Abstract
Objective
This study investigated the association between maternal ages at birth of last child and the likelihood of survival to advanced ages.
Methods
A nested case-control study using Long Life Family Study (LLFS) data. Three hundred and eleven women who survived past the oldest 5th percentile of survival according to birth cohort matched life tables were identified as cases and 151 women who died at ages younger than the top 5th percentile of survival were identified as controls. A Bayesian mixed-effect logistic regression model was used to estimate the association between maternal age at birth of last child and exceptional longevity among these 462 women.
Results
A significant association for later maternal age was found whereby women who had their last child beyond the age of 33 years had twice the odds of survival to the top 5th percentile of survival of their birth cohorts compared to women who had their last child by age 29 (OR=2.08, 95%CI 1.13; 3.92 for age between 33 and 37 years and OR=1.92, 95% CI 1.03; 3.68 for older age).
Conclusion
The study supports the findings from other studies demonstrating a positive association between older maternal age and greater odds of the mother surviving to unusually old age.
Keywords: maternal age, menopause, centenarian, familial longevity, aging, evolution
Introduction
Several studies have observed an association between later maternal age and exceptional longevity. Analysis of New England Centenarian Study cohort data revealed that women who gave birth to a child after the age of 40 had four times greater odds of being a centenarian compared to women from the same birth cohort who had their last child at younger ages [1]. A study of both Mormon pedigrees (siblings born before 1870) and historical demographic data from Quebec residents (siblings born between 1670 and 1750) documented that women who had their last child between ages 41-44 and between 42-44 years respectively had mortality hazard ratios of 0.94 and 0.93 compared to women who gave birth before the ages of 41 and 42 years [2]. In both samples, if the mothers had their last child at age 45 years or later, the hazard ratios were even lower at 0.86 and 0.83 respectively. Analysis of the Mormon pedigrees further documented that the brothers and sisters of women who had children at older ages also lived longer. Northernmost Finnish parish registers were used to study maternal ages of women born between 1679 and 1839, their ages at death and key covariates such as age at first reproduction, total number of offspring born, average length of inter-birth intervals and spouse’s age at death. The authors found that women who had children at the oldest ages also lived the longest, and that every additional year of maternal age translated into a 28% reduction in post-reproductive mortality [3]. These studies suggest that the ability of women to achieve exceptional longevity and the ability to have children at substantially older than average ages have determinants in common and that prolonged fertility may be a marker of slower aging.
The Long Life Family Study (LLFS) is a longitudinal phenotypic and genetic study of 551 families with family members demonstrating clustering for exceptional longevity. The LLFS sample provides an opportunity to further test the association between older maternal age and exceptional survival.
Materials and Methods
Study Participants
The LLFS consists of 4,875 participants from 551 families in the United States and Denmark. Enrolled participants belong to one of two generations, the older generation (G1) comprising probands and their siblings, and the offspring generation (G2). Spouses in both generations were also enrolled as referent controls. In order to be eligible for the LLFS, G1 members of a family must have attained old enough ages to collectively be defined as exceptional based upon birth-cohort specific life tables. Exceptionality was ranked according to a Family Longevity Selection Score (FLoSS) which takes into account the percentile rank survival of each sibling in the G1 sibship for all those who survived beyond the age of 40 years [4] and if family members were alive to participate in the study. Families with G1 sibships that achieved a FLoSS score of at least 7 were asked to enroll in the LLFS. Fewer than 1% of Framingham Heart Study families achieve a Floss ≥7, an indication of the exceptionality of the LLFS sample. Participants have been followed longitudinally since enrollment, which ran from 2006 to 2009 in three locations in the United States (Boston, New York City and Pittsburgh) and one location in Denmark. The aim of the LLFS is to identify genetic and phenotype traits that contribute to survival to extreme ages and subphenotypes of exceptional survival such as measures of healthy aging. Age reporting in the US LLFS sample has been shown to be of high quality [5]. Additional details about the LLFS such as phenotypic measures have been well described [6,7].
