Abstract
The study of thrombus formation has increasingly applied in vivo tools such as genetically modified mice and intravital microscopy to the evaluation of molecular and cellular mechanisms of thrombosis. Among several unexpected findings of this approach was the discovery that protein disulfide isomerase serves an essential role in thrombus formation at sites of vascular injury. The observation that the commonly ingested quercetin flavonoid, quercetin-3-rutinoside, inhibits protein disulfide isomerase and blocks thrombus formation in pre-clinical studies has set the stage for clinical trials using protein disulfide isomerase antagonists as antithrombotics. Even though the mechanisms by which protein disulfide isomerase facilitates platelet activation and fibrin formation have yet to be elucidated, protein disulfide isomerase antagonists are currently being developed as antithrombotics. This review will consider what is known about the role of protein disulfide isomerase in platelet accumulation and fibrin generation with a focus on pharmacological strategies for blocking protein disulfide isomerase activity in the context of thrombus formation. Potential indications and clinical trial design for testing the efficacy of protein disulfide isomerase inhibition to reduce the incidence of thrombosis will be considered.
Protein disulfide isomerase
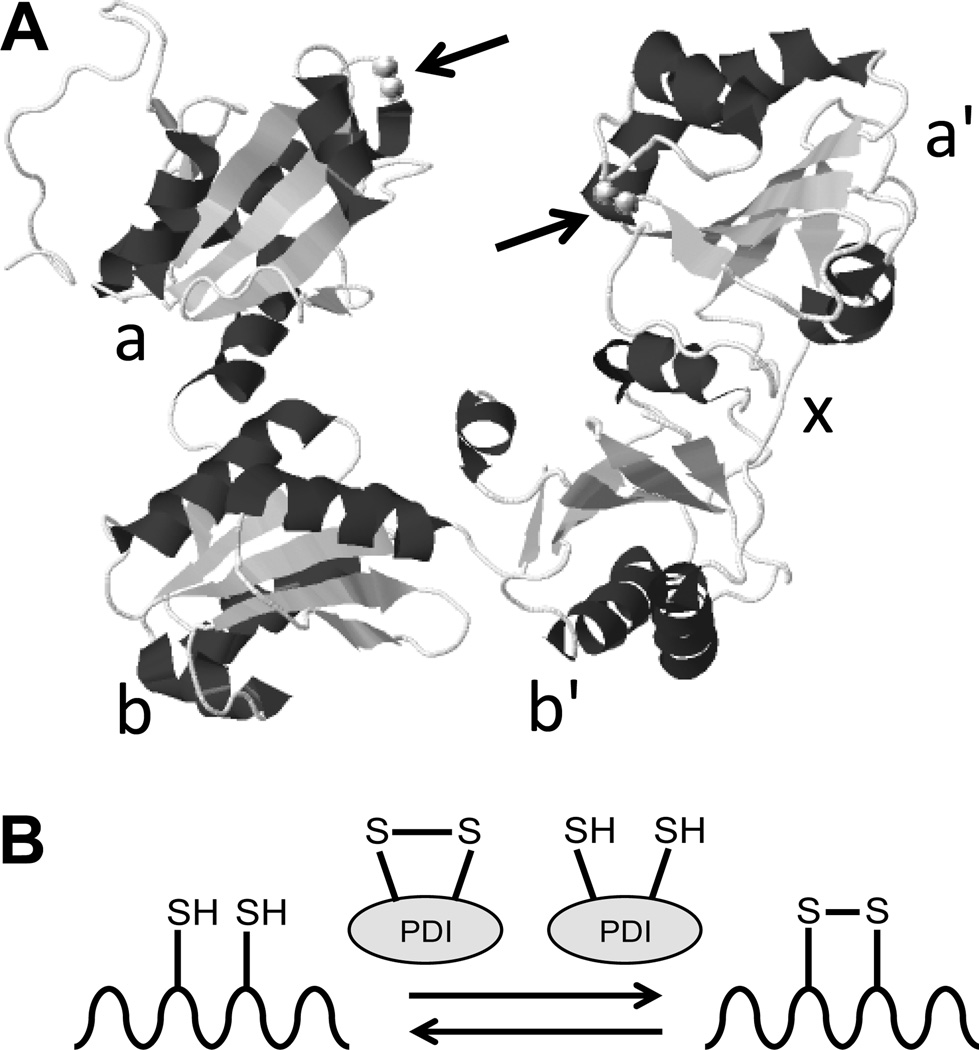
Protein disulfide isomerase (PDI) is the archetypal member of a family of thiol isomerases original identified for their role in modifying disulfide bond formation during protein synthesis and folding (for more detailed information on the biochemistry and cell biology of thiol isomerases please refer to recent reviews1, 2). It is a 57 kD protein that possesses an a-b-b'-x-a'-c domain structure (Fig. 1). The a and the a' domains contain the active CGHC motifs, which face each other in the crystal structure of PDI (Fig. 1).3 These motifs catalyze oxidoreductive activities. The b and b'domains are substrate binding and the × domain consists of a short linker that connects the b' and a' domains. The C-terminal c domain functions in chaperone activity4 and terminates with a KDEL sequence. These domains are attached in an U-shaped structure that is open in the oxidized state and closed in the reduced state (Fig. 1).3
Figure 1. Structure and function of protein disulfide isomerase.
A, The structure of protein disulfide isomerase (PDI) as determined by x-ray crystallography. The a, b, b', x, and a' domains are indicated. Arrows denote the location of the CGHC catalytic motifs (adapted from Wang et al., Antioxid. Redox Signal., 2013).3 B, The primary function of the CGHC motifs is to catalyze the oxidation and reduction of disulfide bonds to facilitate proper folding of proteins as they are synthesized in the endoplasmic reticulum. However, PDI can also be secreted from vascular cells and extracellular PDI is essential for thrombus formation.
PDI is capable of several different distinct activities. It can act as a reductase or an oxidase depending on the redox potential of its substrate (Fig. 1). Such reactions facilitate the isomerase activity of PDI, which is essential for proper folding of nascent proteins as they are synthesized in the endoplasmic reticulum (ER). PDI also acts as a chaperone and its binding can promote proper folding even in proteins that lack disulfide bonds.5–7 The vicinal cysteines in the CGHC motif can undergo S-nitrosylation or glutathionylation, regulating their activity.8, 9 Likewise, PDI can act as a denitrosylase, removing nitric oxide from a substrate protein, or as a transnitrosylase, transferring nitric oxide into cells.10, 11 These varied activities are influenced by the redox environment, pH, allosteric modulators, and substrate characteristics.
