Abstract
The field of hematopoietic stem cell biology has become increasingly dominated by the pursuit and study of highly purified populations of hematopoietic stem cells (HSCs). Such HSCs are typically isolated based on their cell surface marker expression patterns and ultimately defined by their multipotency and capacity for self-generation. However, even with progressively more stringent stem cell separation techniques, the resultant HSC population remains heterogeneous with respect to both self-renewal and differentiation capacity. Critical studies on un-separated whole bone marrow (WBM) have definitively shown that long-term engraftable hematopoietic stem cells are in active cell cycle and thus continually changing phenotype. Therefore, they cannot be purified by current approaches dependent on stable surface epitope expression because the surface markers are continually changing as well. These critical cycling cells are discarded with current stem cell purifications. Despite this, research defining such characteristics as self-renewal capacity, lineage-commitment, bone marrow niches, and proliferative state of HSCs continues to focus predominantly on this small sub-population of purified marrow cells. This review discusses the research leading to the hierarchical model of hematopoiesis and questions the dogmas pertaining to HSC quiescence and purification.
Keywords: Hematopoietic stem cells, Tissue stem cells, Microenvironment, Hematopoietic progenitor cells, Colony-forming units, Extracellular Vesicles
Introduction
This is a consideration of four H words. Hierarchy has been the standard description of marrow stem/progenitor systems. We have used this H word in this light in our benighted past. There are 4 definitions of hierarchy in the Oxford English Dictionary. The first three relate to “divisions of angels and ecclesiastical organization.” The fourth refers to “... a body of persons or things ranked in grades, orders or classes, one above another.” We assume that when hierarchy is used to describe stem cell systems the fourth meaning is implied, although given the religious adherence of some to the current stem cell dogmas, perhaps the first three might be appropriate. Another important H word is homogeneity. This seems to be the goal of most hardcore stem cell purificationists. Homogeneous is defined as “consisting of parts or elements all of the same kind,” a characteristic probably unobtainable in biologic systems. Our concepts could be and have been described as heterodox, i.e. “holding opinions other than the right.” The first definition in the OED is “...of doctrines, opinions, etc. not in accordance with established doctrines or opinions or those generally recognized as right or ‘orthodox’.” The orthodox view of marrow stem cell biology is that the marrow/progenitor system is hierarchical in nature, that the stem cell is a noncycling cell in G0 unless it is “stimulated” and that the key to knowledge in this system is purification of the marrow stem cell to homogeneity. Our heterodoxy is that the stem cell is in a continuum of change and not hierarchical, that the marrow stem cell population is an actively cycling population and, because the stem cell is cycling, it is continually changing phenotype and therefore cannot be purified to homogeneity. This brings us to the final H word, a very important one; heterogeneity. This is, of course, the opposite of homogeneity and is best thought of as “difference or diversity in kind from other things.” In the seminal initial work, first defining marrow stem cells and studying colony-forming unit spleen (CFU-s)(1), a disregarded, but still excellent stem cell assay, Till, McCulloch and Siminovitch (2) found that CFU-s were totally heterogeneous as to renewal and the system appeared stochastic in nature. They compared CFU-s to radioactive isotopes; these have a precisely defined half-life, but the decay rates of individual nuclei are completely heterogeneous. Thus a population could be defined, but looking at individual nuclei in the population would be uninformative. This is not a bad description of the current state of stem cell biology. Most investigators are pursuing the nuclei without regard to the population. We will return to this later.
