Abstract
Aim
Differences in genetic background between control mice and mice with targeted gene mutations have been recognized as a potential cause for phenotypic differences. In the present study we have used A1AR-deficient mice in a C57Bl/6 and SWR/J congenic background to assess the influence of background on the effect of A1AR-deficiency on cardiovascular and renal functional parameters.
Methods
In A1AR+/+ and A1AR−/− mice in C57Bl/6 and SWR/J congenic backgrounds we assessed blood pressure and heart rate using radio-telemetry, plasma renin concentrations, and tubuloglomerular feedback.
Results
We did not detect significant differences in arterial blood pressure (MAP) and heart rates (HR) between A1AR+/+ and A1AR−/− mice in either C57Bl/6, SWR/J, or mixed backgrounds. MAP and HR were significantly higher in SWR/J than in C57Bl/6 mice. A high NaCl intake increased MAP in A1AR−/− mice on C57Bl/6 background while there was less or no salt sensitivity in the SWR/J background. No significant differences in plasma renin concentration were detected between A1AR−/− and A1AR+/+ mice in any of the strains. Tubuloglomerular feedback was found to be absent in A1AR−/− mice with SWR/J genetic background.
Conclusions
While this study confirmed important differences between inbred mouse strains, we did not identify phenotypic modifications of A1AR-related effects on blood pressure, heart rate, and plasma renin by differences in genetic background.
Keywords: adenosine, blood pressure telemetry, micropuncture, C57Bl/6 mice, SWR/J mice
Introduction
The availability of mice with targeted gene deletions has greatly expanded the investigative options in many areas of experimental biology. However, the issue of genetic background diversity has been of long-standing concern in murine physiology (Hackbarth and Hackbarth, 1981, Hackbarth and Hackbarth, 1982), and this issue has therefore become an unavoidable “side-effect’ of using mice with genetic modifications. Although it is well recognized that genetic differences besides the targeted ones can have unexpected biological consequences, general realization of the need to study control and gene modified animals with otherwise identical backgrounds has proved to be a rather intractable problem in practical experimentation (Gerlai, 1996, Sigmund, 2000). If the phenotype under study shows differences between wild type and gene modified mice that are large, consistent, biologically plausible, and demonstrable in mice with diverse genetic backgrounds, one may assume that they are the consequence of the mutation rather than of differences in unspecified modifier genes. Although not always practical for time and cost reasons, a reasonable approach to minimize the potential fallacy of ascribing an observed difference between strains to a specific gene deletion is to maintain the mutation in the background of one or more inbred mouse strains for a sufficient number of generations. After more than 6 generations, a genetic homogeneity of more than 99 % will be achieved between control and mutant animals with the exception of the region flanking the targeted mutation.
Studies in mice with targeted null mutations of the adora1 gene coding for the A1 adenosine receptor (A1AR) have been performed widely and have yielded novel insights into the functional roles of this nucleoside receptor. The majority of these studies were performed in animals in which the genetic background consisted of a mix between 129J and C57Bl/6 DNA (Johansson et al., 2001, Sun et al., 2001). In the present study we have generated two congenic mouse strains that carry the A1AR null mutation in a C57Bl/6 or SWR/J genetic background. We used these strains in addition to the original mixed background strain to re-assess the effect of A1AR deficiency on arterial blood pressure and its sensitivity to changes in dietary salt intake. Previous reports on blood pressure in A1AR-deficient mice have been contradictory so that a reevaluation of this issue in mice with defined genetic backgrounds seemed useful. Our data show clear strain effects in that SWR/J mice have higher blood pressures than C57Bl/6 mice. On the other hand, significant differences between wild type and A1AR-deficient mice were not detectable in these congenic strains. Furthermore, a high salt intake increased arterial blood pressure in A1AR-deficient mice on a C57Bl/6 background whereas no significant effect could be detected in SWR/J animals. We also used SWR/J congenic animals to assess the prominent phenotype of absence of tubuloglomerular feedback (TGF) previously observed in A1AR-deficient mice with mixed background. We confirmed that A1AR-deficiency is associated with abolished or even reversed TGF responses in these genetically homogeneous mice.
