative abundance. (B) Linear regression of junctional zone expression of TRα, Dio2 and Ucp2 mRNA against maternal serum leptin levels at dpc 18.5 shows significant negative associations (P < 0.05). (C) Immunoblot analysis of DIO2, TRα, TRH, UCP2 and α-tubulin protein levels in total lysates from junctional zone of lean and obese dams. Three biologically distinct pools each representing N=3–4 dams per group of were used for analysis. Data are expressed relative to the lean group. Data are expressed as mean ± SEM. * indicates a statistically significant difference between Lean and Obese (P < 0.05).
Maternal OB alters expression of thyroid signaling mediators in offspring
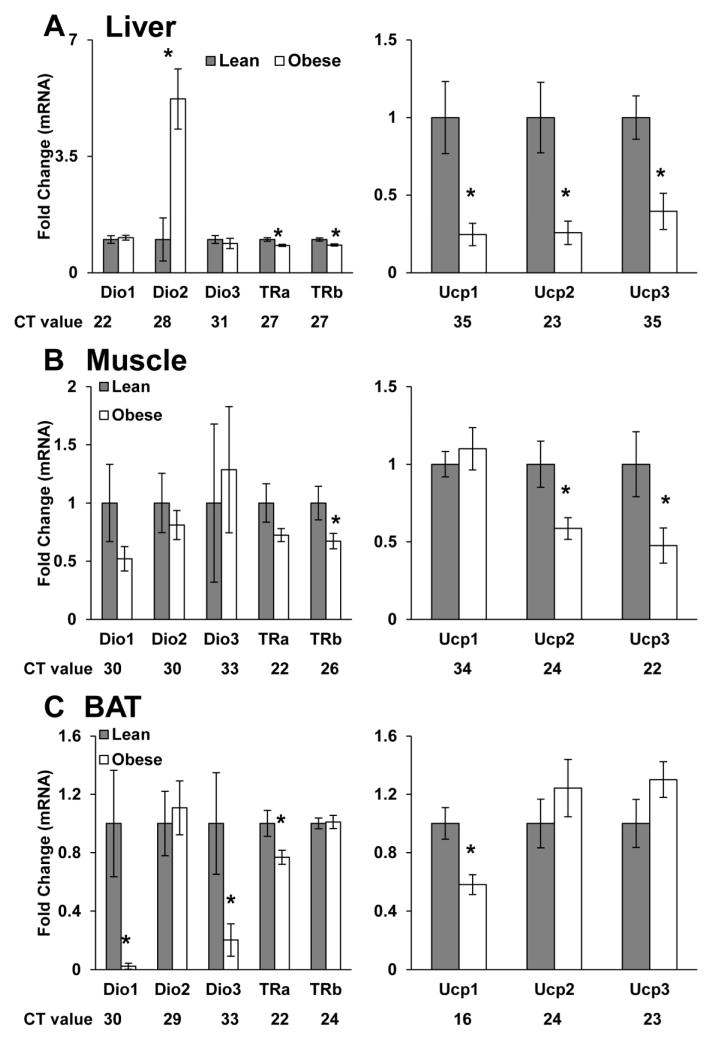
To determine the potential effects of maternal obesity on offspring thyroid hormone signaling, we assessed circulating T3 and T4 in weanling offspring. Serum T3 (6.1 ± 0.39 pg/ml-Lean vs. 5.65 ± 0.22 pg/ml-OB) and T4 (12.3 ± 1.6 pg/ml-Lean vs. 9.5 ± 0.7 pg/ml-OB) were slightly reduced in OB-dam offspring, however these differences did not reach significance (Figure S1). Thyroid hormone responsive genes in liver, muscle, BAT were also examined in weanling offspring. Although, hepatic expression of Dio2 mRNA was increased in OB-dam offspring, expression of Trα, Trβ, Ucp1, Ucp2, and Ucp3 (P < 0.01) were significantly decreased, suggesting impaired thyroid hormone signaling (Figure 5A). Similarly, Trβ, Ucp2, and Ucp3 (P<0.05) mRNA expression was significantly decreased in the gastrocnemius muscle from offspring of OB-dam offspring (Figure 5B). Finally, BAT from offspring of OB dams also showed reduced Dio1, Dio3, Trα, and Ucp1 (P < 0.05) mRNA expression (Figure 5C). Collectively, these findings suggest persistent impairments in thyroid signaling in tissues from offspring of OB dams.