The evolution of hierarchical models, in which we were involved, grew naturally out of experimental work in mice. CFU-s was defined in irradiated mice (1). Marrow cells injected into lethally irradiated mice gave rise to clonal bumps on the spleen 8-12 days after irradiation and these lumps contained combinations of primitive erythroid, myeloid, megakaryocytic and probably lymphoid cells. When dissected out, they would give rise to secondary colonies in irradiated mice in a very heterogeneous fashion. Following this work, in vitro progenitors were characterized, first for granulocytes and macrophages (3, 4), but eventually for virtually all marrow cell classes and in all combinations (5-11). This suggested a very orderly model of marrow stem/progenitor regulation in which CFU-s differentiated into progenitors with multiple lineages and these then differentiated into progenitors with progressively restricted lineages. Then came elegant stem cell purification approaches by a number of investigators and a massive body of work (12-33) that appeared to characterize a beautiful hierarchical system. Researchers purified stem cells by incubating marrow cells with lineage specific monoclonal antibodies, removing the antibody labeled cells by magnetic bead attachment or FACS, and then selecting cells with so-called stem cell antigens- c-Kit, Sca-1, Thy1.1 and more recently CD150, and negatively selecting for other antigens. The work has approached “homogeneity” of these purified cells, with a high percentage being able to repopulate an irradiated mouse. The cell itself was characterized as being predominantly quiescent (33-35). For example, Passegué et al., in a series of elegant studies, isolated long-term hematopoietic stem cells (LT-HSC; Lineage negative (Lin-)/c-Kit+/Sca-1+/Thy1.1int) and further separated these highly purified stem cells into G0, G1, and S/G2/M fractions using the supravital RNA and DNA dyes, Pyronin Y and Hoechst 33342 respectively. They then tested each cell cycle phase-specific fraction for stem cell function in competitive bone marrow transplantation models. Only G0 cells were found to give long-term multilineage engraftment. The model that evolved here is that LT-HSC, a primitive marrow stem cell with tremendous proliferative, renewal and differentiative capacity and in G0, gives rise to classes of stem progenitors which are progressively restricted in lineage choice and are more proliferative (Figure 1).
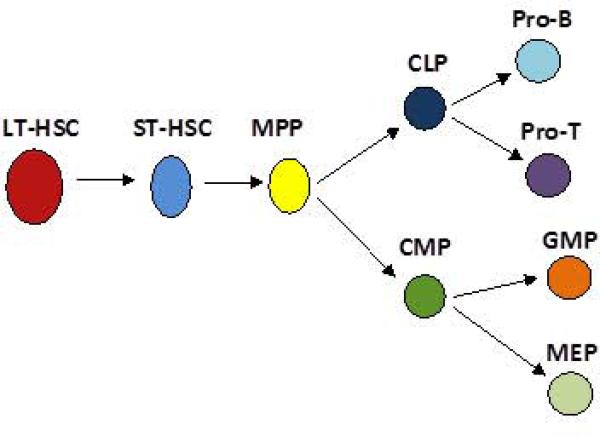
Figure 1.
Traditional stem cell hierarchy.
In this model, pluripotent stem cells with both self-renewal and differentiative properties, give rise to increasingly lineage-restricted progenitors in a hierarchical fashion.
LT-HSC = long-term hematopoietic stem cell, ST-HSC = short-term hematopoietic stem cell, MPP= multipotent progenitor cell, CLP = common lymphoid progenitor, CMP =common myeloid progenitor, Pro-B = pro B cell progenitor, Pro-T = pro T cell progenitor, GMP= granulocyte- macrophage progenitor, MEP = megakaryocyte-erythroid progenitor.
There were early warnings that purification and seeking homogeneity might not be the best way to approach understanding marrow stem cell biology and that a strict hierarchical model might not be correct. We summarized this in an editorial in Experimental Hematology in 1991, “The Blueness of Stem Cells” (36). Elegant work by Ogawa and colleagues (37) indicated that daughters of primitive marrow stem cells could pursue dissimilar differentiation fates through one cell cycle transit. This of course was not consistent with a hierarchical model of stem cell biology. Work by Nilsson and colleagues (38) studying Lin-/rhodamine low/Hoechst low (LRH) stem cells indicated that up to 99% of the whole marrow stem cell capacity was lost with the purification. These considerations were ignored in the aggressive pursuit of a purified homogeneous population of hematopoietic stem cells.
A large body of work on cytokine impact, transcriptional regulation and genetic characterization has been carried out on so-called homogeneous purified hematopoietic stem cells. The details of purification have varied between laboratories, but the general consensus at present is that one of the best candidates for the homogeneous purified stem cell is the Lin-/c-Kit+/Sca-1+ /CD150+/CD48- hematopoietic stem cell (39). All this work has ignored the population of stem cells discarded from whole marrow. The current dogma relates to a primitive long-term multilineage repopulating cell which gives rise to a series of progenitor stem cells with progressively restricted differentiation capacity. However, this model cannot fully account for all the experimental data in the field and is therefore not wholly accurate. Although all the described stem cells exist, they are likely not strictly in a hierarchy, but rather on a continuum of change linked to cell cycle phase and environmental stimuli including extracellular vesicle interactions (more below).
