Abstract
Background: Seventy-five percent of patients with pyridoxine-dependent epilepsy (PDE) due to Antiquitin (ATQ) deficiency suffer from developmental delay and/or intellectual disability (IQ < 70) despite seizure control. An observational study showed that adjunct treatment with a lysine-restricted diet is safe, results in partial normalization of lysine intermediates in body fluids, and may have beneficial effects on seizure control and psychomotor development.
Methods: In analogy to the NICE guideline process, the international PDE Consortium, an open platform uniting scientists and clinicians working in the field of this metabolic epilepsy, during four workshops (2010–2013) developed a recommendation for a lysine-restricted diet in PDE, with the aim of standardizing its implementation and monitoring of patients. Additionally, a proposal for a further observational study is suggested.
Results: (1) All patients with confirmed ATQ deficiency are eligible for adjunct treatment with lysine-restricted diet, unless treatment with pyridoxine alone has resulted in complete symptom resolution, including normal behavior and development. (2) Lysine restriction should be started as early as possible; the optimal duration remains undetermined. (3) The diet should be implemented and the patient be monitored according to these recommendations in order to assure best possible quality of care and safety.
Discussion: The implementation of this recommendation will provide a unique and a much needed opportunity to gather data with which to refine the recommendation as well as improve our understanding of outcomes of individuals affected by this rare disease. We therefore propose an international observational study that would utilize freely accessible, online data sharing technologies to generate more evidence.
Electronic supplementary material
The online version of this chapter (doi:10.1007/8904_2014_296) contains supplementary material, which is available to authorized users.
Introduction
Pyridoxine-dependent epilepsy (PDE, MIM #266100) is an autosomal recessive epileptic encephalopathy characterized by resistance to conventional anti-epileptic drugs but responsiveness to pharmacological dosages of pyridoxine (Mills et al. 2010). In 2006, the underlying genetic defect was identified as deficiency of α-aminoadipic semialdehyde dehydrogenase (antiquitin), which is involved in cerebral lysine catabolism (MIM #107323) (Mills et al. 2006). Antiquitin (ATQ) deficiency results in the accumulation of intermediates arising from lysine degradation proximal to the deficient enzyme activity including α-aminoadipic semialdehyde (AASA), Δ-1-piperideine-6-carboxylate (P6C), and pipecolic acid (see Fig. 1). Inactivation of pyridoxal 5′phosphate (PLP) via chemical reaction with P6C is the pathophysiological mechanism of pyridoxine dependency. While treatment with pyridoxine compensates for PLP deficit, intermediates from lysine degradation remain elevated. These compounds may contribute to the observation that despite adequate seizure control, 75–80% of patients suffer from developmental delay or intellectual disability (IQ < 70) despite pyridoxine treatment (Basura et al. 2009; Bok et al. 2012). Approximately 20% of patients need additional anti-epileptic drugs for seizure control. Further, there is an increase in the white matter abnormalities over time (Gospe 2012). Recently, a magnetic resonance spectroscopy study suggested ongoing neuronal damage with reduction in the n-acetylasparatate (NAA)/choline ratio in a single patient with PDE between the ages of 9 and 18 years, despite pyridoxine treatment (Dogan et al. 2012).
Fig. 1.
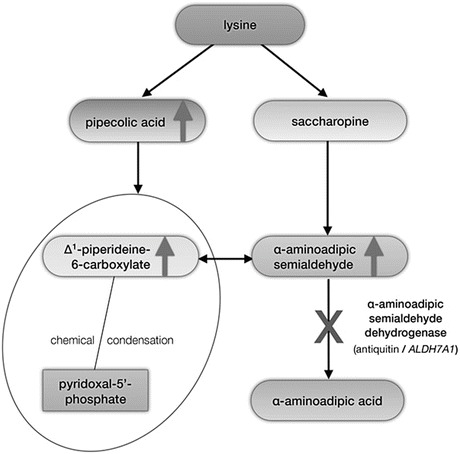
Schematic overview of the metabolism of l-lysine via the saccharopine and pipecolic acid (PA) pathways and biochemical pathophysiology of ATQ deficiency. The two pathways converge where L-Δ1-piperideine 6-carboxylate (P6C), produced via the pipecolic acid pathway, and α-aminoadipic semialdehyde (α-AASA), produced via the saccharopine pathway, are in equilibrium. α-AASA is then converted to α-aminoadipic acid (α-AAA) by ATQ. In ATQ deficiency, P6C and α-AASA accumulate due to a block in α-aminoadipic semialdehyde dehydrogenase (Antiquitin, ALDH7A1). P6C undergoes chemical condensation with pyridoxal phosphate (PLP) resulting in PLP deficiency. PA accumulates due to backpressure from the enzymatic block
Standard treatment for inborn errors of metabolism (IEM) affecting catabolic pathways of essential amino acids includes reduction in the substrate of the deficient enzyme through dietary modification. For ATQ deficiency, a similar strategy was pursued to explore whether dietary lysine restriction could reduce the accumulation of lysine-derived intermediates and help improve cerebral function (neurodevelopment, cognition, and behavior).
The first dietary intervention for PDE was reported in an open-label, observational study that evaluated the effectiveness and safety of concomitant dietary lysine restriction and pyridoxine therapy using chemical biomarkers, seizure control, and developmental/cognitive outcomes in seven children with ATQ deficiency (van Karnebeek et al. 2012). The results show that dietary lysine restriction (evidence level IV) in these children: (1) is tolerated without short-term adverse effects; (2) leads to decrease of potentially neurotoxic biomarkers in different body compartments; and (3) has potential benefit for seizure control and neurodevelopmental outcome.
