Abstract
Background/Objectives
Peripheral nerve impairments are highly prevalent in older adults and are associated with poor lower-extremity function. Whether sensorimotor nerve function predicts incident mobility disability has not been determined. We assessed the relationship between sensorimotor nerve function and incident mobility disability over 10 years.
Design
Prospective cohort study with longitudinal analysis.
Setting
Two U.S. clinical sites.
Participants
Population-based sample of community-dwelling older adults with no mobility disability at 2000/01 exam (N = 1680; mean ± SD: age = 76.5 ± 2.9, BMI = 27.1 ± 4.6; 50.2% women, 36.6% black and 10.7% with diabetes).
Measurements
Motor nerve conduction amplitude (poor: <1 mV) and velocity (poor: <40 m/s) were measured on the deep peroneal nerve. Sensory nerve function was measured using 10-g and 1.4-g monofilaments and vibration detection threshold at the toe. Lower-extremity symptoms included numbness or tingling and sudden stabbing, burning, pain or aches. Incident mobility disability assessed semiannually over 8.5 years (IQR: 4.5–9.6) was defined as two consecutive self-reports of a lot of difficulty or inability to walk ¼ mile or climb 10 steps.
Results
Nerve impairments were detected in 55% of participants and 30% developed mobility disability. Worse motor amplitude (HR = 1.29 per SD, 95% CI: 1.16–1.44), vibration detection threshold (HR = 1.13 per SD, 95% CI: 1.04–1.23), symptoms (HR = 1.65, 95% CI: 1.36–2.17), 2 motor (HR = 2.10, 95% CI: 1.43–3.09), 2 sensory (HR = 1.91, 95% CI: 1.37–2.68), and ≥3 nerve impairments (HR = 2.33, 95% CI: 1.54–3.53) predicted incident mobility disability, after adjustment. Quadriceps strength mediated relationships between certain nerve impairments and mobility disability, although most remained significant.
Conclusion
Poor sensorimotor nerve function independently predicted mobility disability. Future work should investigate modifiable risk factors and interventions like strength training for preventing disability and improving function in older adults with poor nerve function.
Keywords: Peripheral nerve function, disability, older adults, longitudinal analysis, muscle strength
INTRODUCTION
Poor peripheral nerve function is common in older adults and may partially account for poorer physical function in individuals with diabetes.1,2 However, even in older adults without diabetes, the incidence3 and prevalence of poor nerve function are high.4 In the 1999–2000 National Health and Nutrition Examination Survey (NHANES) 28% of adults aged 70 to 79 years and 35% of adults aged 80 years or older had impaired peripheral nerve function measured using simple screening for reduced sensation at the foot.4 Poor peroneal motor nerve conduction and sensory nerve function are cross-sectionally associated with poor balance, slower gait speed, lower performance scores,1,5 and lower-extremity quadriceps and ankle dorsiflexion strength.6 These physical function measures predict activities of daily living (ADL) and mobility disability,7–10 suggesting that poor nerve function may affect late-life disability. Moreover, reduced vibration sensation and neurological signs are cross-sectionally related to mobility disability, defined as self-reported difficulty walking or climbing stairs.2,11 These findings have critical implications for older adults, since they experience the highest burden of both poor nerve function and disability.3,4,12
Quantification of the predictive association between sensory and motor nerve function and incident mobility disability has not been undertaken. We assessed whether poor sensory and motor peripheral nerve function predict incident mobility disability in community-dwelling older adults.
METHODS
Study Participants
The Health, Aging and Body Composition (Health ABC) study is a prospective cohort study established in 1997–1998 to investigate changes in body composition and mobility decline in a biracial cohort of older adults (n = 3,075; 48.4% male; 41.6% black, aged 70–79 years at baseline). Participants were recruited by mail from a random sample of white Medicare beneficiaries and all black community residents eligible by age. Eligibility, determined by phone interview, included reporting no difficulty walking a quarter of a mile or walking up 10 steps, no difficulty performing mobility-related activities of daily living, no life-threatening cancers with active treatment within the past three years, and no plans to move from the study area for the next three years. The study protocol was approved by the institutional review boards at the University of Pittsburgh and the University of Tennessee Health Science Center and written informed consent was provided prior to testing. Out of 3075 participants at baseline, 2405 had ≥1 nerve function measure at the 2000/01 visit and 2148 with no mobility disability prior to the 2000/01 visit were analyzed. Reasons for no nerve function measure included refusal of nerve exam (n = 1), home visit (n = 73), phone visit (n = 233), or proxy visit (n = 63), other reason for missed visit (n = 104), deceased (n = 187), or withdrew from study (n = 9). Participants who did not undergo nerve function testing were slightly older (mean ± SD: 77.0 ± 2.9 vs. 76.5 ± 2.9 years, p = 0.004), had higher depression scores (Center for Epidemiologic Studies Depression Scale [CES-D]: 5.4 ± 5.8 vs. 4.3 ± 5.0, p < 0.001), lower physical activity levels (6.6 ± 12.4 vs. 8.7 ± 19.9 kcal/kg/week, p < 0.001), poorer cognitive performance (Modified Mini-Mental State Examination [3MSE]: 87.5 ± 10.4 vs. 91.1 ± 7.4, p < 0.001), lower strength (101.2 ± 35.9 vs. 109.4 ± 38.9 Nm, p < 0.001), and a higher prevalence of diabetes (22.1% vs. 16.5%, p = 0.002), hypertension (67.7% vs. 57.9%, p < 0.001), cardiovascular disease (25.4% vs. 16.5%, p < 0.001), cerebrovascular disease (8.7% vs. 6.02%, p = 0.02), and peripheral arterial disease (19.8% vs. 11.9%, p < 0.001) at baseline.