The Stem Cell Continuum
We were forced into our current concepts of stem cell biology by our own experiments, first showing a significant heterogeneity of results and then showing dramatic shifts in phenotype tied to cell cycle progression. These studies were initiated when we attempted to improve engraftment into non-ablated mice by exposing marrow to a cytokine cocktail of interleukin 3 (IL-3), IL-6, IL-11 and steel factor in liquid culture (40, 41). After 48 hours incubation the engraftment capacity of the marrow, rather than being increased, was virtually lost. We then followed cells over time under the same conditions and found that engraftment was markedly impaired at 48 hours but subsequently recovered (42). This held for long-term engraftment from 2- 6 months. Cycle mapping indicated that engraftment was lost in late S-phase and recovered in the next G1-phase. Altogether in 8 separate experiments we demonstrated loss and recovery of engraftment. This suggested that the gold standard of long-term engraftment defining the hematopoietic stem cells was at least tarnished if not bronze (and, yes, most gold is mixed with base metals and does tarnish). The key here was reversible loss of the multilineage engraftment phenotype with subsequent recovery during cell cycle transit. We then confirmed these phenotype reversibility studies with subsequent studies using different mouse strains, different stem cell purifications and different cytokine cocktails. We found reversible alterations in differentiation (43), marrow homing (44), expression of adhesion factors (45, 46), global gene expression (47), progenitor numbers (48), surface epitope expression (49), and most recently extracellular vesicle modulation (50). All of these reversible changes were in the context of a cytokine induced stem cell cycle transit. However, as discussed above, many studies have indicated that the true marrow renewal stem cell is in G0, raising the possibility that our in vitro cycle studies were artifactual. Accordingly, we re-evaluated the cell cycle state of purified hematopoietic stem cells (Lin-/ Sca-1+/c-Kit+ /Flk2-) using the model of Pyronin Y/Hoechst 33342 to separate LT-HSC into G0, G1 and S/G2/M phases followed by competitive transplantation into lethally irradiated mice. In accordance with the existing literature, we similarly found that most (not all) of the stem cell capacity of LT-HSC was in fact within the G0 fraction. However, on reviewing the extant literature we found that no one had ever evaluated the cell cycle status of long-term engraftable stem cells in whole unseparated marrow. We had previously shown that with stem cell purification of LRH cells, up to 99% of the stem cell capacity was lost (38). We thus evaluated the cycle status of long-term engraftable stem cells in unseparated marrow (51). The results have been quite informative. We initially separated whole bone marrow cells, staining them with Hoechst 33342 and Pyronin Y and sorting by FACS for G0, G1 and S/G2/M or, using Hoechst alone, sorting for G0/G1 and S/G2/M populations. We assessed the long-term multilineage repopulating capacity using competitive transplantation into lethally irradiated mice. At an instantaneous point in time we found that over 50% of the whole bone marrow cells capable of long-term multilineage engraftment were in S/G2/M, suggesting that most, if not all, of these cells were in active cell cycle. We confirmed this work using an alternate approach, employing tritiated thymidine suicide. In this approach, marrow cells were exposed to high-specific activity radioactive tritiated thymidine for 30 minutes and the engraftment compared to cells exposed to unlabeled thymidine. Using this suicide assay, we found an over 65% reduction in long-term engraftment with tritiated thymidine incubation, indicating the specific loss of actively cycling hematopoietic stem cells during the 30-minute incubation. These two approaches indicated that most long-term multi-lineage repopulating marrow cells were cycling. We utilized bromodeoxyuridine (BrdU) techniques to address the dynamics of cell cycle history of murine LT-HSC in vivo. Forty-eight hours after the initiation of BrdU administration, up to 73% of LTHSC were labeled with BrdU and thus had progressed through S-phase during that time interval, suggesting that these cells are actively cycling in vivo. Separate studies indicated that BrdU administration did not induce the stem cells to enter cell cycle (51).