Study Design
A nested case-control study was designed for this analysis to study the association between the age at which a woman had her last child and her age of survival. Therefore we only included women who bore at least one child. Alive and deceased female participants in the LLFS who survived to or beyond the 5th percentile of survival for their birth cohorts (the cases) were compared to female participants who died at an age younger than the 5th percentile of their birth cohorts but older than 70 years of age (the controls). Percentiles of survival were determined from the birth year-matched cohort life tables from the USA Social security administration [8]. Participants younger than 70 years were excluded in order to avoid comparing cases and controls from substantially different birth cohorts and therefore confounding results due to differing secular trends. For hundred and fifty one women from the proband (G1) generation and 11 women from the offspring (G2) generation (who were older than 70 years) met the above inclusion criteria for a total sample of 462 participants. Two G1 participants were excluded because they were younger than 70 years old. Ten G1 but none of G2 participants were excluded because they were nulliparous. Of the 462 participants, 311 were cases and 151 were controls. Descriptive statistics for these two comparison groups appear in Table 1. Histograms of birth year and maternal age at last child birth for the case and control groups are provided in the supplemental material (Figure S1)
Table 1.
Univariate descriptors for alive and deceased cases (top 5th percentile of survival by age) and deceased referent participants (less than the top 5th percentile of survival).
| Characteristic | Cases(n=311) | Controls (n=151) |
|---|---|---|
| Median Age at last contact for cases and median age of death for controls |
100 (97-109) | 92 (75-96) |
| Maternal age at birth of last child | 34 (18-50) | 34 (21-48) |
| Birth year | 1912 (1898-1916) | 1918 (1912-1935) |
| Tobacco use history (ever used)* | 75 (24.1%) | 50 (33.1%) |
| Years of education (≥ 12 years)* | 184 (59.2%) | 107 (70.1%) |
| Study center (DK) | 65 (20.9%) | 32 (21.2%) |
| Familial longevity (Yes)* | 301(96.8%) | 129 (85.4%) |
| Parity (3+ ) | 163 (52.4%) | 87 (57.6%) |
Age at last contact (determined during annual follow-up), maternal age at birth of last child and birth year are summarized by median (and range). Also noted are women’s frequencies and proportions for tobacco use history, years of education, study location (Danish versus US women), familial longevity (an indicator of blood relatives of the family members selected for longevity) and parity (3+ children versus 1-2 children). The 0.05 a-level statistically significant difference of the proportions in cases and controls are marked by *.
Statistical Analyses
Women’s characteristics were summarized by median and range for continuous measures, frequency and proportions for categorical variables (Table 1). Two Bayesian mixed-effect logistic regression models were used to analyze the association between maternal age at birth of last child and exceptional longevity [9]. In addition to fixed effects, the logit function of both models included a normally distributed random effect with mean 0 and variance that followed a Gamma distribution to model the within family correlation. The first model included maternal age at birth of last child as a continuous variable (Table 2). Since this model assumed a linear relationship between the log-odds of exceptional longevity and maternal age, we also fit a second model in which maternal age at birth of last child was stratified into four groups (ages ≤ 29 years, 30 to 32 years, 33 to 37 years, and 37+ years) based on quartiles of the maternal age distribution (Table 3). Both analyses were adjusted for level of education (less than 12 years vs. 12 years or greater), tobacco use history (never used vs. ever used), parity (1-2 vs. 3+ children to have approximately the same number of women in the two groups), and two indicator variables: 1) to indicate whether women in the analysis were blood relatives of the family members selected for longevity (as opposed to the spouses, who were not selected for familial longevity); 2) to indicate whether the women were members of families enrolled in Denmark rather than in the U.S. All parameters were assigned normal prior distributions with mean 0 and prior variance 100, and the family-specific random effect was also assigned a normal distribution. Gibbs sampling was used to generate samples of at least 50,000 values (after an initial burn-in of 2,000 iterations) from the marginal distribution of the parameters of the logistic regression models, and the odds ratios. The Gelman and Rubin statistic was used to verify the convergence of the method. The sample medians and 2.5 and 97.5 percentiles were used to determine point estimates and 95% credible intervals of the parameters (Tables 2 and 3). Statistically significant associations were determined by 95% credible intervals not including 0 (corresponding to an odds ratio different from 1). Interaction between parity and maternal age at birth of last child was tested but did not reach statistical significance. Statistical analyses were conducted using the Openbugs software package (http://www.openbugs.info/w/) and representative scripts used for analysis are available in the supplement material. The robustness of the results to prior assumptions was examined by repeating the analyses for different prior distributions (See Tables 1-2 in the Supplement material).