Mobility Disability
Mobility disability was assessed semiannually over a maximum follow-up of 10 years during clinic visits or over the phone with the participant or an identified proxy and was defined as two consecutive self-reports of a lot of difficulty or inability to walk a ¼ of a mile or climb 10 steps. Time to event was calculated as the time from the 2000/01 nerve function exam to the first self-or proxy-report of mobility disability out of the two consecutive reports. Participants who did not experience disability were censored at their last contact or at death. If death was preceded by one or more missed contacts, information from the decedent proxy was used to determine disability. The estimated date of onset obtained from the proxy was compared to the visit window for the missed contact and if the reported onset occurred before the end of the visit window, incident disability was assigned to the missed contact.
Peripheral Nerve Function
Peripheral sensory and motor nerve function were measured at the 2000/01 visit by a trained and certified clinic examiner as described previously.13 Peripheral motor nerve function was measured objectively as (1) peroneal motor nerve conduction amplitude in millivolts (mV) with stimulation at the popliteal fossa, fibular head and ankle using the NeuroMax 8 (XLTEK, Oakville, Ontario, Canada) and (2) nerve conduction velocity in meters per second (m/s). Sensory nerve function was measured as (1) vibration detection threshold in microns (µ) on the bottom of the large toe with a VSA-3000 Vibratory Sensory Analyzer (Medoc, Ramat Yishai, Israel), and (2) monofilament insensitivity, defined as the inability to feel three to four touches at the dorsum of the large toe with a 10-g and a 1.4-g monofilament. All assessments were performed after the feet were warmed to 30°C. Measures were performed on the right leg unless contraindicated because of knee replacement, amputation, trauma, ulcer, or surgery. If the right leg was contraindicated, measures were performed on the left leg, unless it too was contraindicated. Peripheral neuropathy symptoms were self-reported; these include (1) numbness or tingling, and (2) sudden stabbing, burning, pain or aches, both within the past 12 months.
We used clinically meaningful cut points of <1 mV for motor amplitude and <40 m/s for motor nerve conduction velocity (NCV) to define motor nerve impairment.14Sensory nerve impairment was defined as 1.4-g or 10-g monofilament insensitivity or insensitivity to a vibration threshold >131 µ.
Additional Covariates
We considered several factors known or hypothesized to be associated with both poor nerve function and incident mobility disability. Covariates were measured concurrent with the 2000/01 nerve exam unless otherwise noted. Standing height was measured using a stadiometer and weight was measured using a calibrated balance beam scale. Dual-energy X-ray absorptiometry (DXA; 4500A, Hologic, Inc., Bedford, MA) was used to measure total body bone-free lean and fat mass. Diabetes was defined as self-reported physician diagnosis, hypoglycemic medication use, or fasting glucose >126 mg/dL [47.0 mmol/L] after an 8-hour fast.15 Ankle brachial index (ABI) was used to indicate peripheral arterial disease (<0.9) and arterial stiffening (>1.3).16 Hypertension was determined by self-report, medication use, and diastolic blood pressure ≥90 mmHg or systolic blood pressure ≥140 mmHg. Prevalent cardiovascular disease (bypass/coronary artery bypass graft, carotid endarterectomy, myocardial infarction, angina, or congestive heart failure), cerebrovascular disease (transient ischemic attack or stroke), and osteoporosis were assessed at baseline (1997/98). Knee pain on most days or for ≥1 month in the past 12 months and pain in the legs when walking were self-reported. Interviewers administered the CES-D17 to assess depressive symptoms. Poor vitamin B12 status was defined as < 260 pmol/L.18 Poor renal function was defined as Cystatin-C >1 mg/dL. Smoking status (current/past) was measured during the 1999/00 visit and alcohol consumption (>1 drink/week) was measured at baseline. Physical activity was estimated as kcal/kg/week spent walking and climbing stairs using a questionnaire.19Cognitive function was measured with the 3MSE (1999/00), and attention, psychomotor speed, and executive function were measured with the Digit Symbol Substitution Test (DSST) (1997/98).20 Knee extensor strength was measured concentrically at 60° per second using an isokinetic dynamometer (Kin-Com 125 AP Dynamometer, Harrison, TN). After warm-up at a submaximal effort, 3–6 trials were performed on the right leg unless a participant had knee pain or a knee replacement. Quadriceps strength was calculated as the mean maximal torque (Nm) from the 3 best trials.21
Statistics
Means and frequencies of characteristics from the 2000/01 visit (unless noted in Additional Covariates) were calculated for the overall study population and for groups stratified by number of peripheral nerve impairments. Tests for trend across groups were performed using Jonckheere-Terpstra tests and Generalized Linear Models. Relationships between time to disability and each nerve function predictor were modeled using multivariable Cox proportional hazards regression. We built four sets of models: (1) nerve function adjusting for age, sex, race, study site, height, and weight; (2) Model 1 plus diabetes; (3) Model 2 substituting lean and fat mass for body weight; (4) Model 3 plus additional covariates related to the outcome or the predictor using an alpha of 0.1. To minimize collinearity, additional covariates were subsequently removed from Model 4 if they had a p-value >0.1 and if their removal did not substantially change the effect of the predictor of interest. We tested interactions between nerve function and sex, which were nonsignificant, and nerve function and diabetes, which significantly predicted disability at an alpha of <0.1 for 1.4-g monofilament insensitivity and symptoms. Given that strength is related to mobility disability8–10 and associated with motor and sensory nerve function,6 we added knee extensor strength to the fully adjusted multivariable models to test whether it mediated the relationship between nerve function and disability. As a sensitivity analysis, we excluded participants with prevalent diabetes from the 2000/01 visit and incident diabetes up to the 2007/08 visit to test its influence on the associations between nerve function and disability. The assumption of proportional hazards was assessed for all variables by testing whether the interactions of the predictors with survival time were related to incident disability at a 0.01 level. Using this criterion, all variables met the proportional hazards assumption. We used SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) to perform statistical analyses. This research was supported by the National Institute on Aging, which approved this manuscript.