However, evidence regarding the benefits of a lysine-restricted diet remains limited. Additionally, lysine restriction poses a burden on patients and families, and can conflict with social and cultural traditions (Stockler et al. 2012). Further, it requires monitoring by a specialist and (metabolic) dietitian with regular clinical follow-up, dietary protocols, and laboratory testing.
During the past three decades, important experience has been gained with the implementation and optimization of a lysine-restricted diet to prevent brain injury in Glutaric Aciduria Type I (GA-I). This rare organic aciduria is caused by an inherited deficiency of glutaryl-CoA dehydrogenase, which is involved in the catabolic pathways of l-lysine, l-hydroxylysine, and l-tryptophan (Kölker et al. 2011). Despite distinct differences between PDE and GA-I, the former being associated with chronic toxicity and the latter with a risk of acute metabolic stroke (striatal necrosis) during catabolism requiring acute intervention in illness (especially before the age of 6 years), and a need for l-tryptophan restriction, the experience with a lysine-restricted diet in GA-I provides a critical framework for the following recommendations in PDE. Furthermore, in IEMs, as in other diseases, standardization of treatments and monitoring improves outcomes (Heringer et al. 2010).
Motivated by the positive outcomes of our first study on the one hand and the limited evidence level on the other, the PDE Consortium has developed the following consensus recommendation for implementation, monitoring, and follow-up of a lysine-restricted diet in patients with PDE due to ATQ deficiency, and proposes an observational study to generate further evidence for this adjunctive therapy and the pathophysiology of PDE in general.
Methods
In 2010, an initiative was launched to bring together clinicians and scientists with experience in PDE due to ATQ deficiency to identify research questions, which could be answered via collaborative efforts and data sharing, analogous to the NICE guidelines (http://www.nice.org.uk). Scientists publishing in the field or attending conferences on the topic were invited to join. The PDE Consortium was established, and by 2013 comprised over 50 scientists including: pediatric and adult neurologists; metabolic diseases specialists; laboratory biochemical geneticists; metabolic dieticians; methodologists; and basic and clinical scientists in neurology, genetics and biochemistry. As an initial step, the Consortium published recommendations for diagnosis and standard treatment of PDE based on a review of existing literature and experts’ experience (Stockler et al. 2011).
In analogy to other disorders of amino acid degradation (specifically GA-I), adjuvant treatment of ATQ deficiency with a lysine-restricted diet had been offered to some patients showing neurodevelopmental deficits in Vancouver (Canada), Hannover (Germany), and Denver (Colorado, US), as part of an observational study, with promising results (van Karnebeek et al. 2012). Consequently, the Consortium undertook a systematic review of a lysine-restricted diet in PDE as a basis for recommendations on its application and a proposal for future studies. The specific review question was: “What is the evidence for benefit and costs of an adjunct lysine-restricted diet in PDE?” It was also agreed that the review and recommendation should not cover other issues related to PDE, specifically the optimal dosage of pharmacological treatment with pyridoxine.
The “International PDE Consortium” convened for the 2nd, 3rd, and 4th International PDE workshops in Geneva, CH (2012), Birmingham, UK (2012), and Barçelona, Spain (2013) respectively, to draft and consolidate the dietary protocol. Milupa/Nutricia sponsored the room rental for the Barcelona meeting; a company employee was in attendance but did not contribute to the content or design of the recommendations. During these workshops it was agreed that, based on first and only evidence (van Karnebeek et al. 2012), neonates and children with PDE due to ATQ deficiency may be offered a lysine-restricted diet as part of “improved clinical care.” The importance of systematic outcome monitoring was also acknowledged and options for further studies to generate more evidence were discussed. Randomized-controlled trials designed to generate the highest level of evidence were not deemed possible due to; the inability to blind the patient and physician to the intervention (i.e., use a placebo for a diet restriction); the small number of patients scattered throughout the world; and the lack of funding despite multiple applications. Furthermore, the UBC/BCCHW REBs advised that the 2012 study presented sufficient evidence in favor of the diet, making an RCT unjustified (even in the form of an “n-of-1-trial”).
Results
Literature Search
The literature search (PubMed, 1966–2011), using combinations of the keywords “pyridoxine dependent epilepsy” and “lysine”, “antiquitin” and “lysine”, “antiquitin” and “pipecolic acid”, and “pyridoxine” and “pipecolic acid” yielded 20 results (seven case series, four case reports, three biochemical methodological studies, one animal study, one comment, and four review articles). Except for one review (Stöckler et al. 2011), no article discussed lysine restriction as possible adjunct treatment in ATQ deficiency. A second search using “pyridoxine dependent epilepsy” combined with “treatment” yielded an additional 12 publications (seven case series, two case reports, one biochemical methodological study, two reviews), but no controlled trials. To date, the only evidence (level IV) for this diet in PDE is that of Van Karnebeek et al. (2012). This evidence, together with the updated guidelines for management of GA-1 and clinical expertise, provided the basis for the following recommendations.
Pharmacotherapy: Recommendation
Lifelong treatment with pyridoxine-HCl is the standard treatment for ATQ deficiency. Reported dosages vary considerably, however, and current evidence for an optimum is limited (Haenggeli et al. 1991; Baxter 2001; Nabbout et al. 1999; Basura et al. 2009). Specific recommendations on treatment with pyridoxine-HCl are beyond the scope of this paper. To avoid grossly diverging dosages, which may impede evaluation of the lysine-restricted diet, the Consortium recommends that pharmacotherapy in patients on a lysine-restricted diet should be administered according to the 2011 Consortium publication (Stockler et al.). Furthermore, in view of the uncertainty regarding safety of long-term treatment with high dosages of pyridoxine (Bender 1999), peripheral neuropathy should be screened for via regular clinical neurological examinations and, if in doubt, nerve conduction studies should be performed (Footitt et al. 2013).