Table 2.
Results of multivariate logistic regression using maternal age at birth of last child asa continuous variable.
| Covariates | OR | 95%CI Low | 95%CI Upper |
|---|---|---|---|
| Maternal age at birth of last child | 1.05 | 1.01 | 1.09 |
| Study center (DK) | 0.55 | 0.29 | 1.04 |
| Parity (3+) | 0.59 | 0.36 | 0.94 |
| Familial longevity (yes) | 5.18 | 2.38 | 11.91 |
| Years of education (≥ 12 years) | 0.42 | 0.23 | 0.72 |
| History of tobacco use (ever used) | 0.61 | 0.39 | 0.97 |
Study center (Danish versus US women); Parity (3+ children versus 1-2 children); Familial longevity (spouses versus blood relatives of the family members selected for longevity); Years of education (< 12 years versus ≥ 12 years); Tobacco use history (never used versus ever used). OR (odds ratios for the groups in parenthesis) and 95% CI were estimated using Bayesian mixed-effect logistic models. The Gelman and Rubin statistic was in the range of 0.99 and 1.0001 with 3 chains indicating convergence of the Markov Chain.
Table 3.
Logistic regression results for impact of age at which a woman last had a child upon survival to ages within the top 5th percentile of survival.
| Covariates | OR | 95% CI Lower |
95% CI Upper |
|---|---|---|---|
| 29 < maternal age at birth of last child <= 33 | 1.31 | 0.71 | 2.44 |
| 33 < maternal age at birth of last child <= 37 | 2.08 | 1.13 | 3.92 |
| Maternal age at birth of last child > 37 | 1.92 | 1.02 | 3.68 |
| Study center (DK) | 0.57 | 0.3 | 1.07 |
| Parity (3+) | 0.61 | 0.37 | 0.97 |
| Familial longevity (Yes) | 5.46 | 2.47 | 12.61 |
| Years of education (>= 12 years) | 0.43 | 0.24 | 0.73 |
| History of tobacco use (ever used) | 0.61 | 0.39 | 0.97 |
Maternal age at last child was stratified by quartiles; Study center (Danish versus US women); Parity (3+ children versus 1-2 children); Familial longevity (spouses versus blood relatives of the family members selected for longevity); Years of education (< 12 years versus ≥ 12 years); Tobacco use history (never used versus ever used). OR (odds ratios for the groups in parenthesis) and 95% CI were estimated using Bayesian mixed-effect logistic models. The Gelman and Rubin statistic was in the range of 0.99 and 1.0001 with 3 chains indicating convergence of the Markov Chain.
Results
Table 1 shows summary statistics of the study participants. Compared to cases, controls were more likely to have a history of smoking, to have higher education, and lower frequency of familial longevity. Median maternal age at birth of last child for both cases and controls was 34 years. The two groups had equal proportions of women enrolled in Denmark and the U.S. and comparable proportions of having 3 or more children.
Table 2 shows the increase in odds for exceptional longevity for a one-year increase in maternal age at birth of the last child. Controlling for covariates, each additional year of maternal age is associated with a 5% increase in the odds for exceptional longevity. This effect is much smaller compared to the effect of familial longevity on a woman’s odds for exceptional longevity (OR=5.18). For example, comparing two women with the same level of education, smoking history, study site, and parity, an equivalent increase in the odds of exceptional longevity due to familial longevity would need an unrealistic 33 year difference in maternal age at last birth. Thus, familial longevity is a much stronger predictor for longevity than maternal age at birth of the last child. Note that the estimated effect of maternal age at last birth did not change substantially when the indicator variable for familial longevity was dropped from the model (Supplement Tables 5 and 6).
We did observe that having more children, for example 3+ children, tempered the association between increased maternal age and later survival. For example, about a 5 year increase in the woman’s age at last birth for women with 1-2 children increased the odds ratio for exceptional survival by 1.29 (black line in Figure 1), while an equivalent increase in the odds ratio for women with 3+ children requires a difference in maternal age at last child of about 16 years (red line in Figure 1). Although the interaction between parity and maternal age at last child did not reach statistically significance (log(OR) = −0.04, 95% CI −0.13; 0.04) the negative parameter suggests that increased parity defined as 3+ children decreases the effect of later maternal age at birth of the child on longevity.