FIG 5. Thyroid hormone signaling components are altered in offspring from obese dams at weaning.
mRNA expression of Dio1, Dio2, Dio3, Trα, Trβ, Ucp1, Ucp2 and Ucp3 in (A) liver, (B) mixed gastrocnemius muscle and (C) brown adipose tissue (BAT) of offspring from lean and obese rats at post-natal day 21 (N = 8 per group). Offspring from both lean and obese dams were cross-fostered to lean uncannulated surrogate dams on PND2, restricting exposure to maternal obesity to gestation alone. Average Ct values are provided for each gene as an indicator of relative abundance. Data are expressed as mean ± SEM. * indicates a statistically significant difference between Lean and Obese (P < 0.05).
Placental TH-related gene expression is decreased in human obesity
Table S3 depicts maternal and infant characteristics for the term placenta samples from lean and overweight/obese women. Similar to our findings in rat placenta, maternal obesity was associated with significantly lower placental TRH, TRβ, and UCP1 gene expression (P < 0.05, Figure 6). Expression of DIO1, DIO2, DIO3, UCP2, UCP3, and TRα were not significantly different between groups (Figure S2).
FIG 6. Thyroid hormone signaling components are altered in human placenta from obese women.
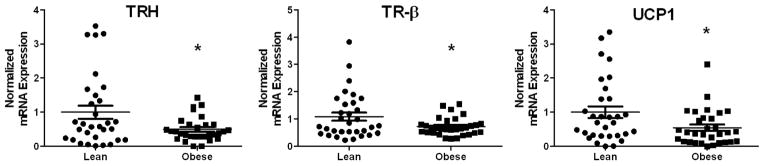
mRNA expression of TRH, TRβ and UCP1 in male human placenta from lean and obese mothers (N=32 per group, normalized to B2M). Data are expressed as mean ± SEM. * indicates a statistically significant difference between Lean and Obese (P < 0.05).
DISCUSSION
In animal models, the susceptibility to obesity and related metabolic co-morbidities is influenced by maternal body composition in the pre-conception and gestational periods (19–22). Nonetheless, the precise mechanisms contributing to long-term changes remain unclear. Several novel observations are evident from the present work. Exposure to maternal obesity led to: 1) distinct changes in gene expression profiles in each of the functionally discrete zones of the placentation site, characterized by pro-inflammatory and acute-phase response changes; 2) a consistent decrease in expression of thyroid hormone pathway, both in the placenta and in fetal tissues, in line with previous findings of decreased energy expenditure; and 3) lower TRH, TRβ, and UCP1 gene expression in placenta from OB women. Together, these results suggest that the role of lower thyroid hormone expression is worthy of further study as a possible pathway leading to low energy metabolism and development of obesity in animals born to obese mothers.
Our goal was to elucidate key changes in the placentation site and offspring liver associated with maternal obesity. We previously reported that each compartment of the late-gestation placenta shows distinct gene expression signatures consistent with the specialized nature of differentiated trophoblast cells populating these zones (10). Hence, we reasoned that the effects of maternal OB on the placenta are likely to be non-uniform and cell-context specific. Certainly, this was evident in the extent of gene expression changes in each zone, with the maternal interacting components showing the most influence of maternal OB (MG and JZ). Of these, response to cytokines, LPS and lipid stimulus predominated within the MG are particularly noteworthy and consistent with our overall understanding of OB-driven metabolic changes in pregnancy. Recent studies in term placenta from non-diabetic OB women (13) and from HFD-fed mice (23) showed increased lipid in the placenta, consistent with the observed GO term enrichment. Likewise, in OB-rat dams, lipid accumulation in the uterine endometrium occurs in early gestation along with gene expression indicative of lipotoxicity (3). Several lines of evidence suggest that maternal OB is associated with an inflammatory placental milieu, characterized by elevated pro-inflammatory cytokines and activation of TLR/JNK/NF-κB signaling (3;13;17;24–28). Likewise, we recently identified JNK and EGR-1 as key regulators of placental cytokine expression in response to lipotoxic insults and observed increased expression of JNK/EGR-1 and NF-κB pathways in villous placenta from obese women (13;17). Consistent with these observations, our results showing increased Tnfα and Egr1 mRNA in the LZ (functionally equivalent to villous tissue) clearly suggest enhanced pro-inflammatory signaling in late-gestation placenta in the rat.
