Abstract
Background
Retinoblastoma has a 5-year survival rate exceeding 95%, yet little is known about long-term functional outcomes for these patients.
Patients/Methods
69 adult survivors of retinoblastoma (33 years of age, 31 years post-diagnosis) enrolled in the St. Jude Lifetime Cohort Study completed clinical cognitive evaluations and questionnaires assessing adult social attainment. Scores on all cognitive measures were converted to z-scores (M=0, SD=1) using age-adjusted normative data. Multivariable linear regression analyses, adjusted for age at diagnosis and disease laterality, were used to examine associations between disease and treatment exposures and cognitive outcomes.
Results
Retinoblastoma survivors performed within normative expectations across most cognitive domains. In multivariable models, adjusted for disease laterality, survivors diagnosed <1 year of age performed significantly better on measures of short-term verbal memory (β=0.87, p<0.01), long-term verbal memory (β=0.66, p=0.02), verbal learning (β=0.67, p=0.02), and verbal reasoning abilities (β=0.79, p<0.01) compared to survivors diagnosed >1 year of age. In multivariable models, restricted to bilateral survivors and adjusted for age at diagnosis, whole brain radiation exposure was significantly associated with poorer performance on tasks of short-term verbal memory (β−0.003, p=0.03) and long-term verbal memory (β=−0.003, p=0.01). Reported social attainment was consistent with adult developmental expectations.
Conclusions
Adult survivors of retinoblastoma demonstrate few cognitive or social attainment deficits decades following diagnosis and treatment. Findings suggest the potential for neural reorganization following early insult to the visual system as well as vulnerability of the developing brain to low dose radiation exposure. Early intervention and rehabilitation will be important for these patients.
Introduction
Retinoblastoma represents approximately 6.1% of childhood cancer under the age of 5 years,1 with approximately 350 cases diagnosed annually in the United States.2 Over 95% of children with this tumor are diagnosed before 5 years of age, with a median age of 24 months in children with unilateral disease and 9 to 12 months in children with bilateral disease. Current treatment for retinoblastoma includes enucleation and/or chemotherapy combined with local ophthalmic therapies. Historically, external beam radiation therapy was used and resulted in high rates of disease control and functional organ preservation,3 but is used less frequently in contemporary treatment protocols.4 Despite a 5-year survival rate estimated at over 95% during the last two decades,5 data on long-term cognitive and functional outcomes for retinoblastoma survivors are limited.
Given the very young age at which retinoblastoma patients are treated, and the intensive multimodal therapies they receive, survivors are likely at risk for disease- and treatment-related late effects. While adverse medical outcomes, including subsequent malignancies, have been well documented,6-8 only limited attention has been paid to cognitive or functional outcomes, particularly in adult survivors. The few studies to report on cognitive function in childhood retinoblastoma survivors have yielded mixed results and are limited by small sample size and narrow assessment of cognitive abilities.9-13 Despite these limitations, evidence of superior verbal intelligence among bilateral survivors has been reported, particularly in survivors blind as a result of their disease.10, 12, 13 As brain development and the potential for neuroplasticity continue well into adulthood, cognitive patterns observed in early childhood may change over several decades following receipt of cancer therapies. Thus, the aims of this study were to (1) report on patterns of cognitive functioning using both direct assessment of specific processes as well as patient reported neurobehavioral outcomes, (2) examine disease and treatment related predictors of neurocognitive outcomes, and (3) report on indicators of adult social attainment in survivors of retinoblastoma.
Methods
St. Jude Lifetime Cohort Study (SJLIFE)
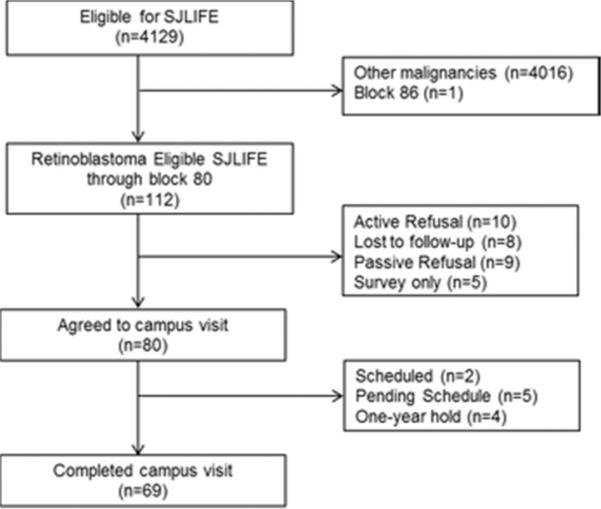
The study design and cohort characteristics of SJLIFE have previously been described.3,4 Briefly, SJLIFE was established as a retrospective cohort with continuous enrollment, to better understand the etiology and severity of long-term adverse effects of treatments for childhood cancer survivors. Survivors eligible for this IRB-approved study include individuals treated at St. Jude Children's Research Hospital (SJCRH) for childhood cancer between 1962 and 1999, and who are ≥18 years of age and ≥10 years since original cancer diagnosis. The SJLIFE study initiated the retrospective component in November of 2007 with participants diagnosed since 1962 randomly allocated for recruitment in blocks of 50. The current study sample was comprised of SJLIFE retinoblastoma survivors recruited through block 80. As of April 30, 2012, the cutoff date for these analyses, 112 potentially eligible retinoblastoma participants were identified, of whom 69 (61.6%) had completed a SJLIFE campus visit (Figure 1).