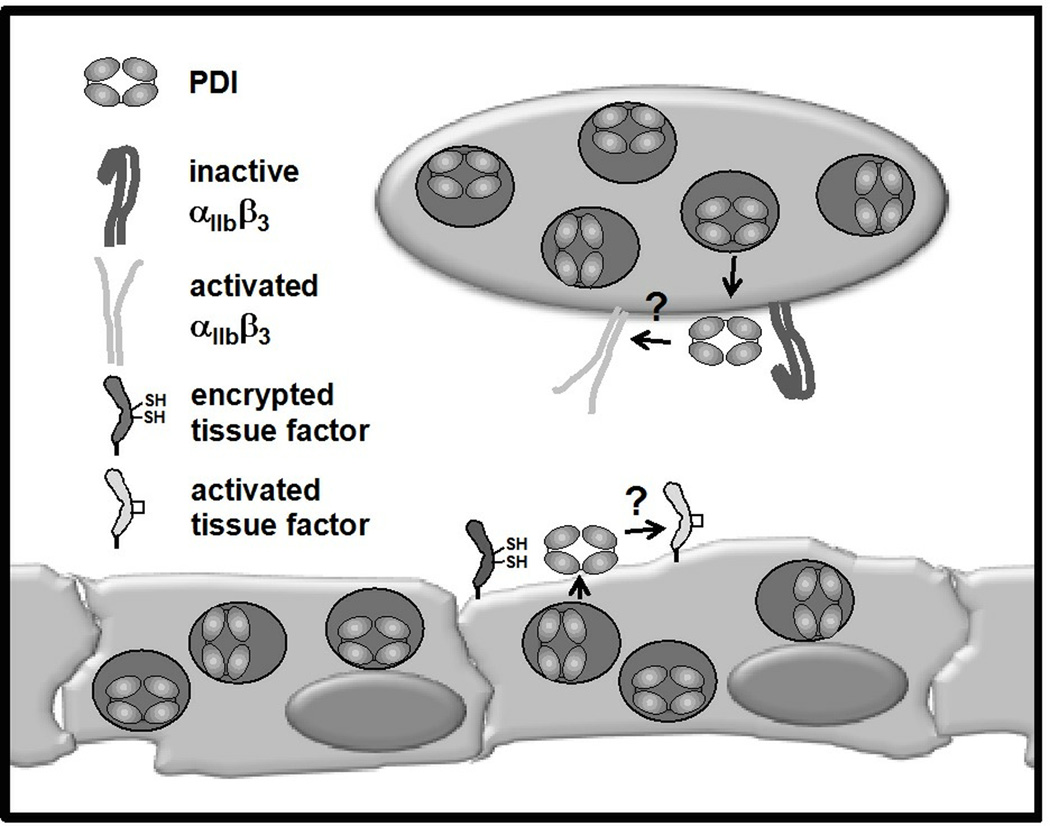
The subcellular localization of PDI also influences its activity. PDI is primarily sequestered in the ER of nucleated cells where it is reported to be concentrated to ~200 µM.12 In platelets, it has been identified within the dense tubular system. Yet in both nucleated cells and platelets, a population of PDI exists in storage granules and on the extracellular surface.13 The mechanism by which PDI is transported to the extracellular is not well-understood. KDEL sequences usually serve as an ER retention signal. However, more recent studies suggest that it may, in some instances, facilitate expression of PDI on the extracellular surface.14 In platelets, PDI co-localizes with toll-like receptor 9 (TLR9) in a novel organelle termed the T-granule (Fig. 2).15 In endothelial cells, PDI co-localizes with chemokines, including growth-related oncogene-α and monocyte chemoattractant protein-1, but not with von Willebrand factor (Fig. 2).16 PDI stores can be released from platelet and endothelial cell granules in an activation-dependent manner. Newly released PDI binds αIIbβ3 on the platelet surface and αvβ3 on the endothelial cell surface.17 Activation-dependent release of PDI is critical for thrombus formation.
Figure 2. Model of potential roles of PDI in thrombus formation.
Little is known about the mechanisms by which PDI functions in thrombus formation. This model illustrates several hypotheses that have been offered. In platelets, PDI localizes to T-granules and is released upon platelet activation. Extracellular PDI is thought to act as an isomerase for platelet receptors, such as αIIbβ3, converting them to an activated conformation. However, the influence of PDI on αIIbβ3 conformation and the importance of PDI in activating αIIbβ3 during thrombus formation are currently unknown. In endothelial cells, PDI localizes to secondary granules (that do not contain von Willebrand factor) and is release with cell activation. One theory is that PDI facilitates the conversion of resting tissue factor to activated tissue factor by facilitating disulfide bond formation between Cys186 and Cys209, but this remains to be proven. The action of PDI on the components of thrombus formation remains to be determined.
PDI in platelet function
Chen et al. first described the release of PDI from platelets more than two decades ago.13 Initial efforts to understand its role in activation were directed at identifying potential substrates of surface-exposed platelet PDI. Thrombospondin 1,18, 19 GPIbα20, α2β1,21 and αIIbβ322 have all been identified as potential substrates. Platelet PDI has also been implicated in the formation of disulfide linkages between proteins, such as the formation of complexes of vitronectin or thrombospondin with thrombin-anti-thrombin.23, 24 However, which of these putative substrates is important for PDI function in platelet activation and thrombus formation is not clear.
Several studies have implicated PDI in platelet activation. Earlier studies demonstrated that the cyclic polypeptide, bacitracin, a non-selective PDI inhibitor or antibodies directed against PDI block platelet aggregation and secretion.22, 25 Although the mechanisms by which PDI participates in platelet activation are not entirely understood, there is evidence that PDI participates in the transformation of αIIbβ3 to an active conformation (Fig. 2).26 Recently, mice with a megakaryocyte/platelet-specific defect in PDI have been generated. These mice demonstrate decreased, but not absent, platelet aggregation in response to multiple agonists.27 PDI-deficient mouse platelets also demonstrate a defect in platelet dense granule secretion in response to low to intermediate concentrations of agonists. All activation defects in PDI-deficient platelets were overcome by either high concentrations of strong agonists or by addition of recombinant PDI. Thrombus formation on collagen at high shear rates was inhibited in PDI-deficient platelets compared with wild-type platelets.27 These results provide strong support for earlier studies implicating PDI in platelet activation.
Observations that PDI contributes to the activation of isolated platelets raises the question of whether PDI is important for platelet activation in vivo. Several lines of evidence demonstrate that PDI mediates platelet accumulation during thrombus formation. Cho et al. demonstrated that infusion of anti-PDI antibody totally inhibited accumulation of platelets following laser injury of mouse cremaster arterioles.28 Inhibition of PDI was subsequently shown to block platelet accumulation in other models of thrombosis, including FeCl3-induced thrombosis.29 Thrombus formation has also been evaluated in mice with megakaryocyte/platelet-specific deficiency of PDI. These mice demonstrated normal initial adhesion of platelets following laser injury, but impaired platelet accumulation.27 Thus, consistent with its role in the activation of isolated platelets, PDI also functions in the activation of platelets in vivo, affecting primarily platelet aggregation and not primary adhesion.