Add-on Dietary Treatment: General Recommendations and Rationale
All patients with confirmed ATQ deficiency are eligible for the diet. Diagnosis must be confirmed by: (1) elevated AASA in plasma or urine (or CSF); and (2) at least one disease-causing mutation (or deletion/duplication) in the ALDH7A1 gene. Measurement of pipecolic acid in plasma is less specific and sensitive. Therefore it is only acceptable as an initial suggestive but not diagnostic test (Mercimek-Mahmutoglu et al. 2013). Confirmation via molecular analysis is important as AASA elevations are not specific for ATQ deficiency, but rather are observed in molybdenum cofactor deficiency and sulphite oxidase deficiency (Mills et al. 2012). In addition to newly diagnosed patients, adjunct dietary lysine restriction may be considered in all patients with a pre-existing diagnosis of ATQ deficiency, except in those who are seizure-free with normal IQs and behaviors on pyridoxine mono-therapy.
Recommendations (Fig. 2)
Fig. 2.
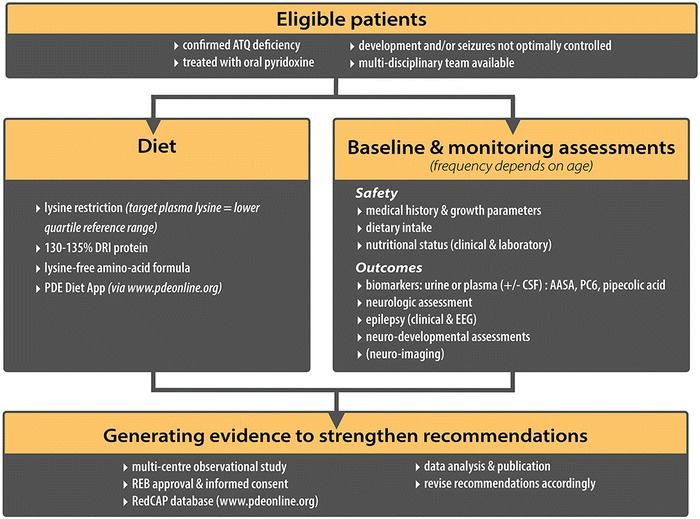
Consortium recommendations for the lysine-restricted diet in PDE patients with confirmed ATQ deficiency
Dietary lysine restriction is an adjunct therapy, not a substitute for pharmacotherapy.
All confirmed ATQ-deficient patients are eligible (unless pyridoxine mono-therapy has resulted in complete symptom resolution with cessation of seizures, and the establishment of normal behavior and development)—regardless of age and gender.
Initiation and duration of treatment: lysine restriction should be started as early as possible, ideally in early infancy (see below). If tolerated without adverse effects, the patient should continue to follow the lysine-restricted diet. The optimal duration of the diet remains to be determined.
Diet and monitoring: see below.
Discontinuation: In case of the unavailability of, or intolerance to, a lysine-free amino acid formula, or severe adverse effects (nutritional, neurological or other), the diet should be terminated. In the former case, a natural protein-restricted diet may be considered.
Quality assurance: A multi-disciplinary team consisting of a medical specialist knowledgeable in PDE treatment (a neurologist and/or a specialist in IEMs), a metabolic dietitian with experience in amino acid/protein restriction, and a nurse should implement dietary treatment and monitoring. Parents or caregivers and the patients should be trained and monitored regularly for dietary compliance.
Rationale
Based on our current understanding of the pathophysiology and derived from experience in conditions with comparable pathogenic backgrounds (accumulation of potentially toxic substrates in the brain), such as phenylketonuria, GA-I, and guanidine-acetate methyltransferase deficiency, the brain may be considered a separate pathogenic compartment which can be influenced by extra-cerebral/systemic metabolic manipulation. The goal of dietary treatment is to restrict lysine as a precursor of potentially neurotoxic intermediates generated by disturbed lysine degradation, whilst avoiding lysine deficiency and maintaining sufficient intake of essential nutrients and energy substrates. Although a decline of pipecolic acid following the supplementation of pharmacological doses of pyridoxine has been observed, the reactive compounds AASA and P6C remain elevated, even during long-term treatment. The potential neurotoxicity of permanently raised AASA or other metabolites could have a substantial impact on neurodevelopmental outcomes. In a cohort of 32 PDE patients, no clear genotype-phenotype correlation could be established (Mills et al. 2010). Lysine restriction should, therefore, be initiated as early as possible in newly diagnosed PDE patients to optimize developmental outcomes, and be maintained over an extended period of time.
Most patients with PDE are diagnosed and followed by pediatric neurologists, often with limited access to dietitians experienced in treating patients with IEMs, which raises inherent challenges for the management of this metabolic epilepsy. These standardized dietary recommendations are intended to support the managing team in this process.
Already routinely used in GA-I, this diet has demonstrated efficacy and safety, if properly monitored. The degree of protein restriction is more liberal than in many IEMs, such as phenylketonuria or maple syrup urine disease, and therefore compliance is easier. This is supported by data from our observational study, which showed dietary adherence in six out seven patients who varied in age from infancy to early teens (van Karnebeek et al. 2012).
Diet Prescriptions (See Online Supplement for More Details)
We recommend the use of a diet based on the restricted lysine intake combined with a lysine-free amino acid supplement to maintain adequate daily total protein and micronutrient intake. This diet has been associated with favorable neurological outcomes and normal growth in several GA-I studies (Heringer et al. 2010; Kölker et al. 2006). The natural protein allowed in the individual’s diet should be based on the daily lysine prescription.