Figure 1.
Consort diagram of study participation
Procedures
SJLIFE participants receive a comprehensive risk-based clinical and laboratory assessment according to the Children's Oncology Group Long-term Follow-up Guidelines (COG LTFU)14 and augmented with a core test battery. Consistent with the COG guidelines, retinoblastoma survivors receive a comprehensive ophthalmic evaluation which includes assessment of visual acuity. For the current study, visual impairment was defined as best corrected visual acuity worse than 20/200 in the better eye.
Cognitive testing was conducted during a two-hour session in dedicated evaluation rooms. Assessed neurocognitive domains (and instruments) included intelligence (Wechsler Abbreviated Scale of Intelligence)15, academics (Woodcock Johnson Tests of Achievement-III: Basic Reading and Math Calculation subtests)16, memory (California Verbal Learning Test-II: Total Learning, Short-Delay Free Recall and Long-Delay Free Recall)17, attention (Trail Making Test Part A18; Conners' Continuous Performance Test-II [CPT-II]19); Digit Span Forward subtest of the Wechsler Adult Intelligence Scale-III [WAIS-III]20), processing speed (Coding Subtest of the WAIS-III;20 CPT-II19), fine motor dexterity (Grooved Pegboard)18 and executive function (Trail Making Test Part B18; Controlled Oral Word Association Test18; Digit Span Backward subtest of the WAIS-III20). Order of testing and survivors' schedules were controlled to limit impact from fatigue and extraneous factors on neurocognitive testing. Survivors also completed self-report behavior ratings to assess symptoms of executive dysfunction (Behavior Rating Inventory of Executive Function-Adult version)21. Each test yields standard scores (age-adjusted means and standard deviations) using test specific standardization norms. If best corrected visual acuity (in the better eye) was less than or equal to 20/200, visually based tasks with fine print were not administered secondary to research demonstrating a significant blur effect on neuropsychological assessments at this reduced level of acuity.22 No other testing accommodations for vision were made.
In addition, survivors completed comprehensive health questionnaires to assess health history and status, social and demographic factors, and psychosocial functioning. Social attainment was measured by survivor self-report of marital status (single, never married or never married living with partner as married; married; living with partner as married; widowed; divorced; separated no longer living as married), current employment (not currently working; working full-time; working part-time; caring for home or family; unemployed and looking for work; unable to work due to illness or disability; retired; student), educational attainment (1-8 years; 9-12 years but did not graduate; completed high school/GED; training after high school other than college; some college; college graduate; postgraduate level), and independent living (live with spouse/partner; live with parents; live with roommates; live with brother and/or sister; live with other relatives; live alone). These questions and response format parallel those used to assess adult social attainment in the Childhood Cancer Survivor Study (CCSS).23
We estimated mean radiation dose to the entire brain and to the individual temporal lobes through review of the treatment data including the dates of treatment, administered doses, and technical parameters of delivery. Radiation therapy data was available for 29 of 30 patients who were treated with a variety of modalities, beam arrangements and fractionation regimens: cobalt-60 was used in 12, 4MV photons in 15 and 10-12MeV electrons in 2 cases. The distribution of dose was estimated by recreating the treatment using age-matched CT and MR data sets of the head with the contoured brain and temporal lobe (left and right) structures. The mean dose was calculated using a 3-dimensional treatment planning system.