The JZ of the rat placenta, located at the maternal-placental interface, houses three cell types: spongiotrophoblasts and trophoblast giant cells, specialized trophoblasts that to secrete hormones and peptides, and glycogen cells. The parietal-trophoblast giant cells form the boundary of the implantation site and are in direct contact with the uterine decidua. Hence, it is likely that changes in the MG (such as lipotoxicity) are directly communicated to the endocrine cells of the JZ. Indeed, recent studies in HFD-fed rats showed more pronounced effects in weights of the JZ, in the absence of changes in the labyrinth compartment (29). The JZ also serves as the source of invasive trophoblasts that migrate into the myometrium, remodel uterine blood vessels, and replace endothelial cells, facilitating greater blood flow to the LZ (9;12). Wnt and TGF-β family proteins (including BMPs) play critical multi-faceted roles in trophoblast lineage commitment, differentiation and migration (30;31). Hence, our results showing decreases in mRNA expression of BMP-4 antagonist, Noggin, and Wnt signaling antagonists, Sfrp4 and Sfrp5, are likely to influence both differentiation and migration of trophoblasts. Most importantly, GO analysis of differentially expressed genes in JZ showed enrichment of cell migration, motility and adhesion related genes.
Consistent with a previous report in non-human primates showing that maternal HFD was associated with alterations in the fetal thyroid axis (32), our studies identified thyroid signaling in the placenta and offspring as a target of maternal OB. Thyroid hormones are important regulators of basal metabolism and mediate pleiotropic effects in virtually all tissues. Systemically, the active hormone triiodothyronine (T3) and its precursor thyroxine (T4) are regulated via TRH-mediated regulation of TSH in the pituitary. In the periphery, T3 binds thyroid hormone receptors alpha (TRα) and beta (TRβ) to transcriptionally regulate thyroid hormone responsive genes (including UCPs, ME1 and MBP) controlling metabolism and fat mass accrual. A critical mechanism regulating thyroid hormone signaling is via activation /inactivation through diiodination by DIO enzymes which either amplify (DIO1 and DIO2) or suppress (DIO3) intracellular T3 levels (33). Collectively, our results are in line with lower basal metabolism, decreased energy expenditure, and an impaired ability to mobilize fatty acids (2;5;6), observed in offspring of OB rats. Interestingly, in a report by Franco et al., weanling offspring born to dams fed a HFD throughout gestation and lactation showed a hyperactive thyroid axis that was accompanied by increased adiposity, central leptin resistance, and decreased β-adrenergic receptors in liver and adipose (37). The discrepancies between previous reports and the present study may be a consequence of the feeding paradigm used (overfeeding-induced obesity vs HFD-induced obesity) or more importantly the timing of offspring exposure to the maternal environment (gestation only vs. gestation and lactation).