Why the discrepancy in results between the purified stem cells and the stem cells present in unseparated whole marrow? The key here is that most stem cells are in the portions discarded during purification and our results show that these discarded cells are proliferating. The purified cells only represent a very small fraction of the total stem cell population, albeit a highly concentrated one. For example, Lin-/c-Kit+/Sca-1+/CD150+/CD48- cells contain highly potent stem cell capacity, with one out of every 2.1 cells able to engraft, giving long-term multilineage reconstitution (52). However, such a purified population only represents approximately 0.0058% ± 0.0012% of the marrow cells in a mouse (52) and its isolation forsakes the majority of stem cell potential within whole bone marrow excluded by the separative techniques. Furthermore, even with the most stringent purification protocols, it has been well appreciated that the resultant population of purified stem cells is still typified by a certain amount of heterogeneity (53-55). Such heterogeneity within these “purified” populations has prompted more concerted efforts to further purify increasingly homogenous sub-populations of highly potent stem cells using additional antibody combinations (56). We propose that such efforts will certainly yield a potent stem cell population but at the great risk of misrepresenting the majority of the stem cell potential in unseparated marrow. We propose that hematopoietic stem cells are, in their entirety, part of an inherently heterogeneous population, therefore defying purification to homogeneity, with stem cell potential and phenotype of hematopoietic cells fluctuating with activation state and developmental stage (as reviewed in 57) as well as cell cycle transit. This concept is graphically presented in Figure 2. We outline a marrow cell population in which stem cells are represented by multicolors and non-stem cells by a blue color (Figure 2). The current separative strategies select out a cell with a particular phenotype, a noncycling G0 cell. This cell will not maintain that phenotype for long as it will change as it passes through cell cycle. Other cells with different phenotypes and cycle stages have varying degrees of the stem cell phenotype-long-term multilineage engraftment in lethally irradiated mice-but some have an equal capacity to the selected stem cell. The composite non-blue cells make up the stem cell population and characterizing this population is the immediate challenge for stem cell investigators.
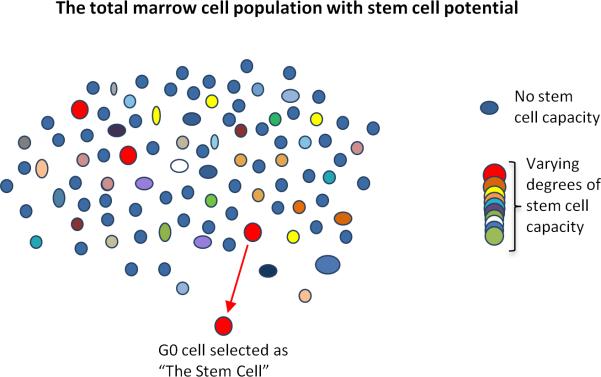
Figure 2.
Stem cell potential within un-separated marrow is lost with stem cell purification. The multi-colored circles represent the total population of marrow cells. The non-stem cells are in blue, the conventional, highly purified stem cells are represented in red, while all other colors represent marrow cells with stem cell potential. Isolation of the small subset of highly purified quiescent stem cells leads to under-representation of the total stem cell potential within marrow.
We tested this concept directly by assessing stem cell content and proliferative status (tritiated thymidine suicide) in the different fractions of a stem cell purification. As expected, highly purified (Lin-/Sca-1+/c-Kit+/CD150+/CD41-/CD48-) stem cells were unaffected by tritiated thymidine, indicating again that they engraft when in G0. In contrast, we found that the majority of long-term repopulating stem cells within both the lineage negative fraction in the absence of further stem cell purification, and the lineage positive cellular fractions were present as cycling cells (51). We estimate that anywhere from 92-99 % of stem cells are in these fractions and that these cells are virtually all proliferating. Thus it appears that the separation discarded almost all the stem cells and these were cycling. In addition, as noted above, the phenotype of the stem cells reversibly changes with cycle passage and thus a specific stem cell could not be reliably and reproducibly purified from such a cycling population of cells. The reversible variations of stem/progenitor cell potential with cell cycle passage are shown in Figure 3.