Data analysis
Descriptive statistics, including means, standard deviations and percentages were calculated for all demographic and treatment variables and for the outcome measures. Scores on all cognitive measures were converted to z-scores (M=0, SD=1) using age-adjusted normative data. Mean scores for the whole cohort of retinoblastoma survivors were compared to normative data using one-sample t-tests. Group differences by disease laterality were examined using two-sample t-tests. Impairment was defined as z-scores >1 SD below the normative mean. Frequency of impairment was examined for all retinoblastoma survivors, and group differences by disease laterality were examined using Chi-square or Fisher's exact test depending on the observed cell size. Pearson correlations were used to examine the relations between age at diagnosis, radiation dosimetry and cognitive performance. For the entire cohort, multivariable linear regression models were used to examine the association between age at diagnosis and cognitive outcomes, adjusting for disease laterality. Multivariable models restricted to bilateral survivors, adjusted for age at diagnosis, were used to examine the association between radiation dosimetry and cognitive outcomes. Our results were not adjusted for multiple comparisons.24, 25
Results
Survivor Characteristics
Survivors were, on average, 33 years of age at follow-up (range 21 to 46 years) and 31 years-post diagnosis (range 17 to 45 years; Table 1). Participants did not differ from nonparticipants on demographic or treatment factors (Supplemental Table 1). Survivors with bilateral disease were significantly younger at age of diagnosis (0.96 vs. 2.44 years, p<0.001), more likely to be treated with radiation therapy (91.7% vs. 18.8%, p<0.001), and more likely to have visual impairment at follow-up (41.6% vs. 0.0%, p<0.001) compared to survivors with unilateral disease. No significant differences were observed in age at follow-up or history of enucleation between survivors of unilateral and bilateral disease.
Table 1.
Descriptive Characteristics
| Cohort |
Unilateral |
Bilateral |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||||
| Sex | |||||||||
| Male | 35 | 50.7 | 24 | 53.3 | 11 | 45.8 | |||
| Female | 34 | 49.3 | 21 | 46.7 | 13 | 54.2 | |||
| Race | |||||||||
| White, non-Hispanic | 51 | 73.9 | 30 | 66.7 | 21 | 87.5 | |||
| Other | 18 | 26.1 | 15 | 33.3 | 3 | 12.5 | |||
| Treatment | |||||||||
| Enucleation only | 26 | 37.7 | 25 | 55.6 | 1 | 4.2 | |||
| Radiation only | 6 | 8.7 | 2 | 4.4 | 4 | 16.7 | |||
| Enucleation & Radiation | 6 | 8.7 | 1 | 2.2 | 5 | 20.8 | |||
| Chemo & Radiation | 3 | 4.4 | 2 | 4.4 | 1 | 4.2 | |||
| Enucleation & Chemotherapy | 13 | 18.8 | 12 | 26.7 | 1 | 4.2 | |||
| Enucleation, Chemo & Radiation | 15 | 21.7 | 3 | 6.7 | 12 | 50.0 | |||
| Visual impairment † | |||||||||
| Yes | 10 | 14.5 | 0 | 0.0 | 10 | 41.7 | |||
| No | 59 | 85.5 | 45 | 100 | 14 | 58.3 | |||
| Subsequent malignancy | 8 | 11.6 | 2 | 4.4 | 6 | 25.0 | |||
| Cranial radiation dose, cGy‡ | M | SD | Range | M | SD | Range | M | SD | Range |
| Left temporal lobe | 470.2 | 618.7 | 6.4-1895.5 | 340.7 | 435.6 | 30.6-1229.3 | 513.4 | 672.3 | 6.4-1895.5 |
| Right temporal lobe | 518.3 | 691.3 | 25.6-2064.2 | 575.7 | 896.3 | 25.6-1924.7 | 499.1 | 634.5 | 41.1-2064.2 |
| Brain | 263.7 | 188.9 | 21.1-543.1 | 236.4 | 210.6 | 32.5-527.2 | 272.8 | 185.7 | 21.1-543.1 |
| Age at evaluation (years) | 32.5 | 6.7 | 20.5-46.2 | 32.7 | 6.3 | 21.0-46.2 | 32.2 | 7.5 | 20.5-45.1 |
| Age at diagnosis (years) | 1.9 | 1.7 | 0.03-7.1 | 2.4 | 1.9 | 0.1-7.1 | 0.96 | 0.8 | 0.03-2.4 |
| Years from diagnosis | 30.6 | 6.6 | 17.2-45.5 | 30.3 | 6.4 | 17.2-45.5 | 31.2 | 7.0 | 19.3-43.2 |
Visual impairment defined as best corrected visual acuity in the best eye worse than 20/200.
Radiation data restricted to patients treated with radiation therapy (cohort n=30, unilateral n=8, bilateral n=22).