Figure 1.
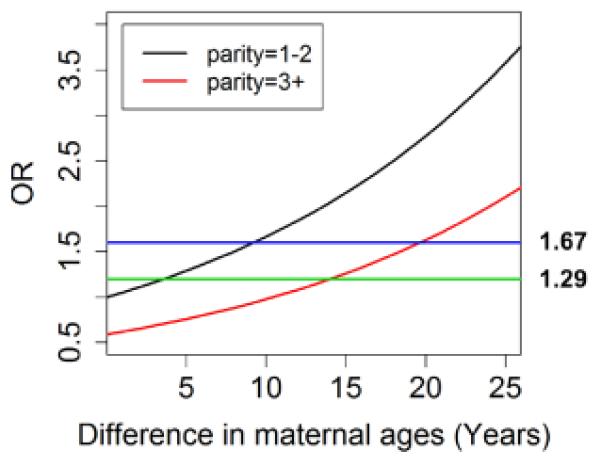
The increasing trend of the two lines shows the positive association of maternal age at last child and exceptional longevity. The downwards shift of the lines for increasing parity shows the negative effect of parity on the odds for exceptional longevity. For example, while a difference in maternal age at last child of about 14 years increases the odds for exceptional longevity by 2 in women with 1 or 2 children, the same effect in women with 3+ children would require a difference of 24 years in age at last child.
To explore whether the assumption of a log-linear association between maternal age at last birth and odds for exceptional longevity is correct, we also conducted an analysis in which maternal age at last birth was stratified into quartiles. Results of the multivariate logistic regression analysis are summarized in Table 3 and show a significant association of later maternal age with exceptional survival: women who had their last child when they were age 33 to 37 had twice the odds of living past the 5th survival percentile of their birth year cohort compared to women who had their last child before age 29 (OR = 2.08, 95% CI 1.13-3.92). The odds were slightly lower for maternal age >37 (OR = 1.92, 95% CI 1.02-3.68) but not significantly different from the age 33-37 group (the small number of women achieving these oldest maternal ages could be influencing the statistical significance). Maternal age at last child between ages 29 and 33 also slightly increased the odds for longevity compared to women who had their last child before age 29, but this association did not reach statistical significance (OR = 1.31, 95% CI 0.71; 2.44).
Sensitivity analysis summarized in Supplement Tables 1—4 showed that the results are robust to varying prior distributions for the parameters.
Discussion
Among female LLFS participants older maternal age at last birth was a predictor of survival past the oldest 5th percentile of their respective birth cohorts. For each additional year in maternal age at last birth, the odds of exceptional longevity increased by 5%. Women who had their last child above the age of 33 years had twice the odds of exceptional survival compared to women who had their last child before age 29. Though significant, the relatively wide confidence intervals for this finding suggest that we would do well to repeat this study when we have more observations (e.g. the LLFS will accumulate more controls as participants pass away and we definitively realize their ages of survival). These findings are at the least consistent with other studies of the relation between maternal age at birth of the last child and exceptional longevity [1,2,10]. The positive association between older maternal age and the odds for exceptional longevity remains significant even after adjusting for familial longevity.
Twin studies have noted that about 20% of the variation in survival to the mid-eighties is likely genetic and the remaining 80% is due to variation in environmental and behavior related exposures [11,12]. The findings from these studies are consistent with findings from the Seventh Day Adventist Health Study which concluded that most people should be able to survive to their mid to late eighties if they engage in healthy behaviors such as daily exercise, no tobacco or alcohol use, vegetarian diet and managing their stress well, for example, by engaging in family and religious activities [13]. Several studies have also established that the influence of genetic variation increases the chances of survival beyond the age of approximately 90 years, and becomes stronger the more exceptional or rare the age of survival. For example, Tan et al. noted that the power to discover genetic variants associated with exceptional longevity is significantly increased when the sample consists of centenarians rather than nonagenarians [14]. Furthermore, several studies have shown that a sibling’s relative risk of survival to extreme old age increases from negligible if the proband survived to his/her 80s to significant if the proband survived to his/her mid to late 90s [15-20]. These risks become even greater if the proband is about 102 years old in which case the probability of survival to 100 years relative to 1900 birth cohort specific average survival rates becomes 8 times greater for female siblings and 17 times greater for male siblings [21]. That this survival advantage is likely to have a genetic component is suggested by results from the New England Centenarian Study (NECS). The NECS discovered genetic profiles, based upon a set of 281 single nucleotide polymorphisms (SNPs), that can accurately differentiate between centenarians and general population controls [22,23]. While the accuracy of this differentiation is about 60% for participants who are 100 years old, it increases to 85% for centenarians aged 106 years and older. This increased accuracy is consistent with the increasing relative influence of genetic variation on survival with increasing age beyond age 90 years.