RESULTS
After a median follow-up time of 8.5 years (Interquartile Range [IQR]: 4.5–9.6) from the 2000/01 nerve exam, 655 participants (30%) developed disability. Participants with a greater number of peripheral nerve impairments tended to be older, have a higher prevalence of peripheral arterial disease, arterial stiffening, and low vitamin B12, and poorer 3MSE and DSST scores (Table 1). Groups with more nerve impairments also had more men, and therefore greater quadriceps strength, and more lean mass. In addition, participants with more nerve impairments had worse motor amplitude, conduction velocity, vibration detection threshold, and a greater prevalence of light and standard monofilament insensitivity (Table 2). However, there was no trend in symptoms by number of peripheral nerve impairments.
Table 1.
Characteristics of Total Study Population and Stratified by Number of Peripheral Nerve Impairments
| Number of Peripheral Nerve Impairments | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Total (n=2148) |
Zero (n=692) |
One (n=589) |
Two (n=191) |
≥ Three (n=60) |
p-value for trend |
| Age, years | 76.5 ± 2.9 | 76.1 ± 2.8 | 76.6 ± 2.9 | 77.1 ±2.9 | 77.2 ±2.9 | 0.001 |
| Women, n (%) | 1079 (50.2) | 426 (61.6) | 306 (52.0) | 48 (25.1) | 15 (25.0) | <0.001 |
| Black race, n (%) | 785 (36.6) | 240 (34.7) | 210 (35.7) | 65 (34.0) | 15 (25.0) | 0.62 |
| Quadriceps strength, Nm | 96.5 ± 34.7 | 93.4 ± 32.8 | 98.1 ±36.0 | 103.1 ± 35.1 | 104.2 ± 37.7 | 0.01 |
| Body Composition | ||||||
| BMI, kg/m2 | 27.1 ± 4.6 | 27.0 ±4.2 | 26.6 ± 4.3 | 26.2 ± 4.5 | 27.1 ± 4.7 | 0.89 |
| Lean mass, kg | 48.3 ±10.2 | 45.8 ±9.2 | 47.8 ±10.4 | 52.2 ±10.2 | 54.9 ±9.6 | <0.001 |
| Fat mass, kg | 26.5 ±8.4 | 26.6±7.9 | 25.8 ±7.9 | 24.7 ±8.3 | 26.5 ± 9.2 | 0.63 |
| Lifestyle characteristics | ||||||
| Current smoker, n (%) | 176 (8.3) | 60 (8.8) | 46 (7.8) | 12 (6.4) | 4 (6.8) | 0.26 |
| Past smoker, n (%) | 974 (46.2) | 302 (44.3) | 266 (45.6) | 103 (54.8) | 28 (47.5) | 0.06 |
| Alcohol consumption > 1/week, n (%) | 1103 (52.4) | 373 (54.9) | 307 (52.7) | 91 (48.7) | 30 (50.9) | 0.14 |
| Physical activity, kcal/kg/week | 6.3 ± 17.5 | 5.6 ±8.5 | 6.4 ± 11.8 | 5.5 ± 7.1 | 4.2 ± 6.8 | 0.19 |
| Chronic conditions | ||||||
| Diabetes, n (%) | 437 (10.7) | 106 (15.5) | 106 (18.2) | 50 (26.9) | 18 (30.0) | <0.001 |
| Impaired fasting glucose, n (%) | 348 (16.5) | 106 (15.5) | 107 (18.4) | 32 (17.2) | 11 (18.3) | 0.24 |
| Cardiovascular disease, n (%) | 344 (17.4) | 95 (14.0) | 103 (17.7) | 30 (16.0) | 11 (19.0) | 0.12 |
| Cerebrovascular disease, n (%) | 126 (6.1) | 34 (5.0) | 36 (6.2) | 14 (7.5) | 3 (5.2) | 0.21 |
| Hypertension, n (%) | 1707 (80.9) | 547 (79.1) | 448 (76.1) | 160 (83.8) | 51 (85.0) | 0.42 |
| ABI <0.9, n (%) | 315 (15.3) | 82 (12.1) | 95 (16.6) | 31 (16.9) | 9 (16.1) | 0.02 |
| ABI >1.3, n (%) | 105 (5.1) | 25 (3.7) | 29 (5.1) | 16 (8.7) | 7 (12.5) | 0.001 |
| Depression, CES-D score | 5.4 ± 5.5 | 5.7 ± 6.0 | 6.2 ± 6.0 | 6.0 ± 6.3 | 5.9 ± 5.6 | 0.84 |
| Osteoporosis, n (%) | 76 (3.6) | 36 (5.3) | 12 (2.1) | 6 (3.2) | 3 (5.0) | 0.002 |
| Knee or leg pain, n (%) | 665 (31.1) | 195 (28.2) | 176 (29.9) | 58 (30.4) | 23 (38.3) | 0.20 |
| Low vitamin B12, n (%) | 345 (16.8) | 92 (13.7) | 100 (17.9) | 41 (22.3) | 9 (15.3) | 0.007 |