Cognitive Outcomes
For the entire cohort, retinoblastoma survivors performed within normative expectations on measures of verbal intelligence, attention, memory, processing speed, and executive functioning (Table 2). Nonverbal reasoning abilities (Mean=0.21, SD=0.73; p=0.03) and the ability to learn new information over a series of trials (Mean=0.39, SD=1.1; p=0.01) were above normative expectations. Survivors performed one standard deviation below the expected mean on a measure of fine-motor dexterity (p<0.001). Survivor self-ratings of cognition and behavior were in the average range; however, survivors reported significantly more problems with working memory (p=0.01) and task completion (p=0.04) compared to similar aged adults. Impairment rates across cognitive domains are shown in Figure 2.
Table 2.
Adult cognitive outcomes
| Mean* | SD* | Impairment %† | 95% CI† | p-value‡ | |
|---|---|---|---|---|---|
| Intelligence | |||||
| Verbal | −0.09 | 1.06 | 13.9 | 6.5-24.7 | 0.51 |
| Nonverbal | 0.21 | 0.73 | 4.9 | 1.0-13.7 | 0.03 |
| Full Scale IQ | 0.10 | 0.80 | 8.2 | 2.7-18.1 | 0.34 |
| Academics | |||||
| Reading | −0.18 | 0.54 | 3.3 | 0.4-11.5 | 0.01 |
| Math | −0.16 | 0.76 | 17.0 | 8.4-29.0 | 0.12 |
| Memory | |||||
| New Learning | 0.39 | 1.11 | 6.2 | 1.7-15.0 | 0.01 |
| Short-term | 0.09 | 1.17 | 26.2 | 16.0-38.5 | 0.53 |
| Long-term | −0.02 | 1.11 | 29.2 | 18.6-41.8 | 0.87 |
| Attention | |||||
| Focus | −0.19 | 1.14 | 34.4 | 22.9-47.3 | 0.20 |
| Sustain | −0.12 | 1.47 | 13.1 | 5.8-24.2 | 0.53 |
| Variability | −0.15 | 1.24 | 21.3 | 11.9-33.7 | 0.35 |
| Processing Speed | |||||
| Cognitive | −0.01 | 0.94 | 10.2 | 3.8-20.8 | 0.96 |
| Response time | −0.18 | 0.89 | 18.0 | 9.4-30.0 | 0.12 |
| Executive Function | |||||
| Cognitive Fluency | −0.24 | 1.20 | 29.2 | 18.6-41.8 | 0.11 |
| Cognitive Flexibility | −0.29 | 1.05 | 22.0 | 12.3-34.7 | 0.04 |
| Working Memory | −0.001 | 0.92 | 9.4 | 3.5-19.3 | 0.99 |
| Motor | |||||
| Fine motor dexterity | −1.09 | 1.37 | 48.3 | 35.2-61.6 | <0.001 |
| Cognitive Rating | |||||
| Initiation | −0.05 | 1.05 | 18.8 | 10.1-30.5 | 0.70 |
| Working Memory | −0.39 | 1.20 | 31.2 | 20.2-44.1 | 0.01 |
| Planning | −0.18 | 0.99 | 21.9 | 12.5-33.9 | 0.16 |
| Task Completion | −0.28 | 1.07 | 25.0 | 15.0-37.4 | 0.04 |
| Organization | −0.11 | 0.89 | 17.2 | 8.9-28.7 | 0.32 |
| Behavior Rating | |||||
| Inhibition | −0.14 | 1.07 | 18.8 | 10.1-30.5 | 0.29 |
| Shift | −0.17 | 1.11 | 26.6 | 16.3-39.1 | 0.22 |
| Emotional Control | 0.02 | 1.10 | 20.3 | 11.3-32.2 | 0.87 |
| Self-Monitor | 0.01 | 1.10 | 18.8 | 10.1-30.5 | 0.92 |
Mean and SD represented in age-adjusted z-scores, referenced to nationally representative norms.
Impairment ≥ 1SD below normative mean, with expected impairment of 16%. 95% confidence intervals for impairment.
p-value compared to expected M=0, SD=1
Figure 2.
