Abstract
Background: Morquio syndrome A (mucopolysaccharidosis type IVA) is an autosomal recessive, life-limiting lysosomal storage disease characterized by deficient activity of the enzyme galactosamine-6-sulfatase. The disease affects multiple body systems, and patients require multidisciplinary care from an early age.
Methods: To better understand the natural progression of the disease, life expectancy and common causes of death, death certificates were evaluated for 27 patients (15 male, 12 female) with Morquio syndrome A in the UK, covering the years 1975–2010.
Results: Mean age at death (±standard deviation) was 25.30 ± 17.43 years, with female patients living longer than male patients (26.55 ± 12.28 years versus 22.95 ± 17.63 years, respectively). Respiratory failure was the primary cause of death in nearly two-thirds of patients (63%). Other causes of death were cardiac failure (11%), post-traumatic organ failure (11%), complications of surgery (11%) and myocardial infarction (4%). Life expectancy increased gradually over time (R2 = 0.0963), and mean age at death due to respiratory failure improved from 17.42 ± 9.54 years in the 1980s to 30.74 ± 10.84 years in the 2000s.
Conclusions: The current data suggest that survival of patients with Morquio syndrome A in the UK has improved in recent decades. It is possible that improvements in multidisciplinary care and referral of patients to specialist centres underlie this trend. It is hoped that novel disease-specific treatments such as enzyme replacement therapy and haematopoietic stem cell therapy will help to extend the lifespan of patients with Morquio syndrome further still.
Introduction
Morquio syndrome A (mucopolysaccharidosis type IVA, MPS IVA; OMIM 253000) is an autosomal recessive lysosomal storage disease characterized by deficient activity of the enzyme galactosamine-6-sulfatase (GALNS). The absence of GALNS activity results in impaired catabolism of two glycosaminoglycans (GAGs), keratin sulphate and chondroitin-6-sulphate (Dorfman et al. 1976; Glössl and Kresse 1982). The progressive accumulation of GAGs in various tissues means that the disease affects multiple body systems. Short stature and skeletal dysplasia are observed in most patients (Wraith 1995), with bone deformity as the most common initial symptom (Montaño et al. 2007). The digestive, cardiovascular and respiratory systems, and visual and auditory function may also be affected (Northover et al. 1996).
The incidence of Morquio syndrome A is estimated to be between 1 in 76,000 and 1 in 450,000 in Europe, and 1 in 200,000 in the USA and Canada (Nelson 1997; Poorthuis et al. 1999; Applegarth et al. 2000). Clinical presentation of the disease ranges from a severe, rapidly progressing form (which represents the classical description of this disorder) to a phenotype that evolves more slowly. Onset of disease symptoms commonly occurs before the age of 1 year in patients with a severe phenotype or as late as the second decade of life in patients with the slowly progressing form of the disease (Montaño et al. 2007).
Cardiac valve disease and respiratory complications leading to limitations in endurance are common in patients with Morquio syndrome A from late childhood (John et al. 1990; Hendriksz et al. 2013), and both are associated with disease involvement in multiple body systems (Hendriksz et al. 2013). GAG accumulation in the upper airways and tonsils predisposes the patient to the development of obstructive sleep apnoea and upper airway obstruction (Walker et al. 2003; Montaño et al. 2007). Respiratory function is further compromised by chest wall deformities and displacement of the diaphragm due to short stature coupled with hepatosplenomegaly (Hendriksz et al. 2013). Atlantoaxial instability and spinal cord compression may also result in respiratory muscle weakness (Tomatsu et al. 2011; Hendriksz et al. 2013). Owing to these changes, patients with Morquio syndrome A may experience recurrent infections, progressive loss of pulmonary function and, ultimately, respiratory failure (Montaño et al. 2007; Pelley et al. 2007; Tomatsu et al. 2011; Hendriksz et al. 2013). Although the central nervous system is not impacted directly by GAG accumulation (Wraith 1995), patients with Morquio syndrome A have a high risk of developing neurological complications owing to skeletal abnormalities (Nelson and Thomas 1988).