These findings raise the question of what might be the evolutionary mechanism or selective pressure that selects for genetic variants that facilitate survival long after the age at which reproduction ceases. Perls and colleagues previously proposed that a selection pressure for longevity-associated genetic variants could be the pressure to facilitate a longer period of time during which women can bear children and therefore have more of them [24,25]. This theory is consistent with the disposable soma theory which asserts that there exists a tradeoff between energy allocation between reproductive fitness and repair/maintenance functions [26] [27]. Women who age more slowly and avoid age-related diseases that adversely affect fertility (and therefore live to much older ages), have biological mechanisms that enhance the efficiency of energy production thus facilitating the delay in this tradeoff. According to the grandparent hypothesis, mechanisms that would facilitate living to old enough ages to be a grandparent would allow grandparents or even older generations to care for their descendants while the parents carried out more labor-intensive activities to ensure their children’s survival [28,29].
Given that increased parity could be one of the benefits of longevity-associated genetic variants, we investigated the relationship between higher parity, which we defined as having 3+ children. Fifty-two percent of the women in the top fifth percentile of survival versus 58% of the controls had 3+ children. Our findings are consistent with those of the Rotterdam Study which noted significantly lower mortality for women who had 2-3 children compared to those who had no children or those who had more than 3 children [30]. Assessing the relationship between parity and exceptional longevity among long-lived individuals who were alive in recent history might not be germane to the conditions in prehistoric times when longevity-associated genes evolved. There are likely very different competing and influencing factors between these two time periods. Of note, unlike the Rotterdam Study, we did not assess relative mortality for women who had no children. Nulliparous women were excluded from this study sample because they were not informative for our estimate of the ages at which women were not reproductively senescent. It is notable however that only 70 women of the entire LLFS sample never had children. This is not surprising given that the LLFS cohort are generally exceptionally healthy. One hypothesis would be that women who had no children had disease(s) or environmental exposures that impair fertility as well as decrease survival (e.g. diabetes, hypertension, autoimmune disorders). Many of these 68 women are currently alive and less than 70 years of age so we are unable to assess mortality risk associated with nullparity at this point in time in the LLFS.
We note some potential limitations to this study. Since we limited the women in the control set to only those who had died (so that we could be certain of their life spans), as we lowered the age of survival, the number of controls that we could include dropped dramatically. For example, if we used the 10th percentile of survival, we were able to include only a quarter of the deceased women. Thus, the top 5 percentile was used as the cutoff. We performed a sensitivity analysis to see if changing the percentile of survival for the control group affected our results. Selecting cases that were even older, such as at least 100 years old, still led to similar results (see Supplementary Tables 3 and 4). An alternative analysis that removes the definition of cases and controls could use survival to age at death as a response variable. However, the LLFS has still limited follow up (average follow up 5 years) and the majority of individuals are still alive so that the power of this analysis would be limited. Also, differences in birth year cohorts between the cases and controls could be a confounder. However, known longevity-associated factors such as years of education, health care, socioeconomic status would have improved (though slightly) for the controls that were born later than the cases. This possible enhanced risk for survival for the controls would bias against our hypothesis of older survival for the cases and therefore these demographic trends would strengthen our findings. Another important limitation that we must consider is the relatively few factors (e.g. education and tobacco use) that we controlled for in assessing the relationship between maternal age at last birth and survival. However factors that predispose to age-related diseases and accelerated aging likely also influence reproductive senescence and that is why we regard the age of such senescence as a marker or predictor of ultimate survival. Still, for future study, a more comprehensive accounting of factors known to influence survival to extreme old age would be helpful.