| Cognition | ||||||
| 3MSE score | 90.7 ± 8.0 | 92.0 ± 6.8 | 91.4 ± 7.0 | 90.2 ± 8.3 | 90.0 ± 7.8 | 0.01 |
| DSST score | 37.2 ± 14.5 | 39.3 ± 14.0 | 37.8 ± 14.9 | 33.9 ±15.2 | 35.4 ± 12.4 | <0.01 |
Data are means ±SD unless otherwise specified; SD = standard deviation; Nm = Newton meters; ABI = ankle brachial index; CES-D = Center for Epidemiologic Studies Depression Scale; 3MSE = Modified Mini Mental State Exam; DSST = Digit Symbol Substitution Test
Table 2.
Peripheral Nerve Characteristics of Total Study Population and Stratified by Number of Peripheral Nerve Impairments
| Number of Peripheral Nerve Impairments | ||||||
|---|---|---|---|---|---|---|
| Peripheral nerve characteristics |
Total (n=2148) |
Zero (n=692) |
One (n=589) |
Two (n=191) |
≥ Three (n=60) |
p-value for trend |
| Motor nerve function | ||||||
| Amplitude, mV | 3.4 ± 2.0 | 3.9 ± 1.8 | 3.6 ± 1.8 | 2.3 ± 1.6 | 0.6 ± 0.3 | <0.001 |
| NCV, m/s | 43.7 ± 5.3 | 46.3 ± 4.0 | 43.1 ± 4.9 | 38.8 ± 4.8 | 36.5 ± 3.9 | <0.001 |
| Sensory nerve function | ||||||
| Vibration detection threshold, µ | 51.2 ± 35.4 | 40.0 ± 28.0 | 50.3 ±31.6 | 72.1 ± 39.6 | 80.1 ± 45.1 | <0.001 |
| Monofilament insensitivity | ||||||
| 10-g, n (%) | 178 (8.4) | 0 (0) | 41 (7.0) | 26 (13.6) | 28 (46.7) | <0.001 |
| 1.4-g, n (%) | 959 (45.5) | 0 (0) | 417 (70.8) | 164 (85.9) | 60 (100.0) | <0.001 |
| Peripheral neuropathy symptoms | ||||||
| Pain, n (%) | 315 (14.8) | 98 (14.2) | 74 (12.6) | 24 (12.6) | 9 (15.0) | 0.50 |
| Numbness, n (%) | 568 (26.6) | 166 (24.1) | 147 (25.0) | 58 (30.4) | 19 (31.7) | 0.09 |
| One, n (%) | 561 (26.3) | 172 (25.0) | 153 (26.1) | 52 (27.2) | 22 (36.7) | 0.14 |
| Two, n (%) | 161 (7.6) | 46 (6.7) | 34 (5.8) | 15 (7.9) | 3 (5.0) | 0.86 |
Data are means ± SD unless otherwise specified; SD = standard deviation; mV = millivolts; m/s = meters per second, µ = microns; NCV = nerve conduction velocity
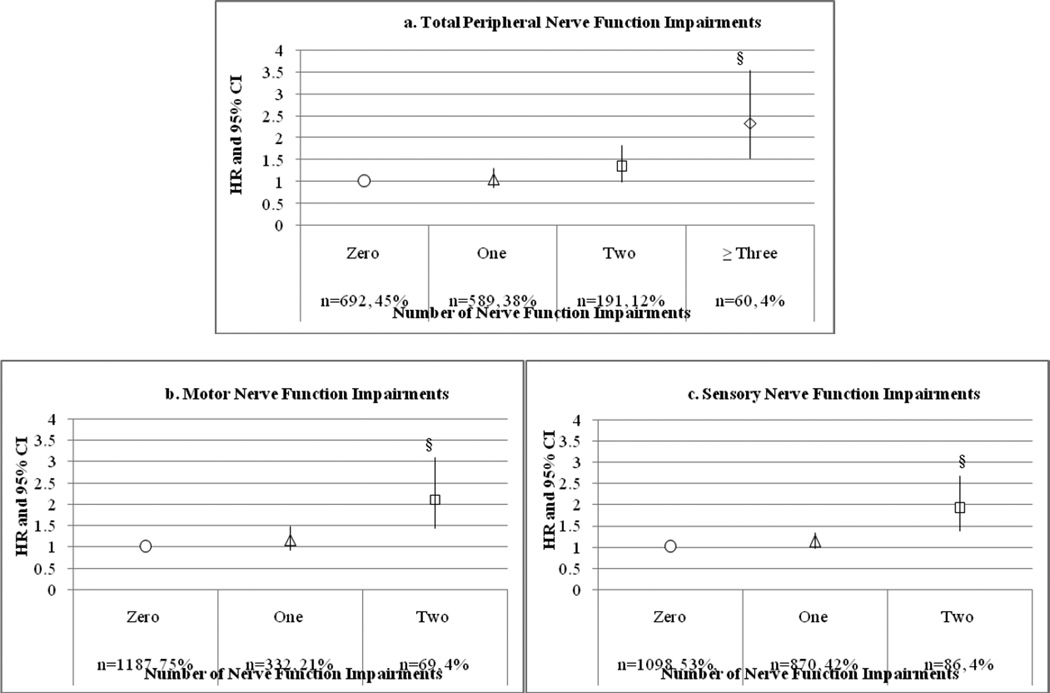
Table 3 shows adjusted hazard ratios and 95% confidence intervals for nerve function measures predicting incident disability. One standard deviation worse motor amplitude (Hazard Ration [HR] = 1.29, 95% Confidence Interval [CI]: 1.16–1.44) and vibration detection threshold (HR = 1.13, 95% CI: 1.04–1.23) and one (HR = 1.34, 95% CI: 1.11–1.63) and two symptoms (HR = 1.65, 95% CI: 1.26–2.17) were associated with incident mobility disability, adjusting for all covariates. Monofilament insensitivity (1.4-g insensitivity with 10-g sensitivity) was associated with disability (HR = 1.19, 95% CI: 1.00–1.40), although the association was attenuated to nonsignificant when adjusted for diabetes and the interaction between monofilament insensitivity and diabetes. Figure 1 shows that having two motor or two sensory nerve function impairments, compared to having none, independently predicted disability (HR = 2.10, 95% CI: 1.43–3.09; HR = 1.91, 95% CI: 1.31–2.88) adjusting for age, race, height, weight, and site. Three or more nerve impairments were associated with incident disability, compared to none (HR = 2.33, 95% CI: 1.54–3.53). These associations remained significant when further adjusting for lifestyle factors, lean and fat mass and chronic conditions (results not shown). Excluding