Patients with Morquio syndrome A require multidisciplinary care from primary care physicians, orthopaedic surgeons, pulmonologists, cardiologists and anaesthesiologists (Algahim and Almassi 2013). Multiple interventions are required to maintain optimal respiratory function, and ongoing management of skeletal manifestations and the associated neurological complications is critically important (Hendriksz et al. 2013). At present, there is no disease-specific treatment for Morquio syndrome A, although enzyme replacement therapy (ERT) is in development (Algahim and Almassi 2013). Owing to a limited effect on skeletal manifestations of other MPS diseases, haematopoietic stem cell therapy (HSCT) is not recommended for patients with Morquio syndrome A (Peters and Steward 2003).
To be able to optimize current management and evaluate the effectiveness of novel treatments for Morquio syndrome A, it is necessary to understand the natural progression of the disease, life expectancy and common causes of death. Here, we analyse survival and causes of death in patients with Morquio syndrome A, and how these have changed in recent decades, using data collected by the Society for Mucopolysaccharide Diseases (UK). These data will be of interest to clinicians, healthcare authorities and commissioning bodies, as well as to patients, their families and patient societies.
Methods
The Society for Mucopolysaccharide Diseases made available death certificates of all deceased patients with Morquio syndrome A held in its database. The death certificates provided information on date of birth, gender, date of death and primary cause of death. The society has aimed to collect data on every patient with Morquio syndrome A in the UK. Although the number of patients missing from the database is unknown, it is estimated by the collators to be very few because most, if not all, individuals with Morquio syndrome A are treated at a small number of designated centres.
Results
Patient Characteristics
Death certificates were available for 27 patients (15 male, 12 female) with Morquio syndrome A, covering the years 1975–2010 (Table 1).
Table 1.
Patient characteristics
| Patient (gender) | Date of death | Age at death (years) | Primary cause of death | Additional information |
|---|---|---|---|---|
| 1 (M) | 07/02/1975 | 4.00 | Post-traumatic organ failure, pneumonia and lung collapse | Fell from swing, head injury |
| 2 (F) | 21/05/1986 | 26.96 | Respiratory failure – bronchopneumonia | |
| 3 (M) | 28/05/1987 | 7.88 | Respiratory failure | Tracheomalacia |
| 4 (M) | 27/02/1989 | 3.08 | Complication of surgery – anoxic brain damage post-surgery | Cervical surgery |
| 5 (F) | 26/10/1989 | 15.16 | Complication of surgery – cerebral haemorrhage | Mitral valve replacement |
| 6 (F) | 18/10/1990 | 10.24 | Post-traumatic organ failure – cervical stenosis | Post-mortem brain stem compression, atlantoaxial subluxation |
| 7 (M) | 28/03/1992 | 75.32 | Respiratory failure – bronchopneumonia | |
| 8 (M) | 13/04/1992 | 22.56 | Respiratory failure – bronchial asthma | |
| 9 (M) | 21/08/1993 | 6.24 | Respiratory failure | Cervical stenosis |
| 10 (M) | 22/10/1993 | 10.08 | Respiratory failure | |
| 11 (F) | 27/09/1995 | 22.48 | Respiratory failure | |
| 12 (F) | 01/04/1996 | 46.24 | Respiratory failure | |
| 13 (F) | 23/03/1996 | 48.56 | Respiratory failure – bronchopneumonia | |
| 14 (M) | 20/05/1997 | 14.16 | Respiratory failure | Bronchopneumonia, obstructive airway disease |
| 15 (M) | 12/03/1998 | 20.80 | Cardiac failure | Aortic valve disease, hypoplastic lung, trachea abnormality seen at post-mortem |