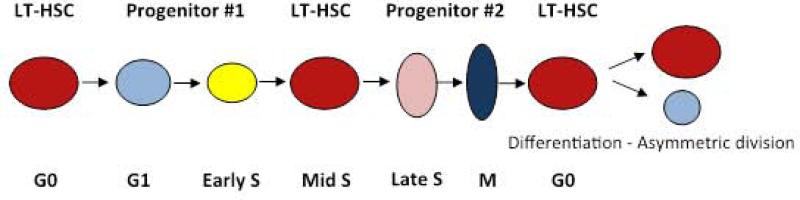
Figure 3.
A Stem Cell Continuum.
This model shows the continuous fluctuation of progenitor/ stem cell phenotype with passage through cell cycle. LT-HSC = long-term hematopoietic stem cell.
Another way of illustrating the situation is to consider the marrow progenitor/stem cells progressing through cycle and continually changing. At any one point in time, a very small percentage of these cells will be transiently in G0 – for example, the magical Lin-/Sca-1+/c-Kit+/ CD150+ cell. This cell is clearly not representative of the whole stem cell population, and studies on this cell are unlikely to give complete insights into true stem cell biology. It should be noted that “stem cells” in other phases of cycle could have equal marrow repopulating potential to the G0 LT-HSC. Here it is relevant that side population cells isolated by virtue of distinct Hoechst staining profiles, although predominantly quiescent as a population, retained equal stem cell potential in S/G2/M when compared to their quiescent counterparts (58).
As indicated in the discussion above, it is probable that the long-term repopulating hematopoietic stem cell cannot be reliably purified to homogeneity with a set of surface markers or characteristics. Rather the goal has to be to define the population, not an individual cell type. Thus heterogeneity, not homogeneity, is the rule and our heterodox theory that stem/progenitor cells exist on a continuum rather than in a strict hierarchy appears to be an appropriate theory.
Vesicle Modulation of Stem Cell Phenotype
There is another important evolving aspect of marrow stem cell fate determination; that of extracellular vesicle phenotype modulation. Extracellular vesicles encompass a range of previously described entities including microvesicles and exosomes (59-62). However, these vesicles are intrinsically heterogeneous and are best described simply as extracellular vesicles. We have shown that lung, liver, brain and heart derived vesicles can alter the genetic phenotype of target marrow cells. Vesicles physically enter target cells to effect phenotype change and initially transfer mRNA and a transcriptional regulator from the originator cell to the target cell. However, long-term genetic changes are due solely to transcriptional alteration of target marrow cells as established with studies on liver and lung vesicles (63,64). Of particular interest with regard to the impact of cell cycle on stem cell fate are studies in which vesicles from normal or irradiated murine lung were incubated with Lin-/Sca-1+ marrow progenitor/stem cells at various points in a cytokine induced cell cycle transit (50). We found that surfactant mRNA was markedly elevated in G0 Lin-/Sca-1+ cells if the vesicles were from irradiated lung but was elevated at the G1/S interface if the vesicles were from normal non-irradiated lungs. Thus the cycle phases and nature of the originating tissue both modulated stem cell phenotype in these experiments.
Conclusion
To summarize, focus in the area of marrow hematopoietic stem cells has been on purifying to homogeneity “The Stem Cell”. A large body of experimental work has been carried out on these purified stem cells. Unfortunately the cell purified is not representative of the true marrow stem population, so that much of this work needs to be reconsidered. As we have outlined above, the marrow long-term multilineage renewal cell is an actively cycling cell, which means it is always changing and cannot be purified to homogeneity. This population is further continuously modulated by exposure to a large variety of tissue derived extracellular vesicles. These studies indicate that most experimental work on purified marrow stem cells is focusing on a non- representative stem cell. The critical need in this field is to define the population and to employ stochastic calculus to structure new models of stem cell biology.
Acknowledgements
The work from the authors’ laboratory discussed in this review was supported by the National Institute of General Medicine (NIGMS) of the National Institutes of Health (NIH) through Grant Number P20GM103468, the NIH Heart, Lung and Blood Institute through Grant Number 01HL103726, and through the National Institute of Diabetes and Digestive and Kidney Diseases through Grant Number UH2TR000880.