individuals with diabetes in the sensitivity analysis, the majority of results remained consistent; however, the association of having two or more nerve function impairments with disability was attenuated to nonsignificant. When adjusting for strength in the mediation analysis, most results remained significant; although average vibration detection threshold was attenuated by 4% (HR = 1.08, 95% CI: 0.98–1.19) and having two motor nerve impairments was attenuated by 8% (HR = 1.49, 95% CI: 0.93–2.41vs. HR = 1.62, 95% CI: 1.03–2.55 when adjusting for demographics, body composition, chronic conditions, and lifestyle factors) and became nonsignificant. The relationship between 1.4-g insensitivity (with 10-g sensitivity) and disability was attenuated by 5% (HR = 1.11, 95% CI: 0.85–1.62) and remained nonsignificant in the final model. Having three or more nerve function impairments was attenuated by 5% (HR = 1.68, 95% CI: 1.01–2.78 vs. HR= 1.76, 95% CI: 1.08–2.86 when adjusting for demographics, body composition, chronic conditions, and lifestyle factors) but remained a significant predictor of disability. Each of these nerve function measures significantly predicted strength6 and strength significantly predicted disability in all models (results not shown).
Table 3.
Type of Nerve Function Measure and Incident Mobility Disability
| HR (95% CI) | ||||
|---|---|---|---|---|
| 1st Models | 2nd Models | 3rd Models | 4th Models | |
| Motor nerve function | ||||
| Amplitude per SD lower | 1.29 (1.17–1.42)§ | 1.29 (1.17–1.42)§ | 1.30 (1.17–1.44)§ | 1.29 (1.16–1.44)§ |
| NCV per SD lower | 1.10 (0.99–1.22) | 1.10 (0.99–1.22) | 1.10 (0.99–1.23) | 1.06 (0.95–1.19) |
| Sensory nerve function | ||||
| Vibration per SD higher | 1.21 (1.11–1.31)§ | 1.18 (1.09–1.29)§ | 1.20 (1.11–1.31)§ | 1.13 (1.04–1.23)† |
| Monofilament sensitivity | ||||
| Felt None | 1.27 (0.96–1.68) | 1.24 (0.93–1.65) | 1.31 (0.98–1.75) | 1.17 (0.87–1.59) |
| Felt 10g only | 1.19 (1.00–1.40)* | 1.18 (0.99–1.39) | 1.20 (1.01–1.43)* | 1.17 (0.98–1.40) |
| Felt 1.4g | Reference | Reference | Reference | Reference |
| Peripheral neuropathysymptoms | ||||
| None | Reference | Reference | Reference | Reference |
| One | 1.43 (1.20–1.70)§ | 1.43 (1.20–1.70)§ | 1.43 (1.20–1.70)§ | 1.34 (1.11–1.63)† |
| Two | 2.03 (1.59–2.60)§ | 2.03 (1.59–2.60)§ | 2.03 (1.59–2.60)§ | 1.65 (1.26–2.17)§ |
P < 0.05;
P < 0.01;
P < 0.001;
NVC = nerve conduction velocity
1st Models – adjusted for age, race, height, weight, site
2nd Models – 1st Models + diabetes
3rd Models – 2nd Models + lean & fat mass instead of weight
4th Models:
Motor nerve function – 3rd Models + cerebrovascular disease, low and stiffening ankle brachial index, knee and leg pain, Center for Epidemiologic Studies Depression Scale, smoking, physical activity, renal function
Sensory nerve function – 3rd Models + cerebrovascular disease, knee and leg pain, Center for Epidemiologic Studies Depression Scale, smoking, physical activity, renal function
Symptoms – 3rd Models + cerebrovascular disease, knee and leg pain, Center for Epidemiologic Studies Depression Scale, smoking, alcohol consumption, physical activity, renal function
Figure 1. Number of Nerve Function Impairments and Incident Mobility Disability.