Currently, changes in placental thyroid signaling in the context of maternal OB have not been previously described. Thyroid hormone transporters, deiodinase enzymes, and thyroid hormone binding proteins localized in placental tissues have bone fide roles in placental development and function (39–42). Consistent with the endocrine nature of the JZ, our studies identified highest expression of Trh and Dio2 genes in this compartment, which were also influenced by maternal OB. While one limitation of our study is that we did not directly measure downstream thyroid hormone signaling, we observed decreased protein levels of DIO2, suggesting that tissue levels of T3 may be reduced in placenta from OB rats. Importantly, placental TRH is predominately released into fetal circulation and may influence the fetal hypothalamic-pituitary (HPT) axis (43), suggesting that lower TRH in OB placenta may play a role in regulating fetal growth and metabolism. Further, changes in genes controlling thyroid signaling and downstream targets showed strong inverse association with maternal leptin. Leptin stimulates hypothalamic TRH via STAT3 phosphorylation in TRH neurons (44) and is thought to play a role in regulating the HPT axis. Additionally, leptin levels increase during normal pregnancy to regulate maternal food intake and promote placental nutrient transport to the fetus (45). Hyperleptinemia and central leptin resistance are characteristics of obesity and evidence is emerging that placental leptin resistance exists in obese pregnancies (46). Another limitation is that leptin regulation of TRH in placental tissue has yet to be shown, though our data suggests that placental leptin resistance may contribute to lower TRH production. Collectively, while these data do not indicate causality, they provide associative evidence for maternal OB-associated changes in placental and offspring thyroid signaling in altering offspring metabolism and obesity susceptibility.
In summary, exposure to maternal OB leads to extensive changes in the placentation site characterized by unique changes in the individual placental compartments. Broadly, maternal OB promotes changes in inflammatory signaling, lipid metabolism, and hormone stimulus in the placenta. Consistent with the premise that in utero programming leads to altered metabolism, offspring of OB dams show alterations in the placental-fetal thyroid axis. In particular, maternal OB-associated decreases in TRH mRNA expression in rat and human placenta may be an underlying mechanism for dysfunctional fetal metabolism. Alterations in expression of thyroid hormone signaling components in OB-dam offspring persist in tissues important in basal metabolism, suggesting that gestational obesity associated alterations in offspring metabolism may originate in early embryonic and placental development.
Supplementary Material
Figure S1. Serum T3 and T4 was assessed via ELISA in dams at dpc 18.5 and offspring at PND21.
TABLE S1 Primer sequences for real-time RT-PCR
Table S2 Maternal Characteristics in Lean and Obese Dams at dpc 18.5
Table S3. Clinical Characteristics of Pregnancies for Placentas Studied
HIGHLIGHTS.
Each utero-placental compartment responds distinctly to maternal obesity (OB)
Inflammation, lipid metabolism and hormone stimulus are predominant effects
Maternal OB negatively influences placental and offspring thyroid hormone regulators
Acknowledgments
We gratefully acknowledge the assistance of Matt Ferguson and the members of the Arkansas Children’s Nutrition Center-Animal Research Facility for their assistance with total enteral nutrition. We also acknowledge the members of the ACNC-Human Studies Core for their assistance in studies using human subjects. These studies were supported in part by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK084225 (K. Shankar) and the USDA Agriculture Research Service Grant CRIS 6251-51000-007-04S. Nursing support for these studies was provided in part by the UAMS Translational Research Institute funded by the National Institutes of Health Clinical and Translational Science Award (CTSA) program, grants UL1TR000039 and KL2TR000063. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ABBREVIATIONS
- BW
body weight
- dpc
days post coitum
- FL
fetal liver
- GO
gene ontology
- JZ
junctional zone
- LZ
labyrinth zone
- MG
metrial gland
- OB
obesity
- RPKM
reads per kilobase per million mapped reads
- TEN
total enteral nutrition
Footnotes
DISCLOSURES
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294:R528–R538. doi: 10.1152/ajpregu.00316.2007. [DOI] [PubMed] [Google Scholar]