| 16 (M) | 13/01/1999 | 33.64 | Respiratory failure | |
| 17 (M) | 17/02/1999 | 18.56 | Cardiac failure | |
| 18 (F) | 17/06/2000 | 52.48 | Cardiac failure – acute left heart failure | |
| 19 (M) | 22/04/2001 | 42.80 | Respiratory failure | |
| 20 (F) | 27/05/2002 | 36.88 | Myocardial infarction | Chest infection |
| 21 (F) | 02/06/2003 | 3.16 | Post-traumatic organ failure – cervical compression after trauma | Fell at school after a seizure |
| 22 (F) | 28/01/2005 | 28.16 | Respiratory failure – chest infection (bronchopneumonia) | |
| 23 (M) | 08/03/2005 | 20.08 | Complication of surgery – aspiration pneumonia | Gastrointestinal haemorrhage, oesophageal tear, post-mortem outcome was misadventure |
| 24 (F) | 01/04/2005 | 15.40 | Respiratory failure | |
| 25 (M) | 03/12/2005 | 20.16 | Respiratory failure | |
| 26 (M) | 24/07/2008 | 44.88 | Respiratory failure – bronchopneumonia, sepsis | Community-acquired pneumonia |
| 27 (F) | 03/10/2010 | 33.06 | Respiratory failure – bronchopneumonia |
Mean Age at Death and Primary Cause of Death
Mean age at death (± standard deviation [SD]) was 25.03 ± 17.43 years. In general, female patients tended to live slightly longer than male patients (mean age at death [±SD], 26.55 ± 12.28 years versus 22.95 ± 17.63 years, respectively), but the difference was not significant. Respiratory failure was the primary cause of death in nearly two-thirds of patients (Fig. 1a). Other reported causes of death were cardiac failure, post-traumatic organ failure, complications of surgery and myocardial infarction. Although respiratory failure was proportionally a more common cause of death in male patients than in female patients (67% versus 59%, respectively), this difference was not considered significant, nor were there other numerical differences in cause of death between the genders (Fig. 1b, c).
Fig. 1.
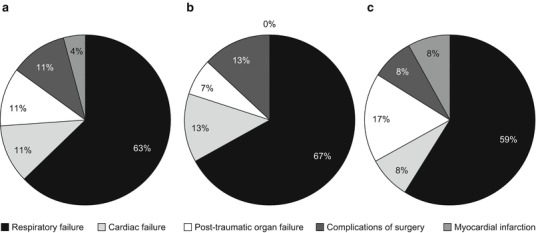
Primary cause of death in patients with Morquio syndrome A. (a) All patients (n = 27); (b) male patients only (n = 15); (c) female patients only (n = 12)
Differences by Age Group
There was a gradual increase in the proportion of deaths caused by respiratory failure with each decade of life (Fig. 2). A high proportion of fatalities in patients younger than 10 years were attributed to post-traumatic organ failure, suggesting that accidental deaths were more common in children than in older individuals. When death owing to post-traumatic organ failure was excluded from the analysis, mean age at death (±SD) increased to 27.73 ± 16.95 years, with females living longer than male patients (mean age at death [±SD], 32.54 ± 12.68 years versus 24.30 ± 18.69 years, respectively). When patients younger than 10 years were excluded from the overall analysis, the mean age at death (±SD) changed to 29.94 ± 16.00 years, with female patients living slightly longer than male patients (mean age at death [±SD], 30.51 ± 13.69 years versus 29.37 ± 17.99 years, respectively).
Fig. 2.
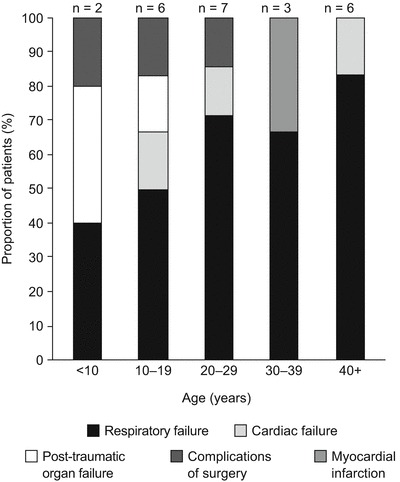
Primary cause of death in patients with Morquio syndrome A, stratified by age group
Changes in Longevity Over Time
An analysis of longevity showed a weak trend over time towards gradual improvement in life expectancy in patients with Morquio syndrome A (R2 = 0.0963; Fig. 3a, b). Importantly, recent deaths due to respiratory failure appear to have occurred later in life than deaths in earlier times, with mean age at death improving from 17.42 ± 9.54 years in the 1980s to 30.74 ± 10.84 years in the 2000s (Fig. 3c).