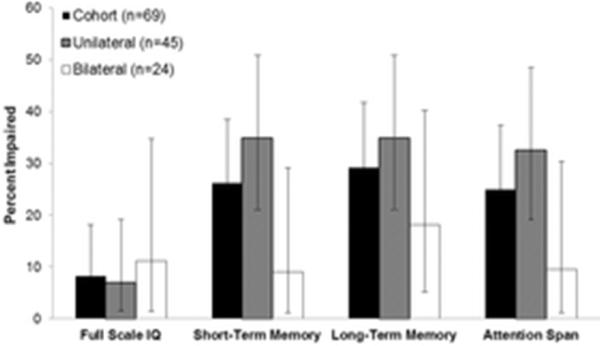
Neurocognitive impairment by disease laterality
Disease laterality
Survivors with bilateral disease performed significantly better than survivors with unilateral disease on measures of verbal learning (p=0.03), short-term verbal memory (p=0.01), and long-term verbal memory (p=0.02). Performance in these domains was superior for survivors of bilateral disease compared to normative expectations (all p's <0.01). A larger proportion of survivors of unilateral disease were impaired on short-term verbal memory (34.9% vs. 9.1%, p=0.04) and attention span (32.6% vs. 9.5%, p=0.07). Cognitive outcomes by disease laterality are presented in Supplemental Table 2.
Age at diagnosis
Age at diagnosis was negatively correlated with performance on measures of verbal learning (r=−0.39, p=0.002), short-term verbal memory (r=−0.47, p<0.001), and long-term verbal memory (r=−0.45, p<0.001). In multivariable models, adjusted for disease laterality, survivors diagnosed less than one year of age performed significantly better on measures of short-term verbal memory (β=0.87, p<0.01), long-term verbal memory (β=0.66, p=0.02), verbal learning (β=0.67, p=0.02), and verbal intelligence (β=0.79, p<0.01) compared to survivors diagnosed when older than one year of age. Importantly, in these multivariable models, disease laterality did not contribute significantly to performance on cognitive tasks. Supplemental Table 3 shows cognitive outcomes by age at diagnosis stratified by disease laterality.
Treatment exposure
Total brain radiation exposure was negatively correlated with performance on measures of verbal learning (r=−0.45, p=0.02), short-term verbal memory (r=−0.47, p=0.01), and long-term verbal memory (r=−0.60, p=0.001). Similar correlations were observed for radiation dose to the right, but not left, temporal lobes. In multivariable models, restricted to survivors of bilateral disease and adjusted for age at diagnosis, whole brain radiation exposure was significantly associated with poorer performance on tasks of short-term verbal memory (β=−0.003, p=0.03) and long-term verbal memory (β=−0.003, p=0.01).
Social Attainment
Seventy percent of survivors were living independently and 62% were married or living as married. Fifty eight percent of survivors completed college or postgraduate education. Three quarters of survivors were employed full time, though over half reported a personal income <$19,999. There were no significant differences in adult social attainment between survivors with unilateral versus bilateral disease (see Table 3), though a larger proportion of those with bilateral disease were never married (41.7% vs. 22.2%).
Table 3.
Adult social attainment
| Unilateral N (%) | Bilateral N (%) | P-value | |
|---|---|---|---|
| Current marital status | 0.13 | ||
| Single, never married | 10 (22.2) | 10 (41.7) | |
| Married or living as married | 30 (66.7) | 13 (54.2) | |
| Previously married | 5 (11.1) | 1 (4.17) | |
| Independent living | 0.25 | ||
| Yes | 34 (75.6) | 15 (62.5) | |
| No | 11 (24.4) | 9 (37.5) | |
| Patient education | 0.82 | ||
| Less than high school | 2 (4.4) | 1 (4.2) | |
| Completed high school/GED | 10 (22.2) | 5 (20.8) | |
| Training after high school, some college | 17 (37.8) | 9 (37.5) | |
| ≥ College graduate | 16 (35.6) | 9 (37.5) | |
| Employment status | 0.59 | ||
| Employed/student | 33 (73.33) | 19 (79.17) | |
| Unemployed | 12 (26.67) | 5 (20.83) | |
| Personal income | 0.41 | ||
| ≤ $19,999 | 23 (51.11) | 15 (62.50) | |
| ≥ $20,000 | 19 (42.22) | 8 (33.33) | |
| Missing | 3 (6.67) | 1 (4.17) |
Discussion
This study reports on cognitive outcomes and social attainment in adults who are, on average, 31 years from diagnosis of retinoblastoma. Overall, retinoblastoma survivors performed well within the average range across most cognitive domains assessed. Younger age at diagnosis (i.e. less than one year) was associated with better performance on tasks of verbal intelligence, learning and memory, after accounting for disease laterality. Increased whole brain radiation dose was associated with poorer performance on verbal memory among survivors with bilateral disease.