The Consortium has adapted the guidelines for GA-I by Kölker et al. (2011), using both the WHO guidelines (FAO/WHO/UNU 1985) and a paper by Yannicelli et al. (2010) as additional references, and presents these recommendations for age-dependent lysine restriction in Table 1. This can be used as a starting point; thereafter, the lysine prescription must be individually tailored (also beyond the age of 6 years) based on the results of plasma lysine levels, adequate nutrition, and growth. Lysine-free amino acid formulas must be used to provide additional protein intake to meet total protein needs and provide micronutrients.
Table 1.
Age-dependent lysine restriction according to the PDE Consortiuma
| Age (year) | Lysineb,c (mg/kg) | Proteind,e (g/kg) | Energy |
|---|---|---|---|
| 0.0 < 0.5 | 70–100 | 2.75–3.50 | 125–145 kcal/kg |
| 1.0 < 1.0 | 55–70 | 2.50–3.25 | 140–115 kcal/kg |
| 1 < 4 | 50–80 | 1.80–2.60 | 900–1,800 kcal/day |
| 4 < 7 | 40–70 | 1.60–2.00 | 1,300–2,300 kcal/day |
| 7 < 11 | 35–65 | 1.55–1.85 | 1,600–2,800 kcal/day |
| Female | |||
| 11 < 15 | 35–40 | 1.50–1.80 | 1,500–2,800 kcal/day |
| 15 < 19 | 33–40 | 1.45–1.75 | 1,200–2,800 kcal/day |
| >19 | 30–40 | 1.45–1.75 | 1,400–2,400 kcal/day |
| Male | |||
| 11 < 15 | 35–40 | 1.45–1.75 | 2,000–3,200 kcal/day |
| 15 < 19 | 33–45 | 1.45–1.75 | 2,100–3,200 kcal/day |
| >19 | 30–40 | 1.45–1.75 | 2,000–3,000 kcal/day |
aThese recommendations are based on the guidelines published for GA-I by Kölker et al. (2011) and Yanicelli (2010), and adapted for PDE by the Consortium based on experience
bThe lysine/protein ratio varies considerably in natural food: thus natural protein intake in children on a low lysine diet is dependent on the source
cThe continued chronic damage model in PDE requires as low as reasonably possible-chronic lysine levels. This contrasts with GA1, where currently the focus is on preventing damage during acute episodes. In some cases, this translates into lower intakes in PDE than currently recommended in GA1
dTryptophan restriction is not needed in the management of PDE, in contrast to GA-I. Lysine-free amino acid formulas developed for managing GA-I are often low in tryptophan. The individual’s diet should be assessed for tryptophan adequacy and, if inadequate, should be supplemented
eLysine-free amino acid mixtures should be supplemented with minerals and micronutrients necessary to maintain normal levels. Adequate intake of essential amino acids is provided by natural protein and lysine-free amino acid supplements. The amount of amino acid supplementation is adjusted to meet 130% of the patient’s age-appropriate DRI (Table C)
Nutritional Aims
The aim is to achieve 130–135% of the daily-recommended intake (DRI) for total protein. The rationale for this recommendation is the rapid digestion and absorption of free amino acids provided in the supplement and the subsequent decreased nitrogen retention.
Daily lysine intake should be prescribed at an amount that maintains the plasma lysine level within the lower normal age-dependent reference range, preferably in the lower quartile. Lysine intake should be kept at the lowest possible levels that allow for adequate growth and nutrition. In contrast to GA-I, tryptophan restriction is not indicated in the management of PDE. As some lysine-free amino acid formulas are also low in tryptophan, this amino acid must be supplemented to meet the daily DRI (FAO/WHO/UNU 1985).
Lysine-free amino acid formulas are often supplemented with vitamins and minerals to provide adequate or significant intakes of these nutrients: an adequate supply of iron, minerals, and vitamins must be confirmed by regular laboratory testing and nutritional evaluation. Possible interactions between the pharmacological treatment with pyridoxine-HCl and altered nutritional B6 vitamers due to the diet, specifically lower PLP intake due to reduced animal protein consumption, are not understood and require further research. If, in an adolescent or adult patient, lysine-free amino acid formulas are abandoned, and a protein-restricted diet is continued, vitamin and mineral supplements become mandatory. Because the consumption of meat, fish, and dairy products will be limited, the use of fats and oils, carbohydrates, and special low protein foods is often necessary to provide adequate caloric intake. Fats and oils also provide essential dietary fatty acids. Low protein foods provide variety to the diet in addition to needed calories (Refer: Online Supplement).
Diet Management in (Breastfed) Infants
Mothers of neonates and infants with PDE should be encouraged to continue breastfeeding. Since the average lysine content of breast milk after the neonatal period (68–86 mg/100 mL) (sources: www.bls.nvs2.de; http://www.ars.usda.gov/nutrientdata) is considerably lower than that of formula milk (120–160 mg/100 mL) a greater amount of breast milk may be consumed.
In order to guarantee normal growth, use of a lysine-free amino acid supplement is also advised for breastfed children. As in organic aciduria (Francis and Smith 1981), this is best achieved by feeding a defined amount of lysine-free formula before breastfeeding ad libitum, or alternatively, by feeding expressed breast milk which can be mixed with the amino acid supplement in the bottle-feedings prepared once daily, and kept refrigerated for a maximum of 24 h. Expressed breast milk may be required when the adequacy of breastfeeding is doubtful, or inconsistent. If breastfeeding is not possible, a combination of an appropriate infant formula and lysine-free amino acid supplement is recommended.
As in healthy infants, measured amounts of solid foods should be introduced from an age of 4 to 6 months onwards with the lysine content included in the daily dietary calculations.