Footnotes
Author Contributions:
PJ Quesenberry: Conception and design, literature review, primary manuscript writing, manuscript review
LR Goldberg: Conception and design, literature review, manuscript writing, manuscript review
MS Dooner: Conception and design, literature review, manuscript writing, manuscript review
Disclaimers: The authors have no potential conflicts of interest.
References
- 1.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–22. [PubMed] [Google Scholar]
- 2.Till JE, McCulloch EA, Siminovitch L. A stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc Natl Acad Sci USA. 1964;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44(3):287–99. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 4.Pluznik DH, Sachs L. The cloning of normal “mast” cells in tissue culture. J Cell Physiol. 1965;66(3):319–24. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- 5.Axelrad AA, McLeod DL, Suzuki S, Shreeve MM. Regulation of the population size of erythropoietic progenitor cells. In: Clarkson B, Marks PA, Till JE, editors. Differentiation of normal and neoplastic hematopoietic cells. Cold Sring Harbor; New York: 1978. p. 155. [Google Scholar]
- 6.Metcalf D, MacDonald HR, Odartchenko N, Sordat B. Growth of mouse megakaryocyte colonies in vitro. Proc Natl Acad Sci USA. 1975;72(5):1744–48. doi: 10.1073/pnas.72.5.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakeff A, Dicke KA, Van Noord MJ. Megakaryocytes in agar culture of mouse bone marrow. Ser Haematol. 1975;8(1):4–21. [PubMed] [Google Scholar]
- 8.Gregory CJ. Erythropoietin sensitivity as a differentiation marker in the hemopoietic system: studies of three erythropoietic colony responses in culture. J Cell Physiol. Oct. 1976;89(2):289–301. doi: 10.1002/jcp.1040890212. [DOI] [PubMed] [Google Scholar]
- 9.Long MW, Gragowski LL, Heffner CH, Boxer LA. Phorbol diesters stimulate the development of an early murine progenitor cell. The burst-forming unit-megakaryocyte. J Clin Invest. 1985;76(2):431–8. doi: 10.1172/JCI111990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley TR, Hodgson GS. Detection of primitive macrophage progenitor cells in mouse bone marrow. Blood. 1979;54(6):1446–50. [PubMed] [Google Scholar]
- 11.Suda T, Suda J, Ogawa M. Single cell origin of mouse hemopoietic colonies expressing multiple lineages in variable combinations. Proc Natl Acad Sci USA. 1983;80(21):6689–93. doi: 10.1073/pnas.80.21.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 13.Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1(3):e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005;102(26):9194–9. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arber C, BitMansour A, Sparer TE, Higgins JP, Mocarski ES, Weissman IL, Shizuru JA, Brown JM. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood. 2003;102(2):421–8. doi: 10.1182/blood-2002-12-3834. [DOI] [PubMed] [Google Scholar]
- 16.Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci USA. 2002;99(18):11872–7. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyamoto T, Iwasaki H, Reizis B, Ye M, Graf T, Weissman IL, Akashi K. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev Cell. 2002;3(1):137–47. doi: 10.1016/s1534-5807(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 18.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98(25):14541–6. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo M, Scherer DC, King AG, Manz MG, Weissman IL. Lymphocyte development from hematopoietic stem cells. Curr Opin Genet Dev. 2001;11(5):520–6. doi: 10.1016/s0959-437x(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 20.Kondo M, Scherer DC, Miyamoto T, King AG, Akashi K, Sugamura K, Weissman IL. Cell fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407(6802):383–6. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- 21.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–7. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 22.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96(6):3120–5. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91(5):661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 24.Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of lineage of multipotent hematopoietic progenitors. Development. 1997;124(10):1929–39. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 25.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythromegakaryocytic potential: A revised road map for adult blood lineage commitment. Cell. 2005;121(2):295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Randal TD, Lund FE, Howard MD, Weissman IL. Expression of murine CD38 defines a population of long-term reconstituting hematopoietic stem cells. Blood. 1996;87(10):4057–67. [PubMed] [Google Scholar]
- 27.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1(8):661–73. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 28.Forsberg EC, Bhattacharya D, Weissman IL. Hematopoietic stem cells: Expression profiling and beyond. Stem Cell Rev. 2006;2(1):23–30. doi: 10.1007/s12015-006-0005-z. Review. [DOI] [PubMed] [Google Scholar]
- 29.Warren L, Bryder D, Weissman IL, Quake SR. Transcription factor profiling in individual hematopoietic progenitors by digital RT-PCR. Proc Natl Acad Sci USA. 2006;103(47):17807–12. doi: 10.1073/pnas.0608512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: The paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169(2):338–46. doi: 10.2353/ajpath.2006.060312., Erratum in: Am. J. Pathol. 169(5), 1899.