PDI in coagulation
Following the demonstration that PDI modulates platelet functions such as αIIbβ3-dependent platelet aggregation, attention turned to the potential regulatory role of PDI in the generation of fibrin. The generation of fibrin by tissue factor in vivo is mediated through the binding of factor VIIa and X to form the tenase complex resulting in a thrombin burst and ultimately fibrin deposition. However, tissue factor in the quiescent vasculature is postulated to be present in an inactive or “cryptic” conformation.30, 31 The description of a Cys186-Cys209 disulfide bond in tissue factor that is susceptible to thiol-dependent changes in procoagulant activity sparked interest in the possibility that PDI serves as the “master switch” regulating thiol-dependent generation of fibrin and platelet accumulation(Fig. 2).32, 33 However, this hypothesis has not been proven and remains highly controversial eight years after its proposal.
A series of experiments conducted using the laser-induced vascular injury model demonstrated that inhibition of PDI by a blocking monoclonal antibody reduces fibrin generation in vivo.28, 34 The generation of fibrin was independent of platelet accumulation as fibrin deposition was normal even in the absence of platelet accumulation in mice that lacked the G protein-coupled platelet receptor Par4. Accordingly, the initial delivery of PDI into a developing thrombus is primarily through its release from endothelial cells rather than accumulating platelets.16, 27 While animal models clearly demonstrate that PDI inhibition diminishes fibrin generation, dissecting these observations performed under a tightly regulated redox environment in vivo is challenging. As such, the specific targets and mechanism by which PDI regulates fibrin generation are actively debated. Inhibition of PDI decreases tissue factor activity in vitro and mutagenesis of either Cys186 and Cys209 impairs tissue factor-VIIa mediated factor X activation.32–34 Conversely, other groups have failed to confirm that tissue factor activity expressed in certain cell lines is regulated by thiol exchange.35, 36 It remains unclear whether the interaction between PDI and tissue factor are mediated directly through disulfide bond exchange or via downstream effectors of other PDI-regulated targets.37 For instance, Furlan-Freguia and colleagues demonstrated that inhibition of PDI prevented the release of procoagulant tissue factor-bearing microparticles via activation of the purigenic P2X7 receptor on macrophages and smooth muscle cells.38 There is also evidence that inhibition of PDI alters the binding of coagulation factors on a negatively charged phospholipid surface and reduces thrombin generation in a tissue factor-independent manner39. The clinical development of PDI inhibitors as antithrombotics will be facilitated by an improved understanding of the mechanisms and targets by which PDI regulates coagulation.
PDI inhibitors as antithrombotics
The discovery that PDI serves a critical role in thrombus formation in vivo raises the question of whether inhibitors of PDI could serve as a new class of antithrombotics. While there are an increasing number of antithrombotic agents with demonstrated clinical efficacy, thrombosis remains the leading cause of mortality in developed countries. While most antithrombotics target either platelet or coagulation activation, PDI inhibitors have the potential to prevent thrombosis in conditions with pathologic activation of both pathways as implicated in complex thrombotic disorders such as myocardial infarction and cancer associated thrombosis.
It is not intuitive that PDI would be a tractable target for antithrombotic therapy. It is ubiquitously expressed and serves an important function in protein folding, as evidenced by the fact that knockdown of the protein is lethal in yeast and mammalian cell lines.40 However, extracellular PDI, not the ER pool that mediates protein folding, functions in thrombus formation. Selective inhibition of extracellular PDI is therefore a viable strategy. Furthermore, extracellular PDI may be more amenable to small molecule inhibition than PDI that is highly concentrated in the ER. Still the strategy of targeting extracellular PDI to block thrombus formation did not appear very appealing until the discovery that commonly ingested quercetin flavonoids found in fruits, vegetables, teas, and grains inhibit PDI.
The discovery that quercetin flavonoids block PDI function occurred during a high throughput screen to identify novel inhibitors of PDI. For this screen, an insulin-based turbidimetric assay was used to identify compounds that inhibited PDI reductase activity.29 This assay is based on the observation that insulin aggregates following cleavage of disulfide bonds in the insulin β-chain. Cleavage of these bonds by PDI results in increased turbidity of the reaction mixture, which can be detected at 650 nm. Quercetin-3-rutinoside was identified as a PDI inhibitor upon screening an annotated small molecule library consisting of 5000 compounds with known biological function.29 Quercetin-3-rutinoside demonstrated the most potent inhibitory activity of all compounds identified. Structure activity relationship assays in which several analogs of quercetin-3-rutinoside were tested showed that only those analogs that possessed carbohydrate at position 3' of the C ring inhibited PDI activity. All compounds with this moiety had similar activity against PDI. When tested in mice, quercetin-3-rutinoside inhibited thrombus formation when infused at concentrations as low as 0.1 mg/kg and with full inhibition of both platelet accumulation and fibrin formation at 0.5 mg/kg. Quercetin-3-rutinoside was also inhibitory when given orally, although approximately 100 times as much compound was required to achieve inhibition.29 Nonetheless, quercetin-3-rutinoside was antithrombotic at concentrations that are safely ingested in humans. These findings provided preliminary proof-of-principle that inhibition of PDI to block thrombus formation could be accomplished safely.
Although quercetin-3-rutinoside is a viable drug for inhibiting PDI in the clinical setting, it is by no means the only PDI inhibitor to which human subjects have been exposed. Bacitracin is a topical antibiotic that has been used to inhibit PDI activity in cell-based studies for more than two decades.41 Despite its widespread use as a PDI antagonist, it is a poor inhibitor of PDI, with an estimated IC50 of ~100 µM. It is also non-selective, blocking the activity of several other thiol isomerases as well as interacting with a large number of proteins. Further, its ingestion is associated with nephrotoxicity. Thus, bacitracin is not useful as either a PDI antagonist in cell-based studies or as a PDI-targeted therapeutic. Aminoglycoside antibiotics (e.g. vancomycin, sisomycin, neomycin, and gentamycin) bind and inhibit PDI, but do so only at concentrations that cannot be achieved safely in patients (>200 µM) and are therefore not adequately potent14. Estrogen binds to PDI with micromolar affinity. However, its systemic estrogenic effects occur in the nanomolar range.42 Similarly, thyroid hormone binds PDI,42 but its side effect profile would be unacceptable. Thus, of the biologically active compounds that block PDI currently used in human, only quercetin flavonoids are tractable drug candidates.
Several synthetic PDI antagonists have been developed in recent years (Table 1; for a comprehensive review of PDI inhibitor please refer to ref. 43). All the new PDI inhibitors have better potency and selectivity than bacitracin and should replace its use as reagents to probe PDI function in biological systems. Whether any of the synthetic PDI inhibitors are potential antithrombotics is worth considering.