- 2.Shankar K, et al. Maternal overweight programs insulin and adiponectin signaling in the offspring. Endocrinology. 2010;151:2577–2589. doi: 10.1210/en.2010-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankar K, et al. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology. 2011;152:4158–4170. doi: 10.1210/en.2010-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borengasser SJ, et al. Maternal Obesity Enhances White Adipose Tissue Differentiation and Alters Genome-Scale DNA Methylation in Male Rat Offspring. Endocrinology. 2013;154:4113–4125. doi: 10.1210/en.2012-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borengasser SJ, et al. Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS One. 2011;6:e24068. doi: 10.1371/journal.pone.0024068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borengasser SJ, et al. High fat diet and in utero exposure to maternal obesity disrupts circadian rhythm and leads to metabolic programming of liver in rat offspring. PLoS One. 2014;9:e84209. doi: 10.1371/journal.pone.0084209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cross JC. Placental function in development and disease. Reprod Fertil Dev. 2006;18:71–76. doi: 10.1071/rd05121. [DOI] [PubMed] [Google Scholar]
- 8.Maltepe E, Bakardjiev AI, Fisher SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120:1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol. 2003;260:176–190. doi: 10.1016/s0012-1606(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 10.Shankar K, et al. RNA-seq analysis of the functional compartments within the rat placentation site. Endocrinology. 2012;153:1999–2011. doi: 10.1210/en.2011-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar K, Harrell A, Kang P, Singhal R, Ronis MJ, Badger TM. Carbohydrate-Responsive Gene Expression in the Adipose Tissue of Rats. Endocrinology. 2009;151:153–164. doi: 10.1210/en.2009-0840. [DOI] [PubMed] [Google Scholar]
- 12.Ain R, Konno T, Canham LN, Soares MJ. Phenotypic analysis of the rat placenta. Methods Mol Med. 2006;121:295–313. doi: 10.1385/1-59259-983-4:293. [DOI] [PubMed] [Google Scholar]
- 13.Saben J, et al. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35:171–177. doi: 10.1016/j.placenta.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saben J, et al. A comprehensive analysis of the human placenta transcriptome. Placenta. 2013;35:125–131. doi: 10.1016/j.placenta.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Res. 1997;7:986–995. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- 16.Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009;10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saben J, et al. Early growth response protein-1 mediates lipotoxicity-associated placental inflammation: role in maternal obesity. Am J Physiol Endocrinol Metab. 2013;305:E1–14. doi: 10.1152/ajpendo.00076.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jonge HJ, et al. Evidence based selection of housekeeping genes. PLoS One. 2007;2:e898. doi: 10.1371/journal.pone.0000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ainge H, Thompson C, Ozanne SE, Rooney KB. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes (Lond) 2011;35:325–335. doi: 10.1038/ijo.2010.149. [DOI] [PubMed] [Google Scholar]
- 20.Alfaradhi MZ, Ozanne SE. Developmental programming in response to maternal overnutrition. Front Genet. 2011;2:27. doi: 10.3389/fgene.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samuelsson AM, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 22.Nathanielsz PW, Poston L, Taylor PD. In utero exposure to maternal obesity and diabetes: animal models that identify and characterize implications for future health. Clin Perinatol. 2007;34:515–26. v. doi: 10.1016/j.clp.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Heerwagen MJ, Stewart MS, de la Houssaye BA, Janssen RC, Friedman JE. Transgenic increase in N-3/n-6 Fatty Acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS One. 2013;8:e67791. doi: 10.1371/journal.pone.0067791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu S, et al. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring) 2011;19:476–482. doi: 10.1038/oby.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu S, Leahy P, Challier JC, Minium J, Catalano P, Hauguel-de Mouzon S. Molecular phenotype of monocytes at the maternal-fetal interface. Am J Obstet Gynecol. 2011;205:265–268. doi: 10.1016/j.ajog.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Challier JC, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29:274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farley D, et al. Feto-placental adaptations to maternal obesity in the baboon. Placenta. 2009;30:752–760. doi: 10.1016/j.placenta.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts KA, et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32:247–254. doi: 10.1016/j.placenta.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Mark PJ, et al. A maternal high-fat diet in rat pregnancy reduces growth of the fetus and the placental junctional zone, but not placental labyrinth zone growth. J DoHAD. 2011;2:63–70. [Google Scholar]