Fig. 3.
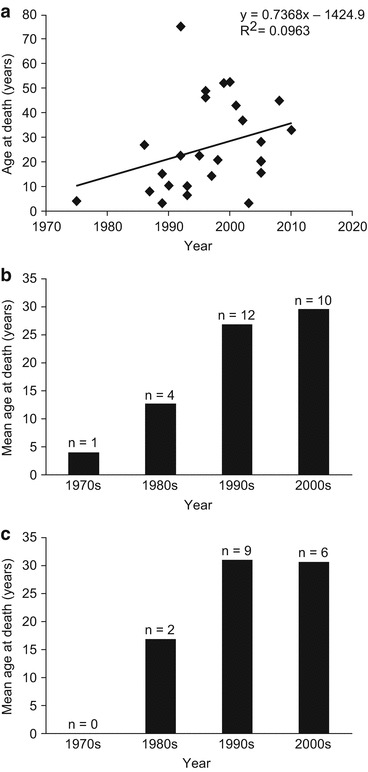
Longevity in patients with Morquio syndrome A between 1975 and 2010. (a) Age at death by individual patient; (b) mean age at death over time; (c) mean age at death due to respiratory failure
One of the greatest advances in the management of patients with Morquio syndrome A has been the implementation of routine prophylactic management of the spinal stenosis and instability in 1990. To evaluate the impact of this development on patient survival, an analysis of longevity and cause of death before 1990 and from 1990 onwards was carried out. Age at death was found to be greater in patients who died from 1990 onwards than in those who died before 1990 (Fig. 4a), although this difference was observed primarily in males. Respiratory failure was found to be a major cause of death both before 1990 and from 1990 onwards (Fig. 4b). Although death due to complications of surgery appears to have been more common before 1990, the dataset is too small to allow any strong association.
Fig. 4.
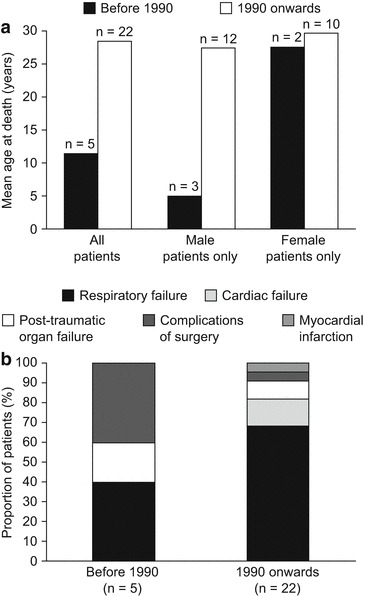
(a) Longevity and (b) primary cause of death in patients with Morquio syndrome A before 1990 and from 1990 onwards
Discussion
This mortality analysis has found that survival in patients with Morquio syndrome A appears to have improved gradually over the past three decades. Although the dataset is too small to allow any strong assertions, it is possible that efforts to improve disease management and multidisciplinary care, and to refer patients to specialist centres, are being reflected in extended lifespans for individuals with this life-limiting condition.
Respiratory failure remains the primary cause of death in patients with Morquio syndrome A; however, the current data suggest that this occurs later in recent years than previously. One reason for this may be advances in pulmonary care for these patients, including regular immunizations, prompt and aggressive treatment of respiratory tract infections, prevention of weight gain and availability of respiratory support (Hendriksz et al. 2013). In addition, improvements in prophylactic neck management in the last two decades may have helped to prevent respiratory compromise due to atlantoaxial instability and spinal cord compression. The introduction of C1/C2 cervical fusion to improve neck stability in the mid-1980s is particularly notable. This was driven by the patient organization investing in expertise and inviting world leaders to collaborate and proactively manage this known risk for the cohort. Once diagnosed, patients now undergo regular assessment to evaluate spinal involvement and monitor its progression. It is recommended that patients undergo a neurological examination at least every 6 months, with annual magnetic resonance imaging and radiological examination every 2–3 years (Solanki et al. 2013). Pathological changes observed during any of these evaluations may indicate a need for surgical intervention (Solanki et al. 2013).