§P<0.001; Models adjusted for age, race, height, weight, site
DISCUSSION
Poor sensory and motor nerve function predicted incident mobility disability over 8.5 years and were partially mediated by strength in this large prospective cohort of older adults. These results suggest that having multiple sensory and motor nerve function impairments may compound their effects on disability. Peripheral nerve function is understudied in older adults, particularly in those without diabetes, yet our sensitivity analysis shows that these measures are important predictors of mobility disability even in those without diabetes. These findings have important implications for preventing and delaying mobility disability by targeting older adults at risk and intervening on modifiable risk factors.
Worse motor amplitude, which may be indicative of axonal degeneration, predicted disability, while worse motor nerve conduction velocity, which is a sign of demyelination,22,23 did not. Our findings suggest that degeneration of the motor axon may play a role in the development of disability. Previously, we showed that motor amplitude but not nerve conduction velocity was cross-sectionally related to poor physical performance and quadriceps strength in this cohort.6 These two measures of nerve function may not decline simultaneously. Low amplitude may result from axonal damage to a proportion of nerves, while normal nerve conduction velocity may persist in the nerves that remain intact.24,25Although poor nerve conduction did not predict disability itself, this in combination with poor motor amplitude was associated with an increased incidence, indicating a compound effect compared to each one alone. Even though nerve conduction studies are used clinically to distinguish between axonal degeneration and demyelination,22,23 other physiological and non-physiological parameters may also impact results.26,27 While we were not able to control for all of these parameters, our standardized protocol was designed to minimize additional factors resulting from variation in measurement procedures.13 In addition, in a subsample of this cohort we previously evaluated the influence of factors such as age, body composition, diabetes, gender, and race on the reliability of nerve conduction measures and found that these factors had no significant impact.13
One limitation of previous studies is the reliance on one measure of peripheral nerve function, often a sensory measure.2,5 This is notable because sensory and motor nerves likely play distinct roles in disability. Since overt peripheral neuropathy is partially characterized by symptoms of weakness and in extreme cases muscle wasting,23 one potential mechanism for the relationship between nerve function and disability is the denervation of muscle fibers. Microscopic examination of muscle tissue in overt neuropathy shows atrophied muscle fibers and a shifting from type II fast twitch to type I slow twitch fibers due to denervation and reinnervation.28 These changes potentially lead to decreased muscle strength and power.29 We adjusted for lean mass and tested whether quadriceps strength mediated the relationship between nerve function and mobility disability. We found that, while lean mass and quadriceps strength were independent predictors of disability, after adjusting for them, the relationship between motor nerve amplitude and mobility disability remained, suggesting an independent effect of motor nerve function on disability beyond the effect of muscle. However, strength attenuated the association of two motor nerve impairments with disability by 8% to nonsignificant. Having two motor nerve impairments is likely a sign of more severely damaged nerves. It is possible that poor motor nerve function must reach a certain threshold before it affects strength, which then becomes a mediator in its relationship with disability.
Interestingly, strength mediated the relationships between average vibration detection threshold and 1.4-g monofilament insensitivity with disability by 4% and 5%, respectively, suggesting it may play a role in the relationship between sensory perception and disability. We previously established that sensory nerve function and strength are cross-sectionally associated.6 Others have found that blocking afferent input in healthy individuals can lead to reduced maximal voluntary contractions,30 believed to occur through impaired proprioception.31 Loss of proprioceptive feedback is also thought to be responsible for the relationship between sensory nerve function and impaired balance, gait, and lower extremity performance.1,31,32
The association between disability and 1.4-g monofilament insensitivity was attenuated when adjusting for diabetes and its interaction with nerve function. The interaction between diabetes and 1.4-g monofilament insensitivity significantly predicted disability (HR for interaction = 1.48, 95% CI: 1.02–2.16). The 1.4-g monofilament is typically used to detect subclinical sensory impairment and is not often used in examining individuals with diabetes. Inclusion of this simple, inexpensive test may potentially identify patients with diabetes who are at risk for developing mobility disability.