Previous studies reporting on cognitive outcomes of retinoblastoma survivors have largely been restricted to children and have yielded mixed results. An early study reported that survivors’ blind as a result of their disease (12 bilateral patients), had verbal IQ scores 10 points above their healthy siblings, although sighted survivors with bilateral disease had significantly lower IQ scores than their sibling controls.13 Ek et al10 reported no cognitive delays in a sample of 22 survivors who were evaluated at 4 and 6 years of age. Notably, the mean IQ for survivors with bilateral disease was in the superior range. Reporting on a sample of 54 survivors, Sheppard et al26 observed that sighted children performed in the average range on tasks measuring verbal intelligence but below average on tasks measuring nonverbal intelligence. Of note, many of these studies were conducted with young children, during a developmental period marked by greater instability on measures of intelligence. Our results extend these findings by reporting differences in specific verbal processes, beyond verbal IQ, between survivors with unilateral versus bilateral disease. Specifically, survivors with bilateral disease performed better than those with unilateral disease and above normative expectations on measures of verbal learning and verbal memory. Despite these differences, our results suggest that adult survivors of retinoblastoma, regardless of disease laterality, are performing within age expectations across most cognitive domains. Importantly, subsequent analyses suggested that disease and treatment variables beyond disease laterality make important contributions to cognitive outcomes in retinoblastoma survivors.
In our sample, after accounting for disease laterality, younger age at diagnosis was significantly associated with better performance on tasks of verbal intelligence, verbal learning and short and long-term verbal memory. That is, in multivariable models including disease location (i.e. bilateral vs. unilateral) and age at diagnosis, younger age at diagnosis contributed significantly to cognitive outcomes whereas disease laterality was no longer significantly associated with cognition. Specifically, survivors diagnosed when less than one year of age performed more than two-thirds of a standard deviation better on these verbally mediated tasks compared to survivors diagnosed when greater than one year of age. These findings are in contrast with those of other cancer survivors that suggest that younger age at diagnosis is associated with increased risk for cognitive impairment, even decades after treatment completion.27 Our findings are, however, consistent with the non-oncology literature on the potential for neural reorganization following early sensory loss.28 For example, individuals blind very early in life may show comparable or even superior cognitive skills, including verbal recall, compared to individuals with intact sensory functioning. In retinoblastoma patients, it is possible that insult to the visual system occurring very early in life (e.g. less than one year of age) results in cross-modal sensory changes. Specifically, reduced visual input may result in neurophysiological changes in the visual cortex and subsequently result in reorganization of this region of the brain to enhance processing of verbal or auditory information. Children diagnosed at a very young age may receive rehabilitation and intervention services during a phase of development marked by increased neural plasticity thereby strengthening the potential for adaptive reorganization in the brain. Future longitudinal research utilizing advanced neuroimaging techniques to quantify potential physiological and morphological changes in the brain will be important to confirm these hypotheses.29
Radiation dose scatter to the brain and pineal gland has been reported following external beam radiation therapy in children treated for retinoblastoma.30 Our dosimetry methods allowed for determination of radiation dose to the whole brain as well as left and right temporal lobes. To our knowledge, no previous study has examined the association between radiation exposure to the brain and cognitive outcomes in retinoblastoma patients. Because only a small number of unilateral participants were treated with radiation (n=8), we examined the association between external beam radiation and cognition within bilateral survivors alone. We found that increased whole brain dose was associated with poorer performance on tasks of short and long-term verbal memory, after adjusting for age at diagnosis. These results suggest that exposure to brain radiation is a risk factor for reduced cognitive performance even within a subgroup of survivors who are cognitively intact. This is particularly striking given the extremely small radiation dose to the brain. Past reports of long-term cancer survivors have consistently documented cognitive impairments following cranial radiation exposure >18Gy.31, 32 Our results may suggest vulnerability of the very young developing brain to even extremely small doses of radiation exposure. However, these findings should be interpreted with caution given the small sample size and modest association between the exposure and outcome.
In our study, most very long-term survivors of retinoblastoma reported attainment of expected adult developmental milestones, with no differences observed based on disease laterality. Reports of social attainment in adult survivors of other childhood malignancies from CCSS provide context for understanding our outcomes. Sixty two percent of retinoblastoma survivors in our study were married or living as married. This is slightly higher than the 47% reported in CCSS.33 With respect to independent living, 82% of adult survivors in CCSS reported living independently compared with 71% of our retinoblastoma survivors.34 This modest difference may be accounted for, in part, by age differences in the two cohorts. Specifically, the CCSS independent living analysis was restricted to survivors older than 25 years while our sample included survivors as young as 20 years of age. Depending on cancer diagnosis, between 7% and 18% of CCSS participants did not complete high school.35 In contrast, only 4.3% of retinoblastoma survivors achieved less than a high school education. We also found fewer non-college graduates in our sample compared with CCSS. Despite these apparent educational attainment differences, unemployment of 25% among our sample of retinoblastoma survivors is consistent with that reported in CCSS (27%).36 This may suggest that retinoblastoma survivors are underemployed, relative to other cancer survivors, given their greater educational attainment.