Assessment of Lysine Content
The lysine content varies considerably in different proteins. Cereals, fruit, and vegetables tend to have a low lysine content, e.g., 2–4% (lysine/protein) in cereals, whereas meat and animal protein is rich in lysine, e.g., 8% in beef, chicken or cow milk, and 9% in fish. Therefore, actual daily lysine intake may be less well controlled by calculation of total protein intake rather than lysine intake. Lysine restriction is preferred to protein restriction, especially during the first 2 years of life, as there is ongoing myelination of the brain, and a reported potential for white matter damage in ATQ-deficient patients (Niermeijer et al. 2012).
Switching to protein counting may be considered only where lysine counting is not feasible, and should be undertaken only after the individual/family has followed a lysine-restricted diet for 1 year (if possible), to allow the patient and caregivers to become familiar with the lysine content of different foods, the taste of the formula, and the rhythm of the diet.
Older patients who refuse the lysine-free formula may still benefit from a protein-restricted diet, which can substantially reduce levels of AASA and P6C.
Lysine-Free Amino Acid Formulas
The use of commercially available formulas with amino acid supplements is recommended, as this ensures a well-balanced diet. It must be noted that some lysine-restricted formulas exist specifically for management of GA-1 patients and so are also tryptophan-restricted. In the Online Supplement (under 10) useful resources are listed that provide information on metabolic diets, nutritional content of foods, and medical formulas.
Monitoring
Clinical Monitoring
Prior to initiating the diet, a detailed medical and nutritional history should be obtained from each patient, including symptoms; date of seizure onset; history of epilepsy; current medical developmental and nutritional status; and documentation of weight, length, and head circumference.
Patient monitoring must include both clinic visits and follow-up by telephone or email. Interim history during the follow-up visits should include questions regarding seizure recurrence, developmental progress, growth parameters, and any change in health status or concomitant medications, to monitor for safety and adverse events.
A nutritional intake history should be reviewed via a 3-day diet record or, if unavailable, a 24-hour dietary recall, for age-appropriate daily nutritional adequacy of energy, macro- and micronutrients, and lysine-restricted diet adherence.
Follow-up clinic visits include, at a minimum, the initial visit, 1-month post dietary initiation, then every 3 months during the first year, and every 6 months thereafter.
Assessments during clinic visits should include vital signs, a physical exam, and anthropometric measures (height, weight, and head circumference), and nutritional assessment and counseling. Weights may be recorded with greater frequency, as guidance for clinical care, i.e., weekly weight will be taken in patients less than 1 year of age, every 4 weeks in patients from ages 1 to 6 years, and every 3 months for patients >6 years of age, to adjust the total dose of pyridoxine and lysine restriction (both calculated per weight).
Biochemical and Routine Laboratory Monitoring
Table 2 provides an overview of the frequency for various laboratory tests. AASA, P6C, and pipecolic acid are the biochemical markers for PDE that will be measured in plasma or urine.
For infants <1 year of age, a plasma sample for the biochemical markers should be taken at least 3 h after a meal; for children >1 year of age, samples should be taken at least 4 h after a meal.
Since AASA and P6C are unstable, plasma and urine samples need to be stored at −80°C, and, if required, transport to the laboratory should be done on dry ice.
If a lumbar puncture is performed for clinical purposes, it is recommended that additional samples be collected to monitor the level of AASA, P6C, and pipecolic acid in the CSF. Levels of PLP, neurotransmitter metabolites, and amino acids can also be analyzed as they may be abnormal. CSF should be frozen immediately, stored at −80°C, and shipped on dry ice. The rationale for the different CSF analyses is provided in Table 3. CSF is the body fluid most closely reflecting cerebral lysine metabolism and possible neurotoxic status in the brain, and provides the most relevant biochemical outcome measure.
Table 2.
summarizes the recommended follow-up for biochemical and routine laboratory monitoring
| Thereafter, frequency at age | ||||
|---|---|---|---|---|
| Parameter | Rationale | Following initiation of diet | 0–3 years | >3 years |
| AASA, P6C, and pipecolic acid (urine and plasma) | Efficacy in reducing metabolites of lysine degradation | 1 month, 3 months | 3 months | 6 months |
| Lysine and amino acids in plasma | Medical adherence, safety | 1 month, 3 months | 1 months (age 0–1 years), 3 months (age 1–3 years) | 6 months |
| Albumin, pre-albumin, iron parameters, calcium, phosphate, 25-OH-vitamin D3, zinc, selenium, complete blood count, folic acid, and vitamin B12 in serum or plasma | Safety panel | 1 month, 3 months | 6 months | 6 months |
Table 3.