Conclusion
Numerous studies have now observed the relationship between older maternal age at birth and exceptional survival, thus providing evidence for sustained reproductive fitness with age as a selective force for genetic variants conducive to longer life. These findings support the conduct of studies of genetic influences of reproductive fitness which may also influence rate of aging and susceptibility to age-related diseases [31,32] .
Supplementary Material
Acknowledgements
The LLFS study is funded by the US National Institute on Aging / National Institutes of Health: NIA/NIH cooperative agreements U01AG023712, U01AG23744, U01AG023746, U01AG023749, and U01AG023755.
Funding: The LLFS study is funded by the US National Institute on Aging / National Institutes of Health: NIA/NIH cooperative agreements U01AG023712, U01AG23744, U01AG023746, U01AG023749, and U01AG023755.
Footnotes
Conflict of Interest and Disclosures: All authors state no potential conflict of interest and there are no disclosures to make
References
- 1.Perls TT, Alpert L, Fretts RC. Middle-aged mothers live longer. Nature. 1997;389:133. doi: 10.1038/38148. [DOI] [PubMed] [Google Scholar]
- 2.Smith KR, Gagnon A, Cawthon RM, Mineau GP, Mazan R, Desjardins B. Familial aggregation of survival and late female reproduction. J Gerontol A Biol Sci Med Sci. 2009;64:740–744. doi: 10.1093/gerona/glp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helle S, Lummaa V, Jokela J. Are reproductive and somatic senescence coupled in humans? Late, but not early, reproduction corelated with longevity in historical sami women. Proc Biol Sci. 2005;272:29–37. doi: 10.1098/rspb.2004.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sebastiani P, Hadley EC, Province M, Christensen K, Rossi W, Perls TT, Ash AS. A family longevity selection score: Ranking sibships by their longevity, size, and availability for study. Am J Epidemiol. 2009;170:1555–1562. doi: 10.1093/aje/kwp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elo IT, Mykyta L, Sebastiani P, Christensen K, Glynn NW, Perls T. Age validation in the long life family study through a linkage to early-life census records. J Gerontol B Psychol Sci Soc Sci. 2013;68:580–585. doi: 10.1093/geronb/gbt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman AB, Glynn NW, Taylor CA, Sebastiani P, Perls TT, Mayeux R, Christensen K, Zmuda JM, Barral S, Lee JH, Simonsick EM, Walston JD, Yashin AI, Hadley E. Health and function of participants in the long life family study: A comparison with other cohorts. Aging (Albany NY) 2011;3:63–76. doi: 10.18632/aging.100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sebastiani P, Sun F, Andersen SL, Lee J, Wojczynski MK, Sanders JL, Yashin AJ, Newman AB, Perls TT. Families enriched for exceptional longevity also have increased health-span: Findings from the long life family study. Front Public Health. 2013 doi: 10.3389/fpubh.2013.00038. doi: 10.3389/fpubh.2013.00038. URL: http://www.frontiersin.org/Journal/00010.03389/fpubh.02013.00038/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell FC, Miller ML. [accessed july 23, 2013];Life tables for the united states social security area 1900-2100, actuarial study no. 120. 2005 Url: Http://www.Ssa.Gov/oact/notes/as120/lifetables_tbl_7_1900.Html.