The results of this study should be considered in the context of several limitations. Survivors who participated in this study may differ on cognitive and functional outcomes compared to survivors who did not participate, thus introducing the potential for selection bias. Although cognition and social attainment are not directly assessable in nonparticipants, participants in this analysis were similar to nonparticipants on key demographic variables and treatment variables. This is consistent with previously reported results from a comprehensive evaluation of potential participation bias from the SJLIFE cohort,37 which reduces concern about possible differential participation. Given our small sample size we had limited power to detect statistical significance, especially in analyses restricted to bilateral survivors. However, our sample is larger than samples in previous studies and reflects the rarity of retinoblastoma. Because survivors were diagnosed nearly 3 decades ago, data on the presence of chromosome 13q deletions were not consistently available. This deletion has been associated with cognitive impairment as well as other central nervous system anomalies; however, phenotypes vary based on region of the gene deletion. We speculate that very few participants who participated in onsite cognitive evaluations had 13q deletions. It is important to note that retinoblastoma survivors are at-risk of developing subsequent malignancies. Because only 8 participants in our cohort developed at least one subsequent malignancy we were unable to assess the potential effect of subsequent treatment on cognitive outcomes; this will be important for future studies to consider. While radiation therapy is less commonly used in contemporary treatment protocols, surgical and chemotherapeutic approaches to retinoblastoma changed very little until the end of the 20th century. Therefore, evaluation of functional outcomes of historic cohorts remains relevant for large numbers of patients and their providers.38
In sum, we found that adult survivors of retinoblastoma demonstrate few cognitive or social attainment deficits several decades following diagnosis and treatment. Survivors diagnosed less than one year of age performed better on measures of verbal reasoning and memory, suggesting the potential for neural reorganization in very young children following an insult to the visual system.
Supplementary Material
Acknowledgments
Funding: This work was supported by St. Jude Children's Research Hospital Cancer Center Support (CORE) grant CA21765 from the National Cancer Institute and by ALSAC.
Footnotes
Disclosures: None
References
- 1.Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975-2004. Br J Ophthalmol. 2009;93:21–23. doi: 10.1136/bjo.2008.138750. [DOI] [PubMed] [Google Scholar]
- 2.Abramson DH. Retinoblastoma in the 20th century: past success and future challenges the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2005;46:2683–2691. doi: 10.1167/iovs.04-1462. [DOI] [PubMed] [Google Scholar]
- 3.Merchant TE, Gould CJ, Hilton NE, et al. Ocular preservation after 36 Gy external beam radiation therapy for retinoblastoma. J Pediatr Hematol Oncol. 2002;24:246–249. doi: 10.1097/00043426-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Galindo C, Chantada GL, Haik BG, Wilson MW. Treatment of retinoblastoma: current status and future perspectives. Curr Treat Options Neurol. 2007;9:294–307. doi: 10.1007/s11940-007-0015-4. [DOI] [PubMed] [Google Scholar]
- 5.Broaddus E, Topham A, Singh AD. Survival with retinoblastoma in the USA: 1975-2004. Br J Ophthalmol. 2009;93:24–27. doi: 10.1136/bjo.2008.143842. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DN, Sklar CA, Oeffinger KC, et al. Long-term medical outcomes in survivors of extra-ocular retinoblastoma: the Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Pediatr Blood Cancer. 2013;60:694–699. doi: 10.1002/pbc.24280. [DOI] [PubMed] [Google Scholar]
- 7.Nahum MP, Gdal-On M, Kuten A, Herzl G, Horovitz Y, Weyl Ben Arush M. Long-term follow-up of children with retinoblastoma. Pediatr Hematol Oncol. 2001;18:173–179. doi: 10.1080/08880010151114769. [DOI] [PubMed] [Google Scholar]
- 8.Bartuma K, Pal N, Kosek S, Holm S, All-Ericsson C. A 10-year experience of outcome in chemotherapy-treated hereditary retinoblastoma. Acta Ophthalmol. 2013 doi: 10.1111/aos.12282. [DOI] [PubMed] [Google Scholar]
- 9.Thurrell RJ, Josephson TS. Retinoblastoma and intelligence. Psychosomatics. 1966;7:368–370. doi: 10.1016/s0033-3182(66)72068-4. [DOI] [PubMed] [Google Scholar]