Methods
Animals
Mice with a targeted null mutation of the adora1 gene had been previously generated in our laboratory and maintained in a C57Bl6/129J mixed genetic background (Sun et al., 2001). Breeder animals of this strain were crossed with C57Bl/6 or SWR/J mice (Jackson Laboratories) for 10 generations before use in these studies. Genotyping was done on tail DNA using PCR as described previously. All mice were maintained on a 12 hour light-dark cycle and kept on a standard rodent chow or high or low salt diets, as described below, and tap water. All diets were provided ad libitum. Animal care and experimentation was approved and carried out in accordance with NIH principles and guidelines for the Care and Use of Laboratory Animals.
Telemetry
The telemetry system of Data Sciences International (St Paul, MN) was used for blood pressure determinations. A detailed description of the technique used has been provided in an earlier publication (Kim et al., 2008). After baseline studies were completed, mice were placed on a high (8.0%) and then on a low NaCl diet (0.04%) in random order. All diets were provided for one week prior to telemetric data collection.
Plasma renin
Blood was taken from conscious mice by tail vein puncture and collection into a 75-µl hematocrit tube that contained 1 µl 125 mM EDTA in its tip. Plasma renin concentration was determined as described previously (Kim et al., 2007).
Tubuloglomerular feedback
Wild type and A1AR−/− mice on SWR/J background were anesthetized with 100 mg/kg thiobutabarbital (inactin) intraperitoneally and 100 mg/kg ketamine subcutaneously. Body temperature was maintained at 38.0° C by placing the animals on an operating table with a servo-controlled heating plate. The trachea was cannulated, and a stream of 100 % oxygen was blown towards the tracheal tube throughout the experiment. The left carotid artery was catheterized with hand-drawn polyethylene tubing for continuous measurement of arterial blood pressure. A catheter was also inserted into the right jugular vein for an intravenous maintenance infusion of saline at a rate of 12 µl/g body weight and hour. The bladder was catheterized for urine collections. Measurements of stop flow pressure (PSF) during perfusion of the loop of Henle were done as described previously (Schnermann et al., 1997, Wright and Schnermann, 1974). When PSF had stabilized, perfusion rate of loop of Henle was increased to 30 nl/min and maximum PSF responses were determined. Two such responses to a saturating flow increase were recorded in each nephron. The perfusion fluid contained (in mM/L) 136 NaCl, 4 NaHCO3, 4 KCl, 2 CaCl2, 7.5 urea and 100 mg/100 ml FD&C green (Keystone, Bellefonte, PA).
Statistics
Data are expressed as means ± SE. Statistical comparisons between WT and A1AR−/− mice were done by paired and unpaired student’s t -test for comparisons of MAP, SAP, DAP, heart rate, pulse pressure and mean activity. P values < 0.05 were considered to indicate a significant difference.