Table 1.
Protein disulfide isomerase inhibitors with therapeutic potential
| Reversibility | IC50(µM) | cell-based & preclinical studies |
comments | ref | |
|---|---|---|---|---|---|
| ML359 | Reversible | 0.3–0.6 | Inhibits platelet aggregation. Not cytotoxic. | Does not inhibit other thiol isomerases tested. | 44 |
| Quercetin-3-rutinoside | Reversible | 6 | Inhibits platelet aggregation and fibrin formation on endothelial cells; blocks thrombus formation in vivo. Not cytotoxic. | Flavonoid quercetins glycosylated at position 3 of the C ring inhibit PDI. | 29 |
| Juniferdin | Reversible | 0.16–3 | Inhibits reduction of HIV-1 gp120. Cytotoxic in several cell lines. | Epoxide analog less cyotoxic. | 62 |
| RB-11-ca | Irreversible | 30–50 | Inhibits proliferation of HeLa cells. | Shows selectivity for Cys53 of the a domain. | 63 |
| PACMA-31 | Irreversible | 10 | Cytotoxic in cancer cell lines. Suppresses tumor growth in mouse xenograft model of ovarian cancer | Orally available. Tolerated in 62 day exposure in mice. | 64 |
| P1 (phenyl vinyl sulfonate) | Irreversible | 1.7 | Cytotoxic in multiple cancer cell lines at 4 mM. | 65 | |
| Adenanthin | Irreversible | 7.3 | Cytotoxic in lymphoma leukemic cell lines. Tolerated in vivo at >20 mg/kg. | Inhibits peroxiredoxins and thioredoxins. | 66 |
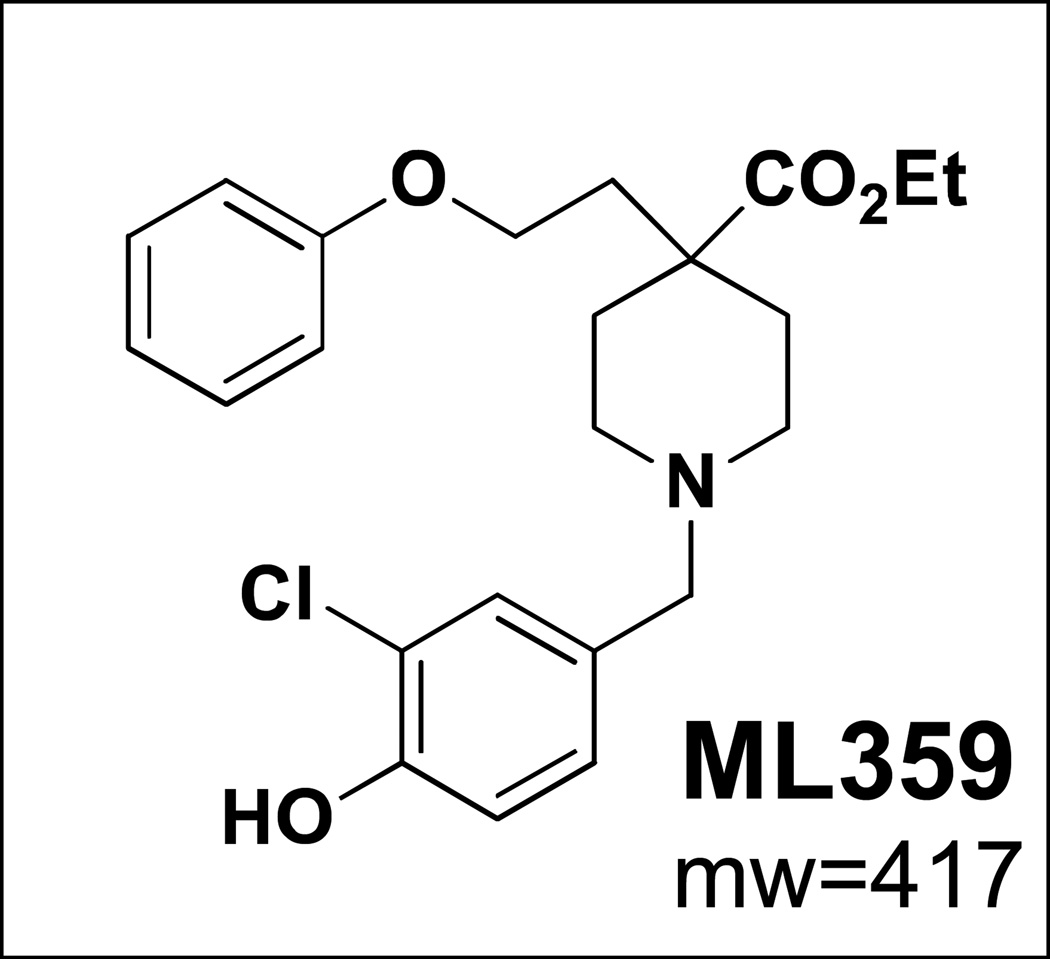
Based on our experience with quercetin-3-rutinoside as an antithrombotic PDI inhibitor, we performed a much larger high throughput screen to identify more potent and selective PDI antagonists. An insulin-based turbidimetric assay was used to screen 348,505 compounds from the Molecular Libraries Small Molecule Repository. This screen identified several novel inhibitors of PDI, some of which blocked PDI with submicromolar potency. ML359 was the most potent and selective inhibitor identified (Fig. 3). It inhibited PDI in the insulin reductase assay with an IC50 of ~250 nM.44 ML359 also demonstrated superior selectivity. It was tested in hundreds of other assays referenced in the PubChem database and was not active at <10µM in any other assay. When tested against other thiol isomerases, ML359 did not inhibit ERp5, thioredoxin, or thioredoxin reductase. ML359 was not cytotoxic when incubated with HEK293, HepG2, or HeLa cells for 48 hours. It demonstrated reversible inhibition of platelet aggregation, indicating that it does not become covalently linked to PDI. Further studies are required to confirm that ML359 is antithrombotic in vivo.
Figure 3. Structure of ML359.
Identifying PDI inhibitors for clinical use
Several properties merit consideration in determining which PDI inhibitors should advance to clinical trials. One property that could be important for an antithrombotic PDI antagonist is reversibility. Reversible inhibition is important for an antithrombotic since reversal of inhibition in the setting of bleeding is a significant and practical advantage. PAMCA-31, 16F16, RB-11-ca, and a novel phenyl vinyl sulfonate-containing (Table 1) all target vicinal cysteines within PDI and demonstrate irreversible inhibition. Although such inhibitors may be useful as antineoplastic agents, their mechanism of action is a liability with regard to antithrombotic therapy.