Rationale for CSF analysis
| CSF Metabolite | Rationale/expected insights |
|---|---|
| AASA and P6C and pipecolic acid | Metabolites reflecting lysine metabolism in the brain/surrogate parameters of toxicity |
| We expect reduction in these metabolites upon lysine-restricted diet | |
| Pyridoxal-5-phosphate (PLP) | Metabolite reflecting the biochemical interaction of accumulating AASA/P6C with PLP |
| We expect increase of this metabolite upon lysine-restricted diet | |
| Monoaminergic neurotransmitter metabolites (HVA, 5-HIAA) | Synthesis of Dopamin and Serotonin requires pyridoxal phosphate as cofactor |
| We expect that these neurotransmitter metabolite levels increase with increasing availability of pyridoxal phosphate upon the lysine-restricted diet | |
| Amino acids | We are primarily interested in CSF lysine, which has been observed in the low normal range in patients on the lysine-restricted diet.7 Other amino acids of interest are (but not restricted to) tryptophan (due to inadequate tryptophan content of most lysine-free amino acid formulas), glycine, serine, and branched chain amino acids (given that many reactions the metabolism of these amino acids requires PLP as a cofactor) |
| Cells count, protein, and glucose | CSF specimen that is bloody, or has an increased protein level have to be excluded from analysis) |
| 1 mL | To store for possible additional research studies such as metabolomics. AASA as a semialdehyde is a reactive substance and its accumulation might result in changes in numerous other metabolites. Metabolomic metabolite patterns might reveal chemical compounds that might contribute to the pathophysiology of ATQ deficiency prior and during treatment with a lysine-restricted diet |
These CSF results may also be of future clinical relevance, specifically, to guide pyridoxine dosing, based on the levels of PLP-dependent neurotransmitters in CSF, in addition to documenting a response to dietary treatment. Moreover, the assessment of lysine in CSF will demonstrate whether lysine restriction decreases CSF lysine levels, and will give insight into whether P6C, AASA, and pipecolic acid are derived from cerebral lysine catabolism or are imported from the circulation. It is important to indicate the interval from the last pyridoxine intake to time of sampling for appropriate interpretation of results.
Nutritional Markers
To ensure adequate nutritional status, regular testing of a number of laboratory parameters should be done, including pre-albumin, albumin, plasma amino acids; complete blood count; comprehensive metabolic panel; and micronutrients (for details see Table 2).
Neurological Monitoring
A pediatric neurological exam should be performed during every clinic visit and be tailored to the age of the individual with a focus on the achievement of developmental milestones; muscle tone; strength; deep tendon reflexes; and coordination. Sensitivity to light touch and vibration should be assessed in children >4 years. If deep reflexes of the Achilles tendon are absent, peripheral neuropathy should be considered and nerve conduction velocity studies performed.
Electroencephalogram (EEG) should be performed every 6 months, or more frequently if clinically indicated, to evaluate the development of age-appropriate background activity and to assess the presence of epileptiform discharges.
Patients who continue to have seizures should maintain a seizure logbook for review during clinic visits.
Neurodevelopmental Monitoring
Table 4 lists the recommended psychometric measures (to be performed by a psychologist) to evaluate the effect of treatment prior to starting the diet and at annual follow-up.
Table 4.
Summarized the standard psychological scales to monitor neurodevelopmental outcome
| Measurement tool | Age group |
|---|---|
| Bayley Scale for infant and toddler development | 0–3 years |
| Vineland Adaptive Behavior Scales | 0–90 years |
| Wechsler Preschool and Primary Scale of Intelligence | 2 years 6 months to 7 years |
| Wechsler Intelligence Scale for Children | 6–16 years 11 months |
Neuro-Radiological Monitoring
Magnetic resonance imaging of the brain should be performed using sedation prior to initiating the diet and considered after 3 years of age, or more frequently, if clinically indicated, to detect structural abnormalities and to monitor for myelination and brain development.
Emergency Treatment
In contrast to IEMs of the intoxication type, such as MSUD or GA-I (Kölker et al. 2011), acute neurological injury resulting in permanent brain damage is not a typical feature of PDE. However, degenerative cerebral MRI findings over time have been reported (Gospe 2012; Niermeijer et al. 2012). Based on this and unpublished individual experience, the Consortium reached the following consensus.
During an acute illness (with inherent increase of catabolism, and potential increase of PLP-inactivating P6C) routine treatment may not sufficiently protect against seizures and neurologic dysfunction, and the following precautions are recommended:
Doubling the dosage of pyridoxine up to a maximum of 40 mg/kg/day (or 500 mg in older children and adults) for up to 3 days.
Maintenance of caloric intake to prevent catabolism of endogenous proteins.
Intravenous fluid, caloric, and pyridoxine supplementation should be administered only if justified by the severity of the acute disease and inability to tolerate medications or fluids orally or enterally.
Discussion
It is the Consortium’s impression that the potential benefits of lysine restriction in PDE could outweigh its risks and burdens, if recommendations such as these are followed closely by a clinical team, including an expert physician (neurologist or IEM specialist), a (metabolic) dietitian, and a nurse or counselor. The greatest risk associated with the diet is lysine over-restriction, which can be avoided by close monitoring and adjustment.
The recommended standardization of treatment and outcome evaluation, together with the creation of an online database with a standard research ethics board (REB) protocol and consent form, paves the way for data collection as part of a multicenter observational study to increase evidence regarding the benefit and optimal duration of the diet in PD. Therefore, the Consortium proposes that an observational study (level III) be done which would include PDE patients of all ages, and compare those on- (active) and off-diet (controls). To this end, we have designed an Internet-accessible RedCAP database for entry of patient data and we will post a standardized REB protocol on our website (www.pdeonline.org), which can be adapted for local submission and approval. With appropriate institutional ethics board approvals, de-identified data on phenotype and genotype will also be collected.
We invite physicians from across the globe to participate. Data entered will be analyzed at periodic intervals to further consolidate evidence and be published with all participants as co-authors. The current recommendations will be adapted according to new insights and evidence.
In support of patients following the lysine-restricted diet and to enhance compliancy, a digital PDE diet-App has been developed for use on handheld devices and desktops. The App will be freely available from the beginning of 2014. It provides the following functions: password coded access; option to enter weight and customized diet plan; lysine-arginine-protein-caloric content of foods; digital calculator for advised and realistic lysine and protein intake; recipes for low protein/lysine-restricted foods; storage and export of daily food records; and tracking of clinical and laboratory values (with charts and graphs).
Not only is this kind of initiative essential for collecting evidence for treatment of rare disorders where it is not practically possible to conduct randomized-controlled studies, it will also provide a framework to generate evidence regarding the evaluation of any new strategies, such as arginine fortification, which might be added in the future (Strauss et al. 2011; Kölker et al. 2012).