Complications of surgery were reported as a cause of death in three patients, one in each of the first three decades of life. It is reassuring that the incidence of fatal complications of surgery is low, although the fact that two of the deaths were probably related to intubation or extubation (patients 4 and 22) highlights the risks associated with general anaesthesia in patients with Morquio syndrome A. Most anaesthetists are not familiar with the issues associated with surgery in individuals with Morquio syndrome A, so it is recommended that these patients should be referred to specialist centres with an experienced paediatric anaesthetist supported by a multidisciplinary team practised in the perioperative management of mucopolysaccharide (MPS) diseases (Walker et al. 2013). In general, the key to successful anaesthesia in patients with MPS diseases is planning, with a thorough preoperative evaluation of anaesthetic risk factors in consultation with a multidisciplinary team (Walker et al. 2013). Assessment of existing respiratory and cardiac manifestations, as well as cervical and tracheolaryngeal anatomy, may help the anaesthetist to anticipate potential problems that may arise during the procedure, such as difficult intubation and ventilation, and cardiac and cervical spine issues. Consideration should also be given to monitoring and postoperative care.
It is notable that the accidental deaths associated with trauma all occurred during the first two decades of life. This is perhaps to be expected, because children and adolescents tend to be more susceptible to falls and accidents than adults. The fact that only one death in this age group was attributed to cardiac failure may also indicate that the cardiovascular complications associated with Morquio syndrome A have yet to become life-threatening in this age group, particularly as cardiac failure is likely to develop secondary to respiratory impairment and loss of mobility due to skeletal and respiratory complications.
There is an opportunity for novel treatment options such as ERT to slow or halt the development of skeletal, respiratory and cardiac complications by preventing the accumulation of GAGs in bodily tissue. It is too early to determine what effect ERT will have on the somatic manifestations of Morquio syndrome A, but functional endpoints (such as the 6-minute walk test and forced vital capacity) will be useful in making these assessments, especially if they could be linked to patient-reported outcomes or quality of life measures.
The current data are based entirely on death certificates from patients with Morquio syndrome A, supplemented with limited information from their families. Although death certificates provide a useful source of information regarding mortality rates and causes of death in patients with rare diseases, a limitation of this study is the absence of clinical notes for each of the patients. Anecdotal reports indicate that all except two patients in the current dataset (patients 2 and 7) had short stature and experienced severe signs and symptoms of Morquio syndrome A. However, there is no information available about each patient’s functional ability and the presence or absence of comorbidities, which may have provided useful context for the trends observed here. For example, it is unclear why the mean age of mortality in boys with Morquio syndrome A is so dramatically different before and after 1990. It can be speculated that this may simply be an anomaly owing to small patient numbers, but without further clinical information, no firm conclusion can be made.
In conclusion, the findings of this study suggest that survival in patients with Morquio syndrome A in the UK has improved in recent decades. It would be of interest to assess whether a similar trend has occurred in other countries. In addition, it would be of interest to evaluate whether changes in multidisciplinary care have led to improvements in patient and carer quality of life. Although quality of life will inevitably decline as the disease progresses, steps can be taken to keep patients active, pain-free and independent for as long as possible. It is hoped that as experience of caring of individuals with Morquio syndrome A increases, this will be reflected in improvements in outcomes for patients and their carers.
Acknowledgements
Scientific editorial assistance was provided by Dr Jonathan Morton (Oxford PharmaGenesis™ Ltd, Oxford, UK) and was funded by an unrestricted grant from BioMarin Europe Ltd, London, UK.
Take-Home message
Evaluation of death certificates from patients with Morquio syndrome A in the UK has shown that survival in this population has improved in recent decades.
Compliance with Ethics Guidelines
Conflicts of Interest
C. Lavery serves on advisory boards for BioMarin Pharmaceutical Inc. and has received travel grants and lecture fees from BioMarin Pharmaceutical Inc.
C. Hendriksz is a consultant and chair of advisory boards for BioMarin Pharmaceutical Inc. and has received travel grants and lecture fees from BioMarin Pharmaceutical Inc.