A second consideration in developing an antithrombotic PDI antagonist is cell permeability. While enhanced cell-permeability may be an asset for developing an anti-neoplastic PDI inhibitor, cell-permeability is not necessary for inhibiting PDI during thrombus formation and could be a liability for an antithrombotic PDI inhibitor. Antibodies that target PDI are potently antithrombotic in vivo, indicating that inhibition of extracellular PDI is sufficient for thrombus formation3 and infusion of recombinant PDI into mice lacking platelet PDI reverses the defect in thrombus formation, indicating that extracellular PDI is necessary for thrombus formation16. In this regard, it is worth noting that the same glycosidic linkage in quercetin flavonoids that is necessary for their anti-PDI activity also impairs their cell permeability.
The mechanism of inhibitor activity is another parameter to consider in deciding which compounds should advance to clinical trials. Allosteric inhibitors that act outside the catalytic domain of PDI could act either by preventing enzyme-substrate interactions or by inducing conformational changes in the enzyme that interfere with its oxidoreductase activity. For example, quercetin-3-rutinoside not only blocks the ability of PDI to reduce disulfide bonds in insulin, which interacts with PDI substrate binding domains, but also inhibits the ability of PDI to reduce the small molecule di-eosin-GSSG, which does not interact with binding domains.29 This observation suggests that quercetin-3-rutinoside has allosteric effects that interfere with the function of the catalytic domain. Allosteric control of PDI using drug-like molecules is in its nascency. However, allosteric modulators of PDI are likely to represent the best strategy for inhibition of PDI in the setting of thrombus formation.
Clinical trials of PDI inhibition
The identification of quercetin-3-rutinoside and related flavonoids as small molecule inhibitors of PDI was fortuitous as this discovery accelerated the clinical evaluation of PDI inhibitors as a target for antithrombotics in humans. The flavonol quercetin and its derivatives are ubiquitously present in fruits and vegetables including in onions, tea, berries, and apples. In Western diets the daily consumption of quercetin is approximately 10-20 mg.45, 46 The structure of the related quercetin compounds differ based on type and location of sugar moieties with quercetin as an aglycone present in only small quantities in food. In contrast, quercetin-3-rutinoside (rutin) with a glycoside group at the 3 position of the pyrone ring, is found in higher concentrations in food sources but is poorly absorbed.47 Although the absorption of the related quercetin compounds differ, all quercetin glycosides, such rutin or quercetin-3-O-β-D-glucoside (isoquercetin), are metabolized in the liver following oral ingestion. Metabolism of orally ingested flavonoid quercetins includes deglycosylation locally at the enterocyte and subsequent additions of glucuronate, methyl or sulfonyl groups, with glucuronated conjugates being a dominant form..48, 49,50, 51 The glucuronidated conjugate of quercetin has been shown to have similar PDI inhibitory activity to quercetin-3-rutinoside.29 Thus, regardless of which quercetin flavonoids are ingested, glucuronated flavonoids can be found in the circulation. While safety of newer PDI inhibitors is not yet known, the flavonoid quercetin has been extensively studied in a number of clinical settings without significant toxicity. For instance, in a randomized clinical trial exploring the anti-viral activity of quercetin, there was no reported toxicity in over 600 individuals consuming 500 mg or 1000 mg daily.52
The efficacy of antithrombotic targeting of PDI using flavonoid quercetins has yet to be established in clinical trails. However, evidence from population-based cohorts suggests that quercetin and related flavonoids significantly reduce cardiovascular mortality. In a longitudinal investigation of elderly men in the Netherlands, the relative risk of death from coronary artery disease was approximately 70% lower in those who consumed diets with high amounts of flavonoid (highest tercile) compared with lower amounts (lowest tercile) even when adjusted for age, diet, and other cardiovascular risk factors (adjusted RR 0.32, 95% CI 0.15-0.71, P=0.003).46 Similarly, in a large prospective cohort of approximately 100,000 adults in the United States, the highest quintile versus lowest quintile flavonoid consumption was associated with a significant 18% reduction in cardiovascular deaths adjusted for age, weight, smoking, activity, hormone use and alcohol (adjusted RR 0.82, 95% CI 0.73-0.92, P=0.01).53 The trend for reduced cardiovascular death has been consistent across a number of large epidemiologic studies (Table 2)54 and flavonoid consumption has also been linked with a decreased incidence of nonfatal and fatal stroke.55, 56 The ascribed cardiovascular bioactivity of quercetin is unlikely to be mediated entirely through PDI inhibition as flavonoids are also known antioxidants and can inhibit collagen-stimulated platelet activation through PDI-independent pathways.57, 58
Table 2.
Risk of cardiovascular mortality in population cohort studies based on dietary flavonoid consumption.
| Study | Year | Country | Sex | Total N (high vs low flavonoid) |
Flavonoid intake mg/ day (high vs low) |
Adjusted RR (high vs low, 95% CI) |
|---|---|---|---|---|---|---|
| Hertog et al46 | 1993 | Holland | M | 535 | ≥29.9 vs≤19 | 0.32 (0.15 – 0.71) |
| Knekt et al67 | 2002 | Finland | M+F | 4,565 | ≥ 26.9*vs 4.3 | 0.92 (0.80 – 1.04) |
| McCullough et al53 | 2012 | U.S. | M+F | 39,387 | ≥ 360 vs≤121 | 0.82 (0.73 – 0.92) |
| Mink et al45 | 2007 | U.S. | F | 13,796 | > 425 vs<133 | 0.93 (0.81 – 1.07) |
| Hirvonen et68 al | 2001 | Finland | M | 58,181 (person years) | 17.8 vs 3.94 | 0.89 (0.71 – 1.11) |
Mean flavonoid for highest quartile for women was 39.5 mg
Based on the in vitro, animal and epidemiologic data supporting antithrombotic efficacy of PDI as therapeutic target, we initiated several proof-of-concept clinical trials in humans with isoquercetin. Quercetin and isoquercetin are attractive small molecule PDI inhibitors due to their established safety profile and improved absorption compared to quercetin-3-rutinoside. These studies serve to lay the groundwork for future clinical studies with more specific PDI inhibitors that are currently under development. An initial clinical study was conducted in healthy individuals to evaluate pharmacokinetic profiles as well as pharmacodynamic inhibition of PDI with isoquercetin. Preliminary data demonstrated significant and sustained inhibition of PDI is achieved following oral ingestion of isoquercetin in healthy individuals.59
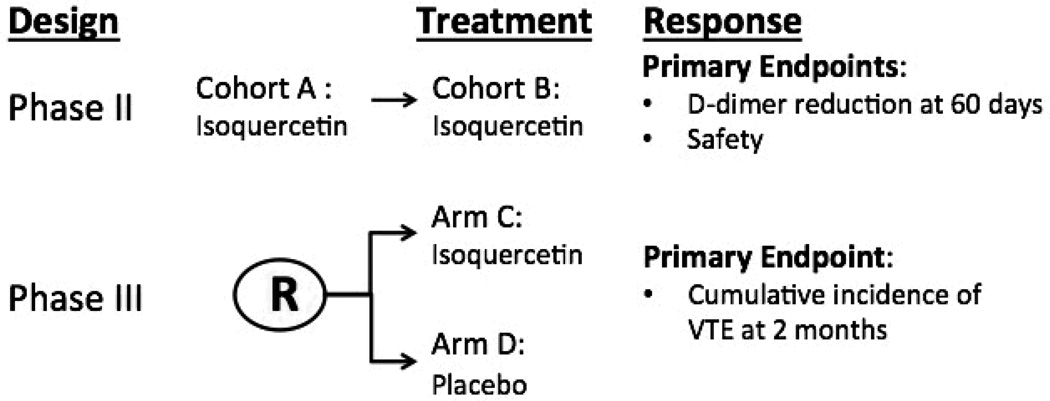
The mechanisms underlying pathologic thrombosis of certain disorders such as malignancy are complex, including increased platelet activation and circulating tissue factor.60, 61 Based on the potential for a PDI inhibitor to mediate antithrombotic activity through inhibition of both platelet activation and fibrin generation, we are currently conducting a phase II/III randomized clinical trial to evaluate whether daily isoquercetin prevents thrombosis in high risk cancer patients (Figure 4). Despite the high incidence of vascular events in cancer patients, the benefit of routine thromboprophylaxis has not been established. The phase II trial will confirm the safety of isoquercetin in cancer patients and evaluate whether there is a significant reduction in thrombotic biomarkers (e.g. D-dimer) at two months. The phase III study will compare the effectiveness of isoquercetin versus placebo in reducing the cumulative incidence of venous thromboembolic events at two months.