The Consortium recognizes that, aside from potential neurotoxic damage, the ID seen in PDE could be explained by the role of ATQ in neuronal migration (Jansen et al. 2013), as well as the increased rate of fetal distress leading to premature birth and/or associated pathology (Mills et al. 2010). Additionally, an association between delay in diagnosis and poor development has been reported (Bok et al. 2012). The latter issue can be addressed by improving the awareness of PDE among neonatologists and pediatric epileptologists, or, in the future, perhaps newborn screening (Jung et al. 2013).
Conclusions
In summary, we realize that for PDE, many challenges remain. These include the limited insights into the natural history and pathophysiology, and the potential impact of long-term hyper-physiologic, high-dose pyridoxine therapy. Through collaboration, standardization of diagnosis and treatment, and the establishment of registries and databases for data collection on natural history as well as interventional outcomes, it will be possible to address these issues and improve the clinical management of individuals affected by PDE.
Electronic Supplementary Material
Acknowledgments
We gratefully acknowledge Mrs. Claire Sowerbutt for text editing, Mrs. Ruth Giesbrecht (B.C. Children’s Hospital) for editorial assistance, and Dr. Beate Szczerbak (Nutricia Advanced Medical Nutrition) for supporting the 3rd PDE Consortium meeting (2013). CvK is supported by TIDE-BC, the 1st Collaborative Area of Innovation grant from BC Children’s Hospital Foundation, Vancouver, Canada. PM is supported by a grant from the GOSHCC Neuroscience Initiative. The content of this article has not been influenced by the funders/supporters.
Synopsis
The first recommendation for the implementation and monitoring of the lysine-restricted diet as adjunct treatment for PDE generated as consensus statement by the international PDE Consortium.
Conflict of Interest
Clara van Karnebeek and Hans Hartmann declare that the room rental for the 4th PDE Consortium meeting in Barcelona, Spain was sponsored by Milupa/Nutricia; a company employee was in attendance but did not contribute to the content or design of the recommendations. Clara van Karnebeek, Sylvia Stockler, Sravan Jaggumantri, Birgit Assmann, Peter Baxter, Daniela Buhas, Levinus A. Bok, Barbara Cheng, Curtis R. Coughlin II, Anibh M. Das, Alette Giezen, Wahla Al-Hertani, Gloria Ho, Uta Meyer, Philippa Mills, Barbara Plecko, Eduard Struys, Keiko Ueda, Monique Albersen, Nanda Verhoeven, Sidney M. Gospe Jr., Renata C. Gallagher, Johan Van Hove, Hans Hartmann declare that they have no (other) conflict interest to declare.
Informed Consent
Not applicable
Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Details of Contributions of Individual Authors’ Contributions
Clara van Karnebeek and Hans Hartmann performed the literature review, led the consensus meetings, and drafted the recommendations, revising it according to the author group’s input. Sylvia Stockler, Sravan Jaggumantri, Barbara Plecko, Sidney Gospe, Renata Gallagher, and Johan van Hove provided critical input for the content and format of the recommendations at each stage, and drafted individual parts of the manuscript. Peter Baxter, Daniela Buhas, Levinus A. Bok, Barbara Cheng, Curtis R. Coughlin II, Anibh M. Das, Alette Giezen, Wahla Al-Hertani, Gloria Ho, Uta Meyer, Philippa Mills, Barbara Plecko, Eduard Struys, Keiko Ueda, Monique Albersen, Nanda Verhoeven provided input for the recommendations specific to their field (pediatric neurology, metabolic diseases, laboratory biochemical genetics, dietetics, and nutrition), both at a clinical and scientific level. All authors are active members of the PDE Consortium and reviewed/edited the manuscript.
Footnotes
Competing interests: None declared
Contributor Information
Clara D. M. van Karnebeek, Email: cvankarnebeek@cw.bc.ca
Hans Hartmann, Email: hartmann.hans@mh-hannover.de.