The magnitudes of the HRs in Figure 1 indicate a possible dose response, with each additional nerve function impairment increasing the HR predicting disability. This is particularly important, given that chronic sensorimotor distal polyneuropathy is one of the most common neuropathies.33 The sensitivity analysis excluding participants with diabetes did not attenuate the relationship between disability and separate nerve function measures but it did attenuate the relationship between disability and having combined nerve function impairments. This is likely because diabetes is a key risk factor for severely impaired nerves and could be a sign that we may be capturing some overt neuropathy with these combined measures.
Strengths of our analysis include the use of multiple sensory and motor nerve function measures, including a gold standard measure of motor nerve conduction that is highly reproducible in a sample of participants from this cohort.13 We investigated the relationship of multiple nerve function impairments individually and together to assess their compounding detrimental effects on disability. However, we did not have sensory nerve conduction measures.
One unique strength is that incident disability was assessed semiannually over a maximum follow-up of 10 years with supplementary proxy report. We used prospective data from a large well-characterized cohort of older adults. One limitation is that participants who were able to come into the clinic for a nerve function exam were healthier that those who were not, potentially resulting in some bias. In addition our participants may have been somewhat healthier than the general population, given they had no disability at the initial nerve exam. Despite this, we found that 55% of participants in the analysis had at least one nerve impairment and that 30% of participants developed disability. These findings illustrate the high prevalence of poor nerve function and the high incidence of disability in older adults, which may be even higher in less healthy populations.
This is the first report that poor peripheral nerve function predicts incident mobility disability in late-life and that this is largely independent of diabetes. The importance of our findings are highlighted by the high incidence of mobility disability and the high prevalence of poor nerve function that we found in this community-dwelling population of older adults.4 It is essential for future work to focus on the prevention of nerve function decline and subsequent disability in those with poor nerve function through known and novel risk factors. More research is needed to develop interventions targeting modifiable risk factors related to peripheral nerve function and their effects on reducing disability. Secondary prevention of disability in those with poor nerve function should also be investigated and our work suggests muscle strength as a potential target for improving physical function in these individuals.
ACKNOWLEDGMENTS
Funding: This research was supported by the National Institute on Aging (contracts N01-AG-6-2101, N01-AG-6-2103, and N01- AG-6-2106) grant 1-R01-AG 028050 (to E.S.S.), and NINR grant R01-NR012459 and supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging, the University of Pittsburgh Claude D. Pepper Older Americans Independence Center (P30-AG024827) Pilot Grant (to E.S.S.), and the American Diabetes Association (1-04-JF-46 to E.S.S.).
Conflict of Interest: Rachel Ward, Robert Boudreau, Jane Cauley, Anne Newman, and Eleanor Simonsick declare grant support from National Institute on Aging grants N01AG062101, R01AG028050, R01AG037451, T32AG000181, National Institute of Nursing Research grant R01NR012459, and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30AG024827. SS declares grant support from National Institute on Aging grants N01AG062101, R01AG028050. AIV declares consultancy from Merck and ISIS Pharmaceuticals, and speaker forum support from Merck and PamLab. JAC declares consultant and expert testimony from Merck.
Sponsor's Role: The funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, and preparation of manuscript or in the decision to submit the manuscript for publication.
Footnotes
These data were presented at the Gerontological Society of America 66th Annual Scientific Meeting in New Orleans, LA, USA:
Ward RE, Caserotti P, Harris T, Vinik AI, Simonsick EM, Cauley JA, Newman AB, Strotmeyer ES. Longitudinal Sensory and Motor Peripheral Nerve Function and Incident Mobility Limitation. Gerontologist. 2013. 53(S1):520. The Gerontological Society of America 66th Annual Scientific Meeting, New Orleans, LA, USA.
Author Contributions:
Anne Newman, Stephen Kritchevsky, and Eleanor M. Simonsick were involved in study concept and design, acquisition of data, interpretation of data, and preparation of manuscript. Rachel E. Ward and Robert Boudreau were involved in analysis and interpretation of data and preparation of manuscript. All other coauthors were involved in interpretation of data and preparation of manuscript.