- 10.Ek U, Seregard S, Jacobson L, Oskar K, Af Trampe E, Kock E. A prospective study of children treated for retinoblastoma: cognitive and visual outcomes in relation to treatment. Acta Ophthalmol Scand. 2002;80:294–299. doi: 10.1034/j.1600-0420.2002.800312.x. [DOI] [PubMed] [Google Scholar]
- 11.Eldridge R, O'Meara K, Kitchin D. Superior intelligence in sighted retinoblastoma patients and their families. J Med Genet. 1972;9:331–335. doi: 10.1136/jmg.9.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams M. Superior intelligence of children blinded from retinoblastoma. Arch Dis Child. 1968;43:204–210. doi: 10.1136/adc.43.228.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitt EA, Rosenbaum AL, Willerman L, Levitt M. Intelligence of retinoblastoma patients and their siblings. Child Dev. 1972;43:939–948. [PubMed] [Google Scholar]
- 14.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 15.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 16.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III: Tests of Achievement. Riverside; Itasca, IL: 2001. [Google Scholar]
- 17.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. Second Edition. Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- 18.Strauss ESE, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Third ed. Oxford University Press; New York: 2006. [Google Scholar]
- 19.Conners CK. Conners' Continuous Performance Test II. Multi-Health Systems, Inc; Noth Tonawanda, NY: 2001. [Google Scholar]
- 20.D W . Wechsler Adult Intelligence Scale. Third Edition. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 21.Roth RMIP, Gioia GA. Behavior Rating Inventory of Executive Function - Adult Version. Psychological Assessment Resources, Inc.; Lutz, FL: 2005. [Google Scholar]
- 22.Hunt LA, Bassi CJ. Near-vision acuity levels and performance on neuropsychological assessments used in occupational therapy. Am J Occup Ther. 2010;64:105–113. doi: 10.5014/ajot.64.1.105. [DOI] [PubMed] [Google Scholar]
- 23.Gurney JG, Krull KR, Kadan-Lottick N, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2390–2395. doi: 10.1200/JCO.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 25.Rothman KJ. Six Persistent Research Misconceptions. J Gen Intern Med. 2014 doi: 10.1007/s11606-013-2755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheppard L, Eiser C, Kingston J. Mothers’ perceptions of children's quality of life following early diagnosis and treatment for retinoblastoma (Rb). Child Care Health Dev. 2005;31:137–142. doi: 10.1111/j.1365-2214.2005.00498.x. [DOI] [PubMed] [Google Scholar]
- 27.Krull K, Brinkman T, Li C, et al. Neurocognitive outcomes decades after treatment for childhood leukemia: A report from the St. Jude Lifetime Cohort Study. J Clin Oncol. 2013;31:4407–4415. doi: 10.1200/JCO.2012.48.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci. 2010;11:44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barb SM, Rodriguez-Galindo C, Wilson MW, et al. Functional neuroimaging to characterize visual system development in children with retinoblastoma. Invest Ophthalmol Vis Sci. 2011;52:2619–2626. doi: 10.1167/iovs.10-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;23:2272–2279. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 31.Spiegler BJ, Kennedy K, Maze R, et al. Comparison of long-term neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate. J Clin Oncol. 2006;24:3858–3864. doi: 10.1200/JCO.2006.05.9055. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong GT, Reddick WE, Petersen RC, et al. Evaluation of memory impairment in aging adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy. J Natl Cancer Inst. 2013;105:899–907. doi: 10.1093/jnci/djt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janson C, Leisenring W, Cox C, et al. Predictors of marriage and divorce in adult survivors of childhood cancers: a report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2009;18:2626–2635. doi: 10.1158/1055-9965.EPI-08-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunin-Batson A, Kadan-Lottick N, Zhu L, et al. Predictors of independent living status in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2011;57:1197–1203. doi: 10.1002/pbc.22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitby PA, Robison LL, Whitton JA, et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2003;97:1115–1126. doi: 10.1002/cncr.11117. [DOI] [PubMed] [Google Scholar]
- 36.Kirchhoff AC, Krull KR, Ness KK, et al. Occupational outcomes of adult childhood cancer survivors: A report from the childhood cancer survivor study. Cancer. 2011;117:3033–3044. doi: 10.1002/cncr.25867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2013;60:856–864. doi: 10.1002/pbc.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60:1083–1094. doi: 10.1002/pbc.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.