- 9.Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR. Methods in health service research. An introduction to bayesian methods in health technology assessment. BMJ. 1999;319:508–512. doi: 10.1136/bmj.319.7208.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McArdle PF, Pollin TI, O’Connell JR, Sorkin JD, Agarwala R, Schaffer AA, Streeten EA, King TM, Shuldiner AR, Mitchell BD. Does having children extend life span? A genealogical study of parity and longevity in the amish. J Gerontol A Biol Sci Med Sci. 2006;61:190–195. doi: 10.1093/gerona/61.2.190. [DOI] [PubMed] [Google Scholar]
- 11.Ljungquist B, Berg S, Lanke J, McClearn GE, Pedersen NL. The effect of genetic factors for longevity: A comparison of identical and fraternal twins in the swedish twin registry. J Gerontol A Biol Sci Med Sci. 1998;53:M441–446. doi: 10.1093/gerona/53a.6.m441. [DOI] [PubMed] [Google Scholar]
- 12.McGue M, Vaupel JW, Holm N, Harvald B. Longevity is moderately heritable in a sample of danish twins born 1870-1880. J Gerontol. 1993;48:B237–244. doi: 10.1093/geronj/48.6.b237. [DOI] [PubMed] [Google Scholar]
- 13.Fraser GE, Shavlik DJ. Ten years of life: Is it a matter of choice? Arch Intern Med. 2001;161:1645–1652. doi: 10.1001/archinte.161.13.1645. [DOI] [PubMed] [Google Scholar]
- 14.Tan Q, Zhao JH, Zhang D, Kruse TA, Christensen K. Power for genetic association study of human longevity using the case-control design. Am J Epidemiol. 2008;168:890–896. doi: 10.1093/aje/kwn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willcox BJ, Willcox DC, He Q, Curb JD, Suzuki M. Siblings of okinawan centenarians share lifelong mortality advantages. J Gerontol A Biol Sci Med Sci. 2006;61:345–354. doi: 10.1093/gerona/61.4.345. [DOI] [PubMed] [Google Scholar]
- 16.Perls TT, Bubrick E, Wager CG, Vijg J, Kruglyak L. Siblings of centenarians live longer. Lancet. 1998;351:1560. doi: 10.1016/S0140-6736(05)61126-9. [DOI] [PubMed] [Google Scholar]
- 17.Montesanto A, Latorre V, Giordano M, Martino C, Domma F, Passarino G. The genetic component of human longevity: Analysis of the survival advantage of parents and siblings of italian nonagenarians. Eur J Hum Genet. 2011;19:882–886. doi: 10.1038/ejhg.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perls T, Kohler IV, Andersen S, Schoenhofen E, Pennington J, Young R, Terry D, Elo IT. Survival of parents and siblings of supercentenarians. J Gerontol A Biol Sci Med Sci. 2007;62:1028–1034. doi: 10.1093/gerona/62.9.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoenmaker M, de Craen AJ, de Meijer PH, Beekman M, Blauw GJ, Slagboom PE, Westendorp RG. Evidence of genetic enrichment for exceptional survival using a family approach: The leiden longevity study. Eur J Hum Genet. 2006;14:79–84. doi: 10.1038/sj.ejhg.5201508. [DOI] [PubMed] [Google Scholar]
- 20.Westendorp RG, van Heemst D, Rozing MP, Frolich M, Mooijaart SP, Blauw GJ, Beekman M, Heijmans BT, de Craen AJ, Slagboom PE. Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: The leiden longevity study. J Am Geriatr Soc. 2009;57:1634–1637. doi: 10.1111/j.1532-5415.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- 21.Perls TT, Wilmoth J, Levenson R, Drinkwater M, Cohen M, Bogan H, Joyce E, Brewster S, Kunkel L, Puca A. Life-long sustained mortality advantage of siblings of centenarians. Proc Natl Acad Sci U S A. 2002;99:8442–8447. doi: 10.1073/pnas.122587599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebastiani P, Solovieff N, DeWan AT, Walsh KM, Puca A, Hartley SA, Melista E, Andersen S, Dworkis DA, Wilk JB, Myers RM, Steinberg MH, Montano M, Baldwin CT, Hoh J, Perls TT. Genetic signatures of exceptional longevity in humans. PLoS ONE. 2012;7(1):e29848. doi: 10.1371/journal.pone.0029848. doi:10.1371/journal.pone.0029848. URL: http://dx.plos.org/10.1371/journal.pone.0029848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sebastiani P, Bae H, Sun FX, Andersen SL, Daw EW, Malovini A, Kojima T, Hirose N, Schupf N, Puca A, Perls TT. Meta-analysis of genetic variants associated with human exceptional longevity. Aging (Albany NY) 2013;5:653–661. doi: 10.18632/aging.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perls T, Fretts R. Why women live longer than men. Sci Amer Pres. 1998;100:107. [Google Scholar]