Results
Blood pressure
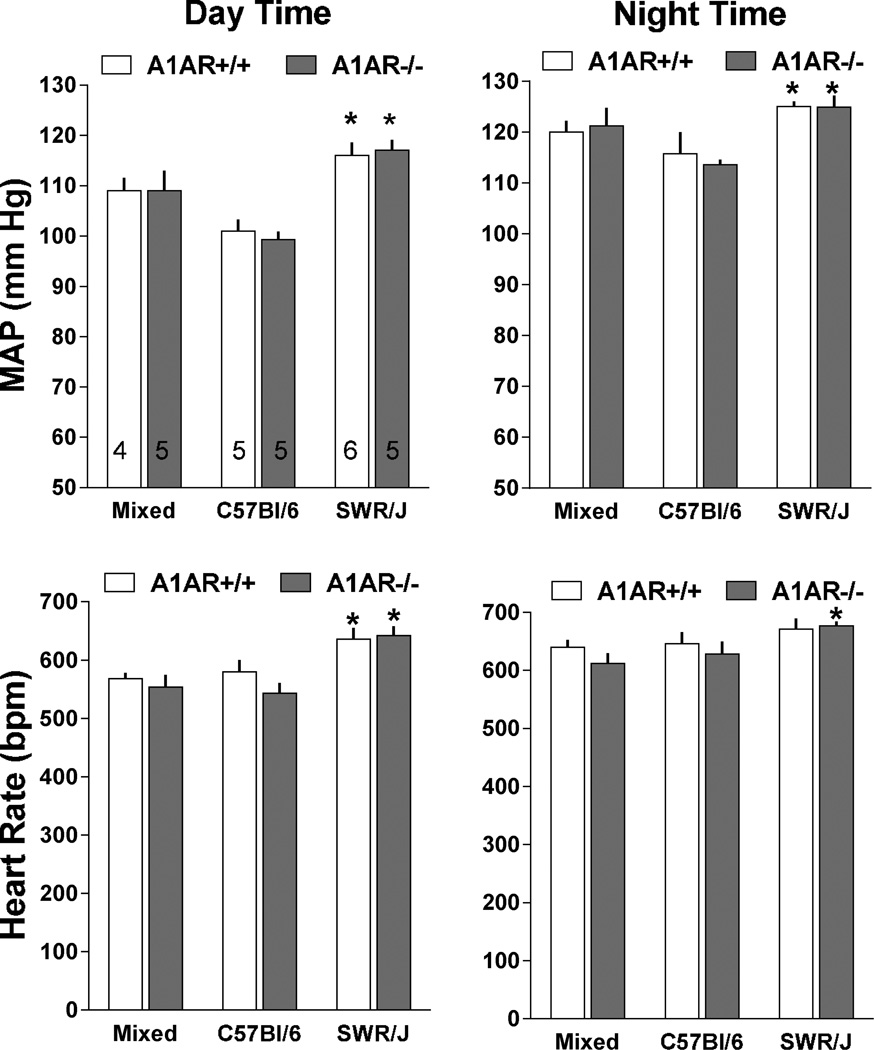
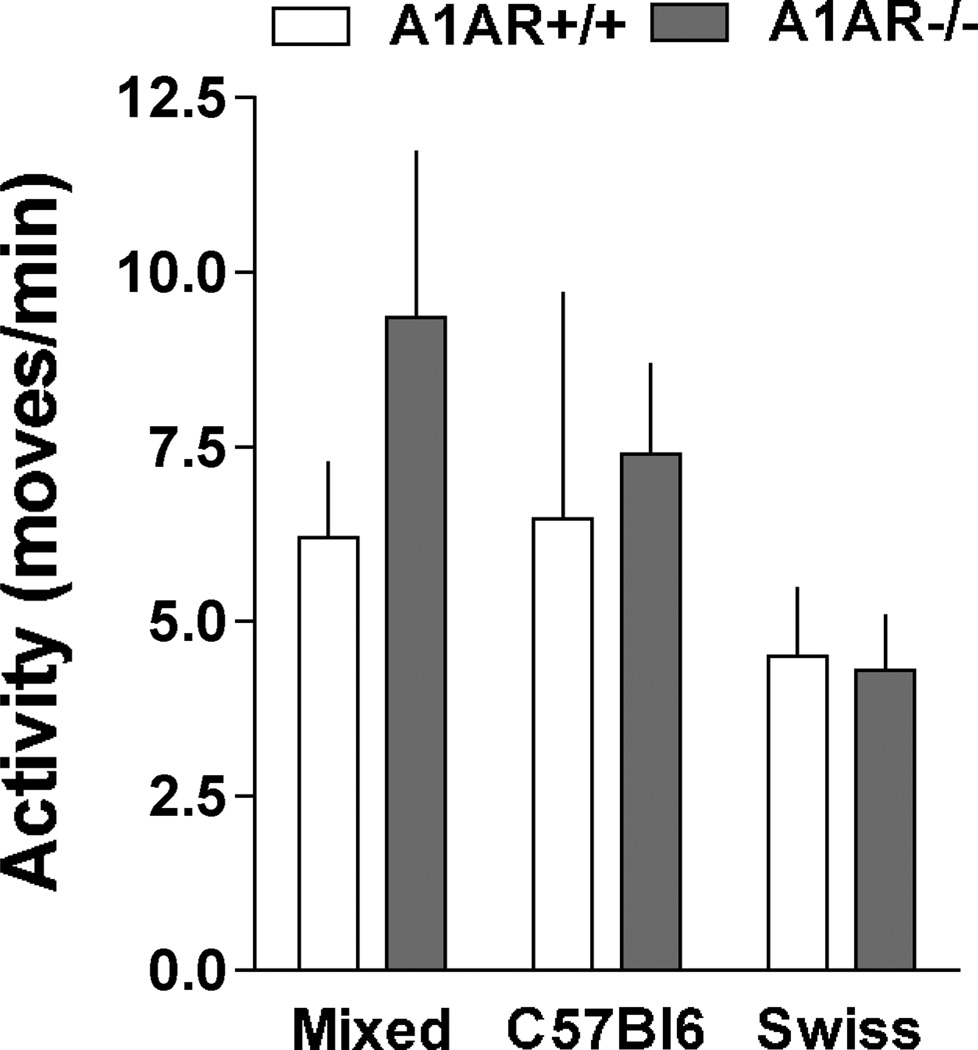
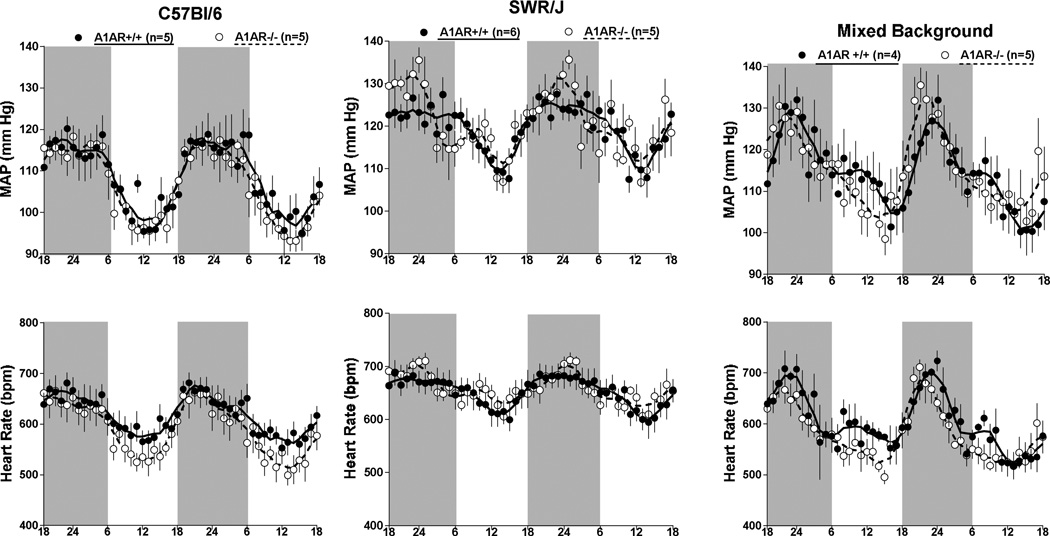
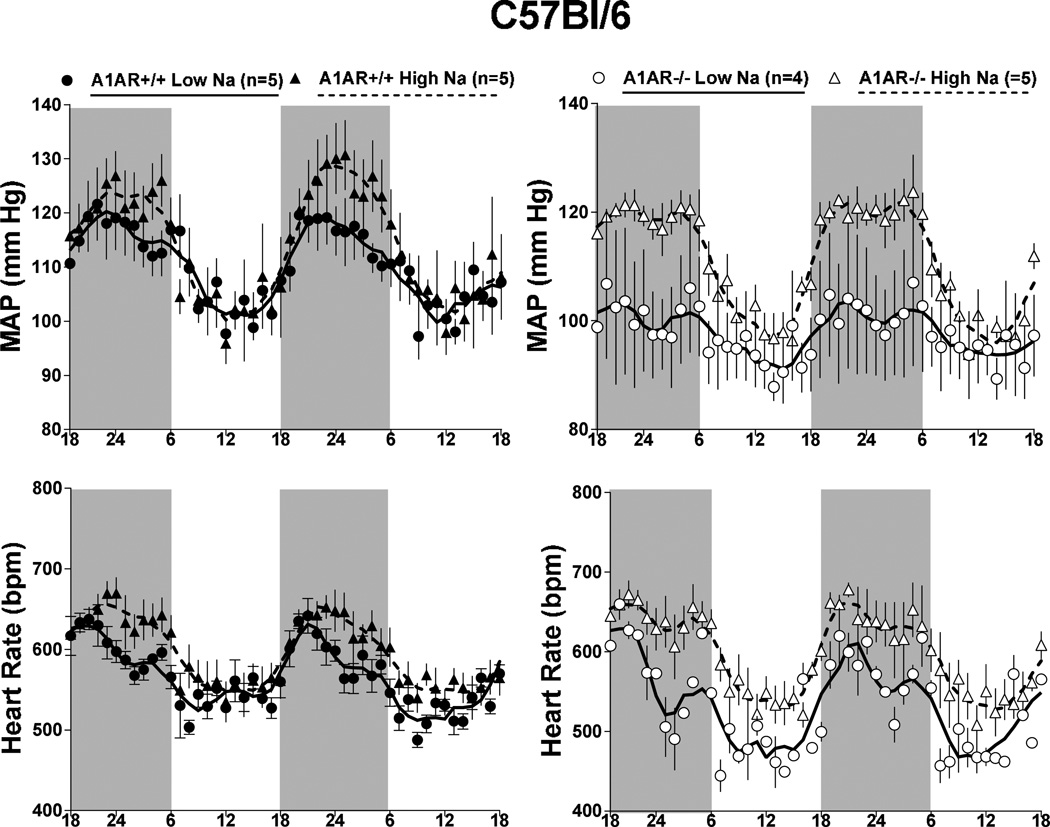
Measurements of mean arterial blood pressure (MAP) and heart rate by radiotelemetry in wild type and A1AR-deficient mice with mixed (n=4 and 5), C57Bl/6 (n=5 and 5), or SWR/J backgrounds (n=6 and 5) are shown in Fig. 1. No detectable differences of MAP (top) or heart rate (bottom) were observed between wild type (open bars) and A1AR-deficient animals (grey bars) during either day (left) or night time (right). However, MAP and HR were significantly higher in SWR/J than in C57Bl/6 mice during both day and night time periods regardless of the A1AR genotype. MAP and HR in mixed background mice were not significantly different from either C57Bl/6 or SWR/J mice. There was a tendency for a higher locomotor activity in A1AR-deficient mice on mixed and C57Bl/6 background, but this difference did not reach statistical significance (Fig. 2). Locomotor activities in SWR/J mice were not higher than in the other strains and therefore cannot explain the blood pressure difference. The presence of circadian variations of MAP and heart rate was not affected by genetic background or deletion of A1AR as shown in Fig. 3. Nevertheless, the pattern of rhythmicity showed a higher degree of complexity in SWR/J mice. Furthermore, in both A1AR+/+ and A1AR−/− genotypes, the amplitude of the night/day difference for MAP and especially for heart rate in SWR/J animals (A1AR+/+: 8.9 ± 2 mm Hg and 35 ± 7.8 bpm; A1AR−/−: 7.8 ± 3.7 mm Hg and 34.7 ± 16 bpm) was only about half that seen in C57Bl/6 (A1AR+/+: 14.7 ± 3.3 mm Hg and 67 ± 8.5 bpm; A1AR−/−: 14.3 ± 1.6 mm Hg and 85 ± 8.5 bpm) or mixed background mice.