REFERENCES
- 1.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: The Health, Aging, and Body Composition (Health ABC) study. Diabetes Care. 2008;31:1767–1772. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volpato S, Blaum C, Resnick H, et al. Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The Women's Health and Aging Study. Diabetes Care. 2002;25:678–683. doi: 10.2337/diacare.25.4.678. [DOI] [PubMed] [Google Scholar]
- 3.Baldereschi M, Inzitari M, Di Carlo A, et al. Epidemiology of distal symmetrical neuropathies in the Italian elderly. Neurology. 2007;68:1460–1467. doi: 10.1212/01.wnl.0000260606.36443.29. [DOI] [PubMed] [Google Scholar]
- 4.Gregg EW, Sorlie P, Paulose-Ram R, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 5.Resnick HE, Vinik AI, Schwartz AV, et al. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: The Women's Health and Aging Study. Diabetes Care. 2000;23:1642–1647. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 6.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. Sensory and motor peripheral nerve function and lower-extremity quadriceps strength: The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2009;57:2004–2010. doi: 10.1111/j.1532-5415.2009.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 8.Rantanen T. Muscle strength, disability and mortality. Scand J Med Sci Sports. 2003;13:3–8. doi: 10.1034/j.1600-0838.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 9.Marsh AP, Rejeski WJ, Espeland MA, et al. Muscle strength and BMI as predictors of major mobility disability in the Lifestyle Interventions and Independence for Elders pilot (LIFE-P) J Gerontol A Biol Sci Med Sci. 2011;66:1376–1383. doi: 10.1093/gerona/glr158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 11.Ferrucci L, Bandinelli S, Cavazzini C, et al. Neurological examination findings to predict limitations in mobility and falls in older persons without a history of neurological disease. Am J Med. 2004;116:807–815. doi: 10.1016/j.amjmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Manton KG, Gu X. Changes in the prevalence of chronic disability in the United States black and nonblack population above age 65 from 1982 to 1999. Proc Natl Acad Sci U S A. 2001;98:6354–6359. doi: 10.1073/pnas.111152298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward RE, Boudreau RM, Vinik AI, et al. Reproducibility of peroneal motor nerve conduction measurement in older adults. Clin Neurophysiol. 2013;124:603–609. doi: 10.1016/j.clinph.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maser RE, Nielsen VK, Dorman JS, et al. Measuring subclinical neuropathy: Does it relate to clinical neuropathy? Pittsburgh epidemiology of diabetes complications study-V. J Diabet Complications. 1991;5:6–12. doi: 10.1016/0891-6632(91)90003-8. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchman AS, Wilson RS, Leurgans S, et al. Vibratory thresholds and mobility in older persons. Muscle Nerve. 2009;39:754–760. doi: 10.1002/mus.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts RE, Vernon SW. The Center for Epidemiologic Studies Depression Scale: Its use in a community sample. Am J Psychiatry. 1983;140:41–46. doi: 10.1176/ajp.140.1.41. [DOI] [PubMed] [Google Scholar]
- 18.Leishear K, Boudreau RM, Studenski SA, et al. Relationship between vitamin B12 and sensory and motor peripheral nerve function in older adults. J Am Geriatr Soc. 2012;60:1057–1063. doi: 10.1111/j.1532-5415.2012.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Mehta KM, Simonsick EM, Rooks R, et al. Black and white differences in cognitive function test scores: What explains the difference? J Am Geriatr Soc. 2004;52:2120–2127. doi: 10.1111/j.1532-5415.2004.52575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 22.Arezzo JC, Zotova E. Electrophysiologic measures of diabetic neuropathy: Mechanism and meaning. Int Rev Neurobiol. 2002;50:229–255. doi: 10.1016/s0074-7742(02)50079-9. [DOI] [PubMed] [Google Scholar]
- 23.National Institute of Neurological Disorders and Stroke. Peripheral Neuropathy Fact Sheet. [Accessed February 13, 2013];2012 Available at: http://www.ninds.nih.gov/disorders/peripheralneuropathy/detail_peripheralneuropathy.htm.
- 24.Lauretani F, Bandinelli S, Bartali B, et al. Axonal degeneration affects muscle density in older men and women. Neurobiol Aging. 2006;27:1145–1154. doi: 10.1016/j.neurobiolaging.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falck B, Stalberg E. Motor nerve conduction studies: Measurement principles and interpretation of findings. J Clin Neurophysiol. 1995;12:254–279. [PubMed] [Google Scholar]
- 26.Keenan KG, Farina D, Merletti R, et al. Influence of motor unit properties on the size of the simulated evoked surface EMG potential. Exp Brain Res. 2006;169:37–49. doi: 10.1007/s00221-005-0126-7. [DOI] [PubMed] [Google Scholar]
- 27.Kimura J. Principles and pitfalls of nerve conduction studies. Ann Neurol. 1984;16:415–429. doi: 10.1002/ana.410160402. [DOI] [PubMed] [Google Scholar]
- 28.Lexell J. Evidence for nervous system degeneration with advancing age. J Nutr. 1997;127(5 Suppl):1011S–1013S. doi: 10.1093/jn/127.5.1011S. [DOI] [PubMed] [Google Scholar]
- 29.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 30.Gandevia SC, Macefield G, Burke D, et al. Voluntary activation of human motor axons in the absence of muscle afferent feedback. The control of the deafferented hand. Brain. 1990;113(Pt 5):1563–1581. doi: 10.1093/brain/113.5.1563. [DOI] [PubMed] [Google Scholar]
- 31.De Luca CJ, Gonzalez-Cueto JA, Bonato P, et al. Motor unit recruitment and proprioceptive feedback decrease the common drive. J Neurophysiol. 2009;101:1620–1628. doi: 10.1152/jn.90245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Rekeneire N, Resnick HE, Schwartz AV, et al. Diabetes is associated with subclinical functional limitation in nondisabled older individuals: The Health, Aging, and Body Composition study. Diabetes Care. 2003;26:3257–3263. doi: 10.2337/diacare.26.12.3257. [DOI] [PubMed] [Google Scholar]
- 33.Vinik AI, Strotmeyer ES, Nakave AA, et al. Diabetic neuropathy in older adults. Clin Geriatr Med. 2008;24:407–435. doi: 10.1016/j.cger.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]