- 31.Forsberg EC, Serwold T, Kogan S, Weissman IL, Passegue E. New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell. 2006;126(2):415–26. doi: 10.1016/j.cell.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Suda T, Suda J, Ogawa M. Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc Natl Acad Sci USA. 1984;81(8):2520–24. doi: 10.1073/pnas.81.8.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202(11):1599–611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleming WH, Alpern EJ, Uchida N, Ikuta K, Sprangrude GJ, Weissman IL. Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J Cell Biol. 1993;122:897–902. doi: 10.1083/jcb.122.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orschell-Traycoff CM, Hiatt K, Dagher RN, Rice S, Yoder MC, Srour EF. Homing and engraftment potential of Sca-1+lin− cells fractionated on the basis of adhesion molecule expression and position in cell cycle. Blood. 2000;96:1380–1387. [PubMed] [Google Scholar]
- 36.Quesenberry PJ. The blueness of stem cells. Exp Hematol. 1991;19(8):725–8. [PubMed] [Google Scholar]
- 37.Suda J, Suda J, Ogawa M. Analysis of differentiation of mouse hemopoietic stem cells in cultures by sequential replating of paired progenitors. Blood. 1984;64(2):393–99. [PubMed] [Google Scholar]
- 38.Nilsson SK, Dooner MS, Tiarks CY, Weier HU, Quesenberry PJ. Potential and distribution of transplanted hematopoietic stem cells in a non-ablated mouse model. Blood. 1997;89(11):4013–20. [PubMed] [Google Scholar]
- 39.Yimez OH, Kiel MJ, Morrison SJ. Slam family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Murine marrow cells expanded in culture with IL-3, IL-6, IL-11, and SCF acquire an engraftment defect in normal hosts. Exp Hematol. 1995;23(5):461–469. (1995) [PubMed] [Google Scholar]
- 41.Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996;87(1):30–37. [PubMed] [Google Scholar]
- 42.Habibian HK, Peters SO, Hsieh CC, Wuu J, Vergilis K, Grimaldi CI, Reilly J, Carlson JE, Frimberger AE, Stewart FM, Quesenberry PJ. The fluctuating phenotype of the lympho-hematopoietic stem cell with cell cycle transit. J Exp Med. 1998;188(2):393–398. doi: 10.1084/jem.188.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colvin GA, Dooner MS, Dooner GJ, Sanchez-Guijo FM, Demers DA, Abedi M, Ramanathan M, Chung S, Pascual S, Quesenberry PJ. Stem cell continuum: Directed differentiation hotspots. Exp Hematol. 2007;35:96–107. doi: 10.1016/j.exphem.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Cerny J, Dooner M, McAuliffe C, Habibian H, Stencil K, Berrios V, Reilly J, Carlson J, Cerny AM, D'Hondt L, Benoit B, Lambert JF, Colvin G, Nilsson S, Becker P, Quesenberry PJ. Homing of purified murine lymphohematopoietic stem cells: A cytokine-induced defect. J Hematother. Stem Cell Res. 2002;11(6):913–922. doi: 10.1089/152581602321080574. [DOI] [PubMed] [Google Scholar]
- 45.Becker PS, Nilsson SK, Li Z, Berrios VM, Dooner MS, Cooper CL, Hsieh CC, Quesenberry PJ. Adhesion receptor expression by hematopoietic cell lines and murine progenitors: Modulation by cytokines and cell cycle status. Exp Hematol. 1999;27(3):533–541. doi: 10.1016/s0301-472x(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 46.Berrios VM, Dooner GJ, Nowakowski G, Frimberger A, Valinski H, Quesenberry PJ, Becker PS. The molecular basis for the cytokine-induced defect in homing and engraftment of hematopoietic stem cells. Exp Hematol. 2001;29(11):1326–35. doi: 10.1016/s0301-472x(01)00734-2. [DOI] [PubMed] [Google Scholar]