Collaborators: Johannes Zschocke and K Michael Gibson
References
- Basura GJ, Hagland SP, Wiltse AM, Gospe SM., Jr Clinical features and the management of pyridoxine-dependent and pyridoxine-responsive seizures: review of 63 North American cases submitted to a patient registry. Eur J Pediatr. 2009;168:697–704. doi: 10.1007/s00431-008-0823-x. [DOI] [PubMed] [Google Scholar]
- Baxter P. Pyridoxine-dependent and pyridoxine-responsive seizures. Dev Med Child Neurol. 2001;43:416–420. doi: 10.1017/S0012162201000779. [DOI] [PubMed] [Google Scholar]
- Bender DA. Non-nutritional uses of vitamin B6. Br J Nutr. 1999;81:7–20. [PubMed] [Google Scholar]
- Bok LA, Halbertsma FJ, Houterman S. Long-term outcome in pyridoxine-dependent epilepsy. Dev Med Child Neurol. 2012;54:849–854. doi: 10.1111/j.1469-8749.2012.04347.x. [DOI] [PubMed] [Google Scholar]
- Dogan M, Dogan DG, Kahraman AS. A 9-year follow-up of a girl with pyridoxine (vitamin B6)-dependent seizures: magnetic resonance spectroscopy findings. Eur Rev Med Pharmacol Sci. 2012;16:695–698. [PubMed] [Google Scholar]
- FAO/WHO/UNU (1985) Energy and protein requirements. In: Report of a joint FAO/WHO/UNU Expert Consultation, World Health Organization Technical Report Series no. 724. WHO, Geneva, Switzerland [PubMed]
- Footitt EJ, Clayton PT, Mills K, Heales SJ, Neergheen V, Oppenheim M, Mills PB. Measurement of plasma B6 vitamer profiles in children with inborn errors of vitamin B6 metabolism using an LC-MS/MS method. J Inherit Metab Dis. 2013;36:139–145. doi: 10.1007/s10545-012-9493-y. [DOI] [PubMed] [Google Scholar]
- Francis DEM, Smith I. Breast-feeding regimen for the treatment of infants with phenylketonuria. In: Bateman C, editor. Applied nutrition London. London: John Libbey; 1981. pp. 82–83. [Google Scholar]
- Gospe SM., Jr Natural history of pyridoxine-dependent epilepsy: tools for prognostication. Dev Med Child Neurol. 2012;54:781–782. doi: 10.1111/j.1469-8749.2012.04354.x. [DOI] [PubMed] [Google Scholar]
- Haenggeli CA, Girardin E, Paunier L. Pyridoxine-dependent seizures, clinical and therapeutic aspects. Eur J Pediatr. 1991;150:452–455. doi: 10.1007/BF01958419. [DOI] [PubMed] [Google Scholar]
- Heringer J, Boy SP, Ensenauer R, et al. Use of guidelines improves the neurological outcome in glutaric aciduria type I. Ann Neurol. 2010;68:743–752. doi: 10.1002/ana.22095. [DOI] [PubMed] [Google Scholar]
- Jansen LA, Heyner RD, Roden WH (2013) Glial localization of antiquitin: implications for pyridoxine-dependent epilepsy. Ann Neurol PMID 24122892 [DOI] [PMC free article] [PubMed]
- Jung S, Tran NT, Gospe SM, Jr, Hahn SH. Preliminary investigation of the use of newborn dried blood spots for screening pyridoxine-dependent epilepsy by LC-MS/MS. Mol Genet Metab. 2013;110:237–240. doi: 10.1016/j.ymgme.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Kölker S, Garbade SF, Greenberg CR, et al. Natural history, outcome, and treatment efficacy in children and adults with glutaryl-CoA dehydrogenase deficiency. Pediatr Res. 2006;59:840–847. doi: 10.1203/01.pdr.0000219387.79887.86. [DOI] [PubMed] [Google Scholar]
- Kölker S, Christensen E, Leonard JV, et al. Diagnosis and management of glutaric aciduria type I–revised recommendations. J Inherit Metab Dis. 2011;34:677–694. doi: 10.1007/s10545-011-9289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölker S, Boy SP, Heringer J, et al. Complementary dietary treatment using lysine-free, arginine-fortified amino acid supplements in glutaric aciduria type I – a decade of experience. Mol Genet Metab. 2012;107:72–80. doi: 10.1016/j.ymgme.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Mercimek-Mahmutoglu S, Donner EJ, Siriwardena K. Normal plasma pipecolic acid level in pyridoxine dependent epilepsy due to ALD7A1 mutations. Mol Genet Metab. 2013;110:197. doi: 10.1016/j.ymgme.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Mills PB, Struys E, Jakobs C, et al. Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nat Med. 2006;12:307–309. doi: 10.1038/nm1366. [DOI] [PubMed] [Google Scholar]
- Mills PB, Footitt EJ, Mills KA, et al. Genotypic and phenotypic spectrum of pyridoxine-dependent epilepsy (ALDH7A1 deficiency) Brain. 2010;133:2148–2159. doi: 10.1093/brain/awq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills PB, Footitt EJ, Ceyhan S, et al. Urinary AASA excretion is elevated in patients with molybdenum cofactor deficiency and isolated sulphite oxidase deficiency. J Inherit Metab Dis. 2012;35:1031–1036. doi: 10.1007/s10545-012-9466-1. [DOI] [PubMed] [Google Scholar]
- Nabbout R, Soufflet C, Plouin P, Dulac O. Pyridoxine dependent epilepsy: a suggestive electroclinical pattern. Arch Dis Child Fetal Neonatal Ed. 1999;81:F125–F129. doi: 10.1136/fn.81.2.F125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niermeijer JMF, Abeling N, Koelman HJ. Pyridoxine dependent epilepsy: clinical features and progressive serial brain MRI abnormalities. JIMD. 2012;35(Suppl):S136. [Google Scholar]
- Stockler S, Plecko B, Gospe SM, Jr, et al. Pyridoxine dependent epilepsy and antiquitin deficiency: clinical and molecular characteristics and recommendations for diagnosis, treatment and follow-up. Mol Genet Metab. 2011;104:48–60. doi: 10.1016/j.ymgme.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Stockler S, Moeslinger D, Herle M, et al. Cultural aspects in the management of inborn errors of metabolism. J Inherit Metab Dis. 2012;35:1147–1152. doi: 10.1007/s10545-012-9455-4. [DOI] [PubMed] [Google Scholar]
- Strauss KA, Brumbaugh J, Duffy A, et al. Safety, efficacy and physiological actions of a lysine-free, arginine-rich formula to treat glutaryl-CoA dehydrogenase deficiency: focus on cerebral amino acid flux. Mol Genet Metab. 2011;104:93–106. doi: 10.1016/j.ymgme.2011.07.003. [DOI] [PubMed] [Google Scholar]
- van Karnebeek CD, Hartmann H, Jaggumantri S, et al. Lysine restricted diet for pyridoxine-dependent epilepsy: first evidence and future trials. Mol Genet Metab. 2012;107:335–344. doi: 10.1016/j.ymgme.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Yannicelli S. Nutrition management of patients with inherited disorders of organic acid metabolism. In: Acosta PB, editor. Nutrition management of patients with inherited metabolic disorders. Boston: Jones and Bartlett publishers; 2010. p. 314. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


