Abstract
Deep brain stimulation (DBS) has been used to treat secondary dystonias caused by inborn errors of metabolism with varying degrees of effectiveness. Here we report for the first time the application of DBS as treatment for secondary dystonia in a 22-year-old male with X-linked adrenoleukodystrophy (X-ALD). The disease manifested at age 6 with ADHD, tics, and dystonic gait, and deteriorated to loss of ambulation by age 11, and speech difficulties, seizures, and characteristic adrenal insufficiency by age 16. DBS in the globus pallidus internus was commenced at age 18. However, after 25 months, no improvement in dystonia was observed (Burke–Fahn–Marsden (BFM) scores of 65.5 and 62 and disability scores of 28 and 26, pre- and post-DBS, respectively) and the DBS device was removed. Treatment with dantrolene reduced skeletal muscle tone and improved movement (Global Dystonia Rating Scores from 5 to 1 and BFM score 42). Therefore, we conclude that DBS was a safe but ineffective intervention in our case with long-standing dystonia, whereas treatment of spasticity with dantrolene did improve the movement disorder in this young man with X-ALD.
Report
Deep brain stimulation (DBS) has demonstrated variable effectiveness in secondary dystonias caused by inborn errors of metabolism (e.g., homocystinuria, PKAN, GM1-gangliosidosis) (Andrews et al. 2010; Aydin et al. 2011). The present report of DBS in a now 22-year-old man with a diagnosis of X-linked adrenoleukodystrophy (X-ALD, OMIM# 300100) (Vidailhet et al. 2005) is its first reported application in X-ALD.
The patient presented at age 6 with ADHD, tics, and a dystonic gait. Neuroimaging revealed symmetrical occipito-parietal demyelination and abnormal signal intensities in the basal ganglia and thalamus (Fig. 1). Diagnosis was confirmed by elevated very long chain fatty acids in plasma. He lost the ability to ambulate within 5 years, and was wheelchair-bound by age 11. His speech deteriorated and was barely intelligible by age 16. Seizure disorder and adrenal insufficiency were effectively controlled with carbamazepine and clobazam, and hydrocortisone, respectively. Initially the dystonia affected only the lower extremities, but quickly generalized. At age 11 years, mild athetoid movements of the head, neck, and extremities were observed. He held the neck in a flexed position, but was able to extend it on request. He had a tendency to elevate the right upper extremity, with his right wrist in a flexed position and fingers extended. There was mild intermittent flexion of the wrist. Knees were held in an extended position, with ankles plantar flexed and feet inverted (left greater than right). Tone was difficult to assess due to the dystonia. Deep tendon reflexes were brisk, especially at the knees; plantar responses were difficult to assess due to dystonia. Hoffman’s sign was absent.
Fig. 1.
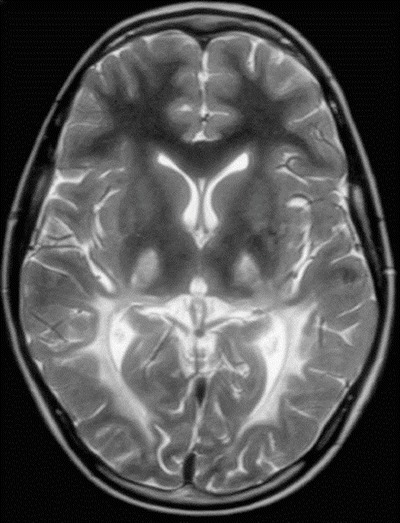
MRI brain scan at 10 years of age. A representative image of the T2-weighted sequence is shown demonstrating changes in signal intensity within the posterior white matter in a bilateral, symmetric fashion as well as increased signal intensity within the thalami
For dystonia, botox, levodopa/carbidopamine, trihexyphenidyl, pergolide, and pramipexole were ineffective. Intrathecal baclofen to reduce muscle tone was refused by the patient.
Repeat gadolinium magnetic resonance imaging revealed no evidence of active disease at age 16, indicating exhaustion of the disease process. The patient’s Loes score was 15 and his IQ was <70 (WISC-III/IV). By then, he exhibited a pronounced cogwheel phenomenon, unresponsive deep tendon reflexes, and dystonic contractures, but was able to manipulate his wheelchair. Deep brain stimulation (DBS) was considered at the request of the patient and his family (Vidailhet et al. 2005); the benefits and risks (i.e., exacerbation of demyelination with external trauma) (Raymond et al. 2010) were carefully considered, and DBS was performed with informed consent at age 18.
DBS leads (Medtronic model 3389-40) were inserted under general anesthesia with frame-based MRI-guided stereotaxy and macro-electrode stimulation and into the globus pallidus internus (GPi) and connected to an implantable neural stimulator (Kinetra). Figure 2 shows the location of the contacts used for stimulation. Continuous mono-polar stimulation began 6 weeks postoperatively with contact 1-positive (second most ventral left) and contact 5-negative (second most ventral right), pulse width 210 μs, 130 Hz, and 3.5 V. Parameters were adjusted on numerous occasions to maximize stimulation and avoid internal capsule response.
Fig. 2.