Informed Consent
Informed consent was not required for this study. This article does not report on any studies with human or animal subjects performed by any of the authors.
Author Contributions
C. Lavery conceived the idea for the study, collected death certificates and contributed to the development of the manuscript.
C. Hendriksz analysed the study data and contributed to the development of the manuscript.
Both authors have seen and approved the final manuscript.
Footnotes
Competing interests: None declared
Contributor Information
Christine Lavery, Email: c.lavery@mpssociety.org.uk.
Collaborators: Johannes Zschocke and K Michael Gibson
References
- Algahim MF, Almassi GH. Current and emerging management options for patients with Morquio A syndrome. Ther Clin Risk Manag. 2013;9:45–53. doi: 10.2147/TCRM.S24771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969−1996. Pediatrics. 2000;105(1):e10. doi: 10.1542/peds.105.1.e10. [DOI] [PubMed] [Google Scholar]
- Dorfman A, Arbogast B, Matalon R. The enzymic defects in Morquio and Maroteaux−Lamy syndrome. Adv Exp Med Biol. 1976;68:261–276. doi: 10.1007/978-1-4684-7735-1_18. [DOI] [PubMed] [Google Scholar]
- Glössl J, Kresse H. Impaired degradation of keratan sulphate by Morquio A fibroblasts. Biochem J. 1982;203(1):335–338. doi: 10.1042/bj2030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksz CJ, Al-Jawad M, Berger KI, et al. Clinical overview and treatment options for non-skeletal manifestations of mucopolysaccharidosis type IVA. J Inherit Metab Dis. 2013;36(2):309–322. doi: 10.1007/s10545-012-9459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John RM, Hunter D, Swanton RH. Echocardiographic abnormalities in type IV mucopolysaccharidosis. Arch Dis Child. 1990;65(7):746–749. doi: 10.1136/adc.65.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30(2):165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet. 1997;101(3):355–358. doi: 10.1007/s004390050641. [DOI] [PubMed] [Google Scholar]
- Nelson J, Thomas PS. Clinical findings in 12 patients with MPS IV A (Morquio's disease). Further evidence for heterogeneity. Part III: Odontoid dysplasia. Clin Genet. 1988;33(2):126–130. doi: 10.1111/j.1399-0004.1988.tb03423.x. [DOI] [PubMed] [Google Scholar]
- Northover H, Cowie RA, Wraith JE. Mucopolysaccharidosis type IVA (Morquio syndrome): a clinical review. J Inherit Metab Dis. 1996;19(3):357–365. doi: 10.1007/BF01799267. [DOI] [PubMed] [Google Scholar]
- Pelley CJ, Kwo J, Hess DR. Tracheomalacia in an adult with respiratory failure and Morquio syndrome. Respir Care. 2007;52(3):278–282. [PubMed] [Google Scholar]
- Peters C, Steward CG. Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant. 2003;31(4):229–239. doi: 10.1038/sj.bmt.1703839. [DOI] [PubMed] [Google Scholar]
- Poorthuis BJ, Wevers RA, Kleijer WJ, et al. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999;105(1–2):151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- Solanki GA, Martin KW, Theroux MC, et al. Spinal involvement in mucopolysaccharidosis IVA (Morquio−Brailsford or Morquio A syndrome): presentation, diagnosis and management. J Inherit Metab Dis. 2013;36(2):339–355. doi: 10.1007/s10545-013-9586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Oikawa H, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr Pharm Biotechnol. 2011;12(6):931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- Walker PP, Rose E, Williams JG. Upper airways abnormalities and tracheal problems in Morquio's disease. Thorax. 2003;58(5):458–459. doi: 10.1136/thorax.58.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R, Belani KG, Braunlin EA, et al. Anaesthesia and airway management in mucopolysaccharidosis. J Inherit Metab Dis. 2013;36(2):211–219. doi: 10.1007/s10545-012-9563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith JE. The mucopolysaccharidoses: a clinical review and guide to management. Arch Dis Child. 1995;72(3):263–267. doi: 10.1136/adc.72.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]