Figure 4. Randomized, phase II/III clinical trial to evaluate the efficacy of oral isoquercetin to prevent thrombosis in advanced cancer patients.
Phase II studies will be completed as two sequential dosing cohorts A and B. Based on evidence of safety in cohort A, patients will then enroll into cohort B using a higher dose of daily isoquercetin. D-dimer will be measured before and following two months of daily isoquercetin administration. Based on safety, efficacy in reducing D-dimer levels as well as preliminary data on venous thromboembolic events, the dose will be selected to proceed to phase III. The phase III trial will involve randomization between placebo and isoquercetin with a primary endpoint of venous thromboemoblic events.
Perspective
The molecular targets of PDI in thrombosis continue to be explored and will be further facilitated by specific PDI small molecule inhibitors under development. The holy grail of antithrombotic therapeutics remains a drug that prevents pathologic thrombosis without increasing the risk of hemorrhage. While it is too soon to know whether oral PDI inhibitors representing a novel class of antithrombotics fit that description, the clinical studies underway will hopefully lead to new therapeutic options for individuals where current standard approaches to thrombosis are either inadequate or ineffective.
Significance.
Vascular thrombosis, including coronary artery disease, stroke, and venous thromboembolic disease, remains the most common cause of morbidity and mortality in the United States. Limitations of current therapies for thrombotic disorders are evidenced by the high incidence of recurrent thrombosis. There is a need for novel therapies targeting alternative components of the blood clotting mechanism based on new knowledge of thrombus formation. PDI is now known to serve an essential role in thrombus formation following vascular injury and inhibition of PDI represents a new approach for the development of antithrombotics. The feasibility of this strategy is underscored by the observation that the widely consumed quercetin flavonoid, rutin, inhibits thrombus formation in preclinical studies at concentrations that are safely ingested by individuals. Inhibition of PDI either by quercetin flavonoids or more potent and selective small molecule inhibitors of PDI is presently being developed as a new modality for antithrombotic therapy.
Acknowledgements
We thank past and present members of the Flaumenhaft, Furie, and Zwicker laboratories for their contributions to the research described in this review and the Broad Institute for work related to synthetic PDI inhibitors.
Sources of Funding
This work has been supported by grants from the National Institutes of Health (U54 HL112302, P01 HL087203, and HL112809)
Footnotes
Disclosures
R.F. is named as an inventor in a patent describing ML359.
J.I.Z. is PI for clinical trials with isoquercetin, which are funded in part by a research grant from Quercegen Pharma.
References
- 1.Butera D, Cook KM, Chiu J, Wong JW, Hogg PJ. Control of blood proteins by functional disulfide bonds. Blood. 2014;123:2000–2007. doi: 10.1182/blood-2014-01-549816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furie B, Flaumenhaft R. Thiol isomerases in thrombus formation. Circ Res. 2014;114:1162–1173. doi: 10.1161/CIRCRESAHA.114.301808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Li W, Ren J, Fang J, Ke H, Gong W, Feng W, Wang CC. Structural insights into the redox-regulated dynamic conformations of human protein disulfide isomerase. Antioxidants & redox signaling. 2013;19:36–45. doi: 10.1089/ars.2012.4630. [DOI] [PubMed] [Google Scholar]
- 4.Tian R, Li SJ, Wang DL, Zhao Z, Liu Y, He RQ. The acidic c-terminal domain stabilizes the chaperone function of protein disulfide isomerase. J Biol Chem. 2004;279:48830–48835. doi: 10.1074/jbc.M407076200. [DOI] [PubMed] [Google Scholar]
- 5.Cai H, Wang CC, Tsou CL. Chaperone-like activity of protein disulfide isomerase in the refolding of a protein with no disulfide bonds. J Biol Chem. 1994;269:24550–24552. [PubMed] [Google Scholar]
- 6.Dai Y, Wang C. A mutant truncated protein disulfide isomerase with no chaperone activity. J Biol Chem. 1997;272:27572–27576. doi: 10.1074/jbc.272.44.27572. [DOI] [PubMed] [Google Scholar]
- 7.Song JL, Wang CC. Chaperone-like activity of protein disulfide-isomerase in the refolding of rhodanese. European journal of biochemistry / FEBS. 1995;231:312–316. doi: 10.1111/j.1432-1033.1995.tb20702.x. [DOI] [PubMed] [Google Scholar]
- 8.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 9.Uys JD, Xiong Y, Townsend DM. Nitrosative stress-induced s-glutathionylation of protein disulfide isomerase. Methods Enzymol. 2011;490:321–332. doi: 10.1016/B978-0-12-385114-7.00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sliskovic I, Raturi A, Mutus B. Characterization of the s-denitrosation activity of protein disulfide isomerase. J Biol Chem. 2005;280:8733–8741. doi: 10.1074/jbc.M408080200. [DOI] [PubMed] [Google Scholar]
- 11.Zai A, Rudd MA, Scribner AW, Loscalzo J. Cell-surface protein disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide. J Clin Invest. 1999;103:393–399. doi: 10.1172/JCI4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyles MM, Gilbert HF. Catalysis of the oxidative folding of ribonuclease a by protein disulfide isomerase: Pre-steady-state kinetics and the utilization of the oxidizing equivalents of the isomerase. Biochemistry. 1991;30:619–625. doi: 10.1021/bi00217a005. [DOI] [PubMed] [Google Scholar]
- 13.Chen K, Detwiler TC, Essex DW. Characterization of protein disulphide isomerase released from activated platelets. Br J Haematol. 1995;90:425–431. doi: 10.1111/j.1365-2141.1995.tb05169.x. [DOI] [PubMed] [Google Scholar]