Figure 1.
Mean arterial blood pressure (MAP) and heart rate averaged for 12 hour day time (left) and 12 hour night periods (right) over 3 days in A1AR+/+ (open bars) and A1AR−/− mice (shaded bars) on mixed, C57Bl/6, and SWR/J genetic backgrounds. Animal numbers are given in the upper left hand graph. Male to female gender distribution in A1AR+/+ was 4 : 1, 5 : 1, and 4 : 0 in C57Bl/6, SWR/J, and mixed strains; in A1AR−/− it was 4 : 1, 4 : 1, and 3 : 2. Values are means ± S.E.
*P< 0.05 (comparing different strains of same A1AR genotype)
Figure 2.
Mean 24 hour locomotor activity measured by telemetry in A1AR+/+ (open bars) and A1AR−/− mice (shaded bars) on mixed, C57Bl/6, and SWR/J genetic backgrounds.
Figure 3.
Telemetric recordings of mean arterial blood pressure (MAP) and heart rate in A1AR+/+ (black dots) and A1AR−/− mice (open circles) on C57Bl/6 (left), SWR/J (center), and mixed genetic backgrounds (right). Data are shown for two consecutive days on 12:12-h light/dark cycles (dark periods from 18 h to 6 h indicated by shaded areas). Lines represent smoothed data using the weighted average of the nearest 9 points.
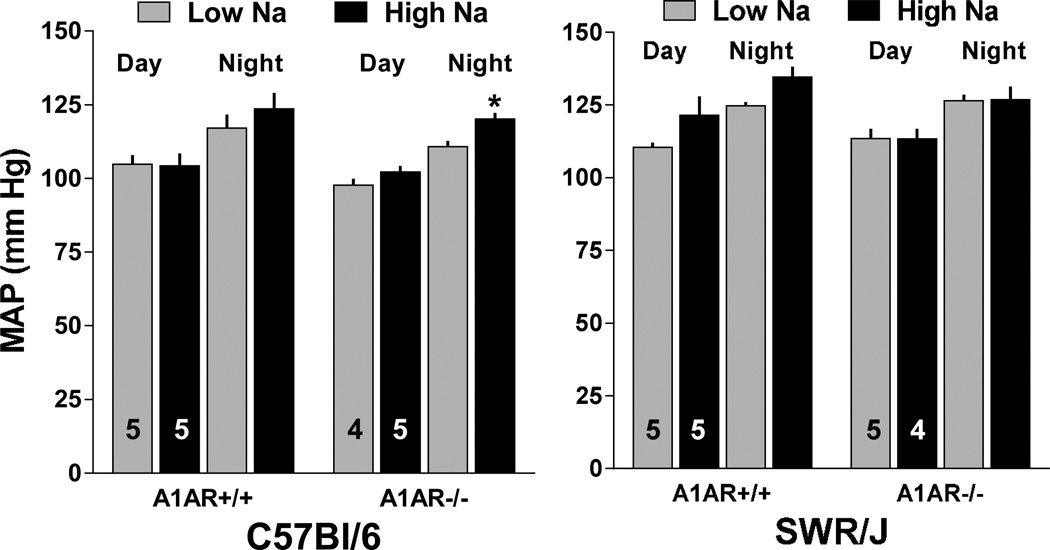