- 25.Perls TT, Fretts RC. The evolution of menopause and human life span. Ann Hum Biol. 2001;28:237–245. doi: 10.1080/030144601300119052. [DOI] [PubMed] [Google Scholar]
- 26.Kirkwood TB, Rose MR. Evolution of senescence: Late survival sacrificed for reproduction. Philos Trans R Soc Lond B Biol Sci. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- 27.Westendorp RG, Kirkwood TB. Human longevity at the cost of reproductive success. Nature. 1998;396:743–746. doi: 10.1038/25519. [DOI] [PubMed] [Google Scholar]
- 28.Hawkes K. Grandmothers and the evolution of human longevity. Am J Hum Biol. 2003;15:380–400. doi: 10.1002/ajhb.10156. [DOI] [PubMed] [Google Scholar]
- 29.Hawkes K. Human longevity: The grandmother effect. Nature. 2004;428:128–129. doi: 10.1038/428128a. [DOI] [PubMed] [Google Scholar]
- 30.Kuningas M, Altmae S, Uitterlinden AG, Hofman A, van Duijn CM, Tiemeier H. The relationship between fertility and lifespan in humans. Age (Dordr) 2011;33:615–622. doi: 10.1007/s11357-010-9202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murabito JM, Yang Q, Fox CS, Cupples LA. Genome-wide linkage analysis to age at natural menopause in a community-based sample: The framingham heart study. Fertil Steril. 2005;84:1674–1679. doi: 10.1016/j.fertnstert.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 32.Stolk L, Perry JR, Chasman DI, He C, Mangino M, Sulem P, Barbalic M, Broer L, Byrne EM, Ernst F, Esko T, Franceschini N, Gudbjartsson DF, Hottenga JJ, Kraft P, McArdle PF, Porcu E, Shin SY, Smith AV, van Wingerden S, Zhai G, Zhuang WV, Albrecht E, Alizadeh BZ, Aspelund T, Bandinelli S, Lauc LB, Beckmann JS, Boban M, Boerwinkle E, Broekmans FJ, Burri A, Campbell H, Chanock SJ, Chen C, Cornelis MC, Corre T, Coviello AD, d’Adamo P, Davies G, de Faire U, de Geus EJ, Deary IJ, Dedoussis GV, Deloukas P, Ebrahim S, Eiriksdottir G, Emilsson V, Eriksson JG, Fauser BC, Ferreli L, Ferrucci L, Fischer K, Folsom AR, Garcia ME, Gasparini P, Gieger C, Glazer N, Grobbee DE, Hall P, Haller T, Hankinson SE, Hass M, Hayward C, Heath AC, Hofman A, Ingelsson E, Janssens AC, Johnson AD, Karasik D, Kardia SL, Keyzer J, Kiel DP, Kolcic I, Kutalik Z, Lahti J, Lai S, Laisk T, Laven JS, Lawlor DA, Liu J, Lopez LM, Louwers YV, Magnusson PK, Marongiu M, Martin NG, Klaric IM, Masciullo C, McKnight B, Medland SE, Melzer D, Mooser V, Navarro P, Newman AB, Nyholt DR, Onland-Moret NC, Palotie A, Pare G, Parker AN, Pedersen NL, Peeters PH, Pistis G, Plump AS, Polasek O, Pop VJ, Psaty BM, Raikkonen K, Rehnberg E, Rotter JI, Rudan I, Sala C, Salumets A, Scuteri A, Singleton A, Smith JA, Snieder H, Soranzo N, Stacey SN, Starr JM, Stathopoulou MG, Stirrups K, Stolk RP, Styrkarsdottir U, Sun YV, Tenesa A, Thorand B, Toniolo D, Tryggvadottir L, Tsui K, Ulivi S, van Dam RM, van der Schouw YT, van Gils CH, van Nierop P, Vink JM, Visscher PM, Voorhuis M, Waeber G, Wallaschofski H, Wichmann HE, Widen E, Wijnands-van Gent CJ, Willemsen G, Wilson JF, Wolffenbuttel BH, Wright AF, Yerges-Armstrong LM, Zemunik T, Zgaga L, Zillikens MC, Zygmunt M, Study TL, Arnold AM, Boomsma DI, Buring JE, Crisponi L, Demerath EW, Gudnason V, Harris TB, Hu FB, Hunter DJ, Launer LJ, Metspalu A, Montgomery GW, Oostra BA, Ridker PM, Sanna S, Schlessinger D, Spector TD, Stefansson K, Streeten EA, Thorsteinsdottir U, Uda M, Uitterlinden AG, van Duijn CM, Volzke H, Murray A, Murabito JM, Visser JA, Lunetta KL. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet. 2012;44:260–268. doi: 10.1038/ng.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