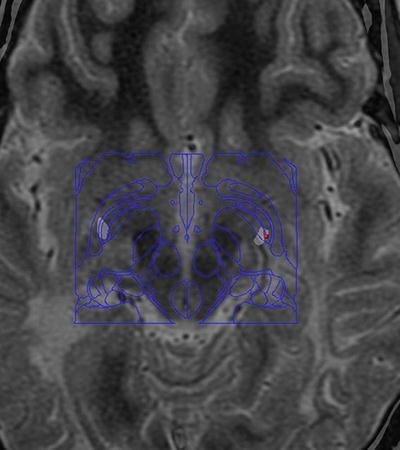
Location of the DBS contacts. Preoperative T2-weighted MRI fused with postoperative CT (windowed to show metal contacts) with Schaltenbrand atlas superimposed using StealthStation S7 (Medtronic) software. Active contacts can be seen in the most ventral posterior pallidum bilaterally (approximately 2 mm anterior, 20 mm lateral, and 4 mm below the mid-commissural point)
After 25 months, the patient had neither deteriorated nor shown improvement of dystonia. His Burke–Fahn– Marsden (BFM) and disability scores were 62 and 26, respectively (baseline scores: 65.5 and 28). No change in either score occurred upon deactivation and removal of DBS (measured after 3 months).
Dantrolene was subsequently initiated at 12.5 mg orally once daily and gradually increased to 25 mg three times daily. Dantrolene sodium reduces skeletal muscle tone at the level of the muscle fiber rather than the neural level (Haslam et al. 1974). After 5 weeks, the patient’s Global Dystonia Rating Scores were consistently 1 out of 10 (pre-treatment: 5 out of 10), and his BFM score was 42. Major improvements included increased flexibility of the right arm and lower limbs, and only occasional episodes of dystonic posturing. No side effects were reported; liver enzymes remained normal. However, his underlying neurometabolic condition has since deteriorated with cognitive decline and swallowing difficulties at age 22.
DBS was safe but ineffective in our patient. Treatment of spasticity may improve DBS outcomes in long-standing dystonia (Lumsden et al. 2013), particularly when supported by occupational and physiotherapies with individualized and goal-directed outcomes.
Acknowledgments
We are grateful to Mr. Bryan Sayson for administrative assistance.
Synopsis
Deep brain stimulation was a safe but ineffective intervention for long-standing dystonia in X-ALD, whereas treatment with dantrolene improved movement and dystonia symptoms at the level of skeletal muscle.
Compliance with Ethics Guidelines
Conflict of Interest
Christopher Honey has received consulting fees from Medtronic, a commercial company that fabricates DBS instruments. The authors Clara van Karnebeek, Gabriella Horvath, Tyler Murphy, Jacqueline Purtzki, Kristin Bowden, Sandra Sirrs, and Sylvia Stockler have no conflict of interest to declare.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). The patient provided informed consent for publication of this report.
Ethics
The institutional review board at BC Children’s Hospital and the University of British Columbia approved the study protocol [H12-00067], and parents provided written consent for publication.
Details of Contributions of Individual Authors
CvK: Clinical biochemical geneticist who was responsible for organization of multidisciplinary meetings to discuss patient treatment and follow-up, data collection, and drafting of the first manuscript with subsequent coordination of authors’ edits.
GH: Clinical biochemical geneticist currently following patient in the Adult Metabolic Clinic, responsible for the symptomatic management with dantrolene, and reviewed/edited the manuscript.
TM: Medical student who contributed to data collection and drafting of the manuscript.
JP: Physiatrist who performed pre- and post-DBS functional evaluation, and reviewed/edited the manuscript.
KB: Technical writer who provided critical revision of the manuscript.
SSirrs: Metabolic diseases specialist who managed and followed the patients in the Adult clinic around the time of DBS: Contributed to data collection, and reviewed/edited the manuscript.
SStockler: Neurometabolic diseases specialist who was responsible for the conception of the case report, contributed to data collection, and reviewed/edited the manuscript.
CRH: Neurosurgical consultant who coordinated surgery and DBS follow-up care, and reviewed/edited the manuscript.
Footnotes
Competing interests: None declared
Contributor Information
Christopher R. Honey, Email: chris.honey@telus.net
Collaborators: Johannes Zschocke and K Michael Gibson
References
- Andrews C, Aviles-Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry. 2010;81(12):1383–1389. doi: 10.1136/jnnp.2010.207993. [DOI] [PubMed] [Google Scholar]
- Aydin S, Abuzayed B, Varlibas F, et al. Treatment of homocystinuria-related dystonia with deep brain stimulation: a case report. Stereotact Funct Neurosurg. 2011;89(4):210–213. doi: 10.1159/000325703. [DOI] [PubMed] [Google Scholar]
- Haslam RH, Walcher JR, Lietman PS, Kallman CH, Mellits ED. Dantrolene sodium in children with spasticity. Arch Phys Med Rehabil. 1974;55(8):384–388. [PubMed] [Google Scholar]
- Lumsden DE, Kaminska M, Gimeno H, et al. Proportion of life lived with dystonia inversely correlates with response to pallidal deep brain stimulation in both primary and secondary childhood dystonia. Dev Med Child Neurol. 2013;55(6):567–574. doi: 10.1111/dmcn.12117. [DOI] [PubMed] [Google Scholar]
- Raymond GV, Seidman R, Monteith TS, et al. Head trauma can initiate the onset of adreno-leukodystrophy. J Neurol Sci. 2010;290(1–2):70–74. doi: 10.1016/j.jns.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352(5):459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]