- 47.Lambert JF, Liu M, Colvin GA, Dooner M, McAuliffe CI, Becker PS, Forget BG, Weissman SM, Quesenberry PJ. Marrow stem cells shift gene expression and engraftment phenotype with cell cycle transit. J Exp Med. 2003;197(11):1563–72. doi: 10.1084/jem.20030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colvin GA, Lambert JF, Moore BE, Carlson JE, Dooner MS, Abedi M, Cerny J, Quesenberry PJ. Intrinsic hematopoietic stem cell/progenitor plasticity: Inversions. J Cell Physiol. 2004;199:20–31. doi: 10.1002/jcp.10436. [DOI] [PubMed] [Google Scholar]
- 49.Reddy GP, McAuliffe CI, Pang L, Quesenberry PJ, Bertoncello I. Cytokine receptor repertoire and cytokine responsiveness of Ho dull / Rh dull stem cells with differing potentials for G1/S phase progression. Exp Hematol. 2002;30(7):792–800. doi: 10.1016/s0301-472x(02)00814-7. [DOI] [PubMed] [Google Scholar]
- 50.Aliotta JM, Lee D, Puente N, Faradyan S, Sears EH, Amaral A, Goldberg L, Dooner MS, Pereira M, Quesenberry PJ. Progenitor/stem cell fate determination: interactive dynamics of cell cycle and microvesicles. Stem Cells Dev. 2012 Jul 1;21(10):1627–38. doi: 10.1089/scd.2011.0550. Epub 2012 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldberg LR, Dooner MS, Johnson K, Papa E, Pereira M, Del Tatto M, Adler DM, Aliotta J, Quesenberry PJ. The murine long-term multi-lineage renewal marrow stem cell is a cycling cell. Leukemia. 2014;28(4):813–22. doi: 10.1038/leu.2013.252. doi: 10.1038/leu.2013.252. Epub 2013 Aug 30. [DOI] [PubMed] [Google Scholar]
- 52.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison S. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 53.Hock H. Some hematopoietic stem cells are more equal than others. J Exp Med. 2010;207(6):1127–1130. doi: 10.1084/jem.20100950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Copley MR, Beer PA, Eaves CJ. Hematopoietic stem cell heterogeneity takes center stage. Cell Stem Cell. 2012;10:690–697. doi: 10.1016/j.stem.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 2010;207(6):1173–1182. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oguro H, Ding L, Morrison S. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13:102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogawa M. Changing phenotypes of hematopoietic stem cells. Exp Hematol. 2002;30:3–6. doi: 10.1016/s0301-472x(01)00770-6. [DOI] [PubMed] [Google Scholar]
- 58.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: From biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosome derived from exocytosis of multivesicular bodies and alpha granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 61.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 62.Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KA, Greer D, Pimentel J, Kolankiewicz LM, Puente N, Faradyan S, Ferland P, Bearer EL, Passero MA, Abedi M, Colvin GA, Quesenberry PJ. Alteration of marrow gene expression, protein production, and engraftment into lung by lung-derived microvesicles: A novel mechanism for phenotype modulation. Stem Cells. 2007;25(9):2245–2256. doi: 10.1634/stemcells.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aliotta JM, Pereira M, Johnson KW, de Paz N, Dooner MS, Puente N, Ayala C, Brilliant K, Berz D, Lee D, Ramratnam B, McMillan PN, Hixson DC, Josic D, Quesenberry PJ. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp Hematol. 2010;38(3):233–45. doi: 10.1016/j.exphem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aliotta JM, Pereira M, Li M, Amaral A, Sorokina A, Dooner MS, Sears EH, Brilliant K, Ramratnam B, Hixson DC, Quesenberry PJ. Stable cell fate changes in marrow cells induced by lung-derived microvesicles. J Extracell Vesicles. 2012 Apr 16; doi: 10.3402/jev.v1i0.18163. 1.doi:10.3402/jev.v1i0.18163. [DOI] [PMC free article] [PubMed] [Google Scholar]