- 14.Wan SW, Lin CF, Lu YT, Lei HY, Anderson R, Lin YS. Endothelial cell surface expression of protein disulfide isomerase activates beta1 and beta3 integrins and facilitates dengue virus infection. Journal of cellular biochemistry. 2012;113:1681–1691. doi: 10.1002/jcb.24037. [DOI] [PubMed] [Google Scholar]
- 15.Thon JN, Peters CG, Machlus KR, Aslam R, Rowley J, Macleod H, Devine MT, Fuchs TA, Weyrich AS, Semple JW, Flaumenhaft R, Italiano JE., Jr T granules in human platelets function in tlr9 organization and signaling. The Journal of cell biology. 2012;198:561–574. doi: 10.1083/jcb.201111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jasuja R, Furie B, Furie BC. Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo. Blood. 2010;116:4665–4674. doi: 10.1182/blood-2010-04-278184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho J, Kennedy DR, Lin L, Huang M, Merrill-Skoloff G, Furie BC, Furie B. Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of beta3 integrins. Blood. 2012;120:647–655. doi: 10.1182/blood-2011-08-372532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotchkiss KA, Chesterman CN, Hogg PJ. Catalysis of disulfide isomerization in thrombospondin 1 by protein disulfide isomerase. Biochemistry. 1996;35:9761–9767. doi: 10.1021/bi9603938. [DOI] [PubMed] [Google Scholar]
- 19.Huang EM, Detwiler TC, Milev Y, Essex DW. Thiol-disulfide isomerization in thrombospondin: Effects of conformation and protein disulfide isomerase. Blood. 1997;89:3205–3212. [PubMed] [Google Scholar]
- 20.Burgess JK, Hotchkiss KA, Suter C, Dudman NP, Szollosi J, Chesterman CN, Chong BH, Hogg PJ. Physical proximity and functional association of glycoprotein 1balpha and protein-disulfide isomerase on the platelet plasma membrane. J Biol Chem. 2000;275:9758–9766. doi: 10.1074/jbc.275.13.9758. [DOI] [PubMed] [Google Scholar]
- 21.Lahav J, Wijnen EM, Hess O, Hamaia SW, Griffiths D, Makris M, Knight CG, Essex DW, Farndale RW. Enzymatically catalyzed disulfide exchange is required for platelet adhesion to collagen via integrin alpha2beta1. Blood. 2003;102:2085–2092. doi: 10.1182/blood-2002-06-1646. [DOI] [PubMed] [Google Scholar]
- 22.Essex DW, Li M, Miller A, Feinman RD. Protein disulfide isomerase and sulfhydryl-dependent pathways in platelet activation. Biochemistry. 2001;40:6070–6075. doi: 10.1021/bi002454e. [DOI] [PubMed] [Google Scholar]
- 23.Essex DW, Miller A, Swiatkowska M, Feinman RD. Protein disulfide isomerase catalyzes the formation of disulfide-linked complexes of vitronectin with thrombin-antithrombin. Biochemistry. 1999;38:10398–10405. doi: 10.1021/bi990694s. [DOI] [PubMed] [Google Scholar]
- 24.Milev Y, Essex DW. Protein disulfide isomerase catalyzes the formation of disulfide-linked complexes of thrombospondin-1 with thrombin-antithrombin iii. Arch Biochem Biophys. 1999;361:120–126. doi: 10.1006/abbi.1998.0963. [DOI] [PubMed] [Google Scholar]
- 25.Essex DW, Li M. Protein disulphide isomerase mediates platelet aggregation and secretion. Br J Haematol. 1999;104:448–454. doi: 10.1046/j.1365-2141.1999.01197.x. [DOI] [PubMed] [Google Scholar]
- 26.Manickam N, Sun X, Li M, Gazitt Y, Essex DW. Protein disulphide isomerase in platelet function. Br J Haematol. 2008;140:223–229. doi: 10.1111/j.1365-2141.2007.06898.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim K, Hahm E, Li J, Holbrook LM, Sasikumar P, Stanley RG, Ushio-Fukai M, Gibbins JM, Cho J. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood. 2013;122:1052–1061. doi: 10.1182/blood-2013-03-492504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jasuja R, Passam FH, Kennedy DR, Kim SH, van Hessem L, Lin L, Bowley SR, Joshi SS, Dilks JR, Furie B, Furie BC, Flaumenhaft R. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. The Journal of clinical investigation. 2012;122:2104–2113. doi: 10.1172/JCI61228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh JD, Geczy CL. Discordant expression of tissue factor antigen and procoagulant activity on human monocytes activated with lps and low dose cycloheximide. Thromb Haemost. 1991;66:552–558. [PubMed] [Google Scholar]
- 31.Le DT, Rapaport SI, Rao LV. Relations between factor viia binding and expression of factor viia/tissue factor catalytic activity on cell surfaces. J Biol Chem. 1992;267:15447–15454. [PubMed] [Google Scholar]
- 32.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45:12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 33.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pendurthi UR, Ghosh S, Mandal SK, Rao LV. Tissue factor activation: Is disulfide bond switching a regulatory mechanism? Blood. 2007;110:3900–3908. doi: 10.1182/blood-2007-07-101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kothari H, Nayak RC, Rao LV, Pendurthi UR. Cystine 186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption. Blood. 2010;115:4273–4283. doi: 10.1182/blood-2009-09-241356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;282:25416–25424. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 38.Furlan-Freguia C, Marchese P, Gruber A, Ruggeri ZM, Ruf W. P2x7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. J Clin Invest. 2011;121:2932–2944. doi: 10.1172/JCI46129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jurk K, Lahav J, VANA H, Brodde MF, Nofer JR, Kehrel BE. Extracellular protein disulfide isomerase regulates feedback activation of platelet thrombin generation via modulation of coagulation factor binding. J Thromb Haemost. 2011;9:2278–2290. doi: 10.1111/j.1538-7836.2011.04509.x. [DOI] [PubMed] [Google Scholar]
- 40.Appenzeller-Herzog C, Ellgaard L. The human pdi family: Versatility packed into a single fold. Biochim Biophys Acta. 2008;1783:535–548. doi: 10.1016/j.bbamcr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Mandel R, Ryser HJ, Ghani F, Wu M, Peak D. Inhibition of a reductive function of the plasma membrane by bacitracin and antibodies against protein disulfide-isomerase. Proc Natl Acad Sci U S A. 1993;90:4112–4116. doi: 10.1073/pnas.90.9.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Primm TP, Gilbert HF. Hormone binding by protein disulfide isomerase, a high capacity hormone reservoir of the endoplasmic reticulum. J Biol Chem. 2001;276:281–286. doi: 10.1074/jbc.M007670200. [DOI] [PubMed] [Google Scholar]