- 30.Lichtner B, Knaus P, Lehrach H, Adjaye J. BMP10 as a potent inducer of trophoblast differentiation in human embryonic and induced pluripotent stem cells. Biomaterials. 2013;34:9789–9802. doi: 10.1016/j.biomaterials.2013.08.084. [DOI] [PubMed] [Google Scholar]
- 31.Knofler M, Pollheimer J. Human placental trophoblast invasion and differentiation: a particular focus on Wnt signaling. Front Genet. 2013;4:190. doi: 10.3389/fgene.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suter MA, et al. Maternal high-fat diet modulates the fetal thyroid axis and thyroid gene expression in a nonhuman primate model. Mol Endocrinol. 2012;26:2071–2080. doi: 10.1210/me.2012-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams GR, Bassett JH. Deiodinases: the balance of thyroid hormone: local control of thyroid hormone action: role of type 2 deiodinase. J Endocrinol. 2011;209:261–272. doi: 10.1530/JOE-10-0448. [DOI] [PubMed] [Google Scholar]
- 34.de Jesus LA, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108:1379–1385. doi: 10.1172/JCI13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christoffolete MA, et al. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes. 2004;53:577–584. doi: 10.2337/diabetes.53.3.577. [DOI] [PubMed] [Google Scholar]
- 36.Marsili A, et al. Mice with a targeted deletion of the type 2 deiodinase are insulin resistant and susceptible to diet induced obesity. PLoS One. 2011;6:e20832. doi: 10.1371/journal.pone.0020832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franco JG, et al. Maternal high-fat diet induces obesity and adrenal and thyroid dysfunction in male rat offspring at weaning. J Physiol. 2012;590:5503–5518. doi: 10.1113/jphysiol.2012.240655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowell BB, Flier JS. Brown adipose tissue, beta 3-adrenergic receptors, and obesity. Annu Rev Med. 1997;48:307–316. doi: 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- 39.Chan SY, Vasilopoulou E, Kilby MD. The role of the placenta in thyroid hormone delivery to the fetus. Nat Clin Pract Endocrinol Metab. 2009;5:45–54. doi: 10.1038/ncpendmet1026. [DOI] [PubMed] [Google Scholar]
- 40.Vasilopoulou E, et al. Monocarboxylate transporter 8 modulates the viability and invasive capacity of human placental cells and fetoplacental growth in mice. PLoS One. 2013;8:e65402. doi: 10.1371/journal.pone.0065402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canettieri G, et al. Activation of thyroid hormone is transcriptionally regulated by epidermal growth factor in human placenta-derived JEG3 cells. Endocrinology. 2008;149:695–702. doi: 10.1210/en.2007-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel J, Landers K, Li H, Mortimer RH, Richard K. Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab. 2011;22:164–170. doi: 10.1016/j.tem.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Bajoria R, Babawale M. Ontogeny of endogenous secretion of immunoreactive-thyrotropin releasing hormone by the human placenta. J Clin Endocrinol Metab. 1998;83:4148–4155. doi: 10.1210/jcem.83.11.5216. [DOI] [PubMed] [Google Scholar]
- 44.Huo L, Munzberg H, Nillni EA, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic trh gene expression by leptin. Endocrinology. 2004;145:2516–2523. doi: 10.1210/en.2003-1242. [DOI] [PubMed] [Google Scholar]
- 45.von Versen-Hoynck F, Rajakumar A, Parrott MS, Powers RW. Leptin affects system A amino acid transport activity in the human placenta: evidence for STAT3 dependent mechanisms. Placenta. 2009;30:361–367. doi: 10.1016/j.placenta.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tessier DR, Ferraro ZM, Gruslin A. Role of leptin in pregnancy: consequences of maternal obesity. Placenta. 2013;34:205–211. doi: 10.1016/j.placenta.2012.11.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Serum T3 and T4 was assessed via ELISA in dams at dpc 18.5 and offspring at PND21.
TABLE S1 Primer sequences for real-time RT-PCR
Table S2 Maternal Characteristics in Lean and Obese Dams at dpc 18.5
Table S3. Clinical Characteristics of Pregnancies for Placentas Studied