- 43.Xu S, Sankar S, Neamati N. Protein disulfide isomerase: A promising target for cancer therapy. Drug discovery today. 2014;19:222–240. doi: 10.1016/j.drudis.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 44.VerPlank L, Khodier C, Nag PP, et al. Probe reports from the nih molecular libraries program. Bethesda, MD: National Center for Biotechnology Information (US); 2010. Identification of ML359 as a small molecule inhibitor of protein disulfide isomerase. [PubMed] [Google Scholar]
- 45.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 46.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: The zutphen elderly study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 47.Hollman PC, van Trijp JM, Buysman MN, van der Gaag MS, Mengelers MJ, de Vries JH, Katan MB. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418:152–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- 48.Gee JM, DuPont MS, Day AJ, Plumb GW, Williamson G, Johnson IT. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J Nutr. 2000;130:2765–2771. doi: 10.1093/jn/130.11.2765. [DOI] [PubMed] [Google Scholar]
- 49.Walle T, Otake Y, Walle UK, Wilson FA. Quercetin glucosides are completely hydrolyzed in ileostomy patients before absorption. J Nutr. 2000;130:2658–2661. doi: 10.1093/jn/130.11.2658. [DOI] [PubMed] [Google Scholar]
- 50.Mullen W, Edwards CA, Crozier A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br J Nutr. 2006;96:107–116. doi: 10.1079/bjn20061809. [DOI] [PubMed] [Google Scholar]
- 51.Sesink AL, O'Leary KA, Hollman PC. Quercetin glucuronides but not glucosides are present in human plasma after consumption of quercetin-3-glucoside or quercetin-4'-glucoside. J Nutr. 2001;131:1938–1941. doi: 10.1093/jn/131.7.1938. [DOI] [PubMed] [Google Scholar]
- 52.Heinz SA, Henson DA, Austin MD, Jin F, Nieman DC. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol Res. 2010;62:237–242. doi: 10.1016/j.phrs.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of us adults. Am J Clin Nutr. 2012;95:454–464. doi: 10.3945/ajcn.111.016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2003;57:904–908. doi: 10.1038/sj.ejcn.1601624. [DOI] [PubMed] [Google Scholar]
- 55.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: The zutphen study. Arch Intern Med. 1996;156:637–642. [PubMed] [Google Scholar]
- 56.Hollman PC, Geelen A, Kromhout D. Dietary flavonol intake may lower stroke risk in men and women. J Nutr. 2010;140:600–604. doi: 10.3945/jn.109.116632. [DOI] [PubMed] [Google Scholar]
- 57.Wright B, Moraes LA, Kemp CF, Mullen W, Crozier A, Lovegrove JA, Gibbins JM. A structural basis for the inhibition of collagen-stimulated platelet function by quercetin and structurally related flavonoids. British journal of pharmacology. 2010;159:1312–1325. doi: 10.1111/j.1476-5381.2009.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mulvihill EE, Huff MW. Antiatherogenic properties of flavonoids: Implications for cardiovascular health. Can J Cardiol. 2010;26(Suppl A):17A–21A. doi: 10.1016/s0828-282x(10)71056-4. [DOI] [PubMed] [Google Scholar]
- 59.Stopa J, Furie B, Flaumenhaft R, Zwicker J. Human clinical trials evaluating protein disulfide isomerase as an antithrombotic target: Pharmacodynamic and pharmacokinetic studies of oral quercetin and isoquercetin. XXIV Congress of the International Society of Thrombosis and Haemostasis. 2013 [Google Scholar]
- 60.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830–6840. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ay C, Simanek R, Vormittag R, Dunkler D, Alguel G, Koder S, Kornek G, Marosi C, Wagner O, Zielinski C, Pabinger I. High plasma levels of soluble p-selectin are predictive of venous thromboembolism in cancer patients: Results from the vienna cancer and thrombosis study (cats) Blood. 2008;112:2703–2708. doi: 10.1182/blood-2008-02-142422. [DOI] [PubMed] [Google Scholar]
- 62.Khan MM, Simizu S, Lai NS, Kawatani M, Shimizu T, Osada H. Discovery of a small molecule pdi inhibitor that inhibits reduction of hiv-1 envelope glycoprotein gp120. ACS chemical biology. 2011;6:245–251. doi: 10.1021/cb100387r. [DOI] [PubMed] [Google Scholar]
- 63.Banerjee R, Pace NJ, Brown DR, Weerapana E. 1,3,5-triazine as a modular scaffold for covalent inhibitors with streamlined target identification. Journal of the American Chemical Society. 2013;135:2497–2500. doi: 10.1021/ja400427e. [DOI] [PubMed] [Google Scholar]
- 64.Xu S, Butkevich AN, Yamada R, Zhou Y, Debnath B, Duncan R, Zandi E, Petasis NA, Neamati N. Discovery of an orally active small-molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proc Natl Acad Sci U S A. 2012;109:16348–16353. doi: 10.1073/pnas.1205226109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ge J, Zhang CJ, Li L, Chong LM, Wu X, Hao P, Sze SK, Yao SQ. Small molecule probe suitable for in situ profiling and inhibition of protein disulfide isomerase. ACS chemical biology. 2013;8:2577–2585. doi: 10.1021/cb4002602. [DOI] [PubMed] [Google Scholar]
- 66.Muchowicz A, Firczuk M, Chlebowska J, Nowis D, Stachura J, Barankiewicz J, Trzeciecka A, Klossowski S, Ostaszewski R, Zagozdzon R, Pu JX, Sun HD, Golab J. Adenanthin targets proteins involved in the regulation of disulphide bonds. Biochem Pharmacol. 2014;89:210–216. doi: 10.1016/j.bcp.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 67.Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 68.Hirvonen T, Pietinen P, Virtanen M, Ovaskainen ML, Hakkinen S, Albanes D, Virtamo J. Intake of flavonols and flavones and risk of coronary heart disease in male smokers. Epidemiology (Cambridge, Mass.) 2001;12:62–67. doi: 10.1097/00001648-200101000-00011. [DOI] [PubMed] [Google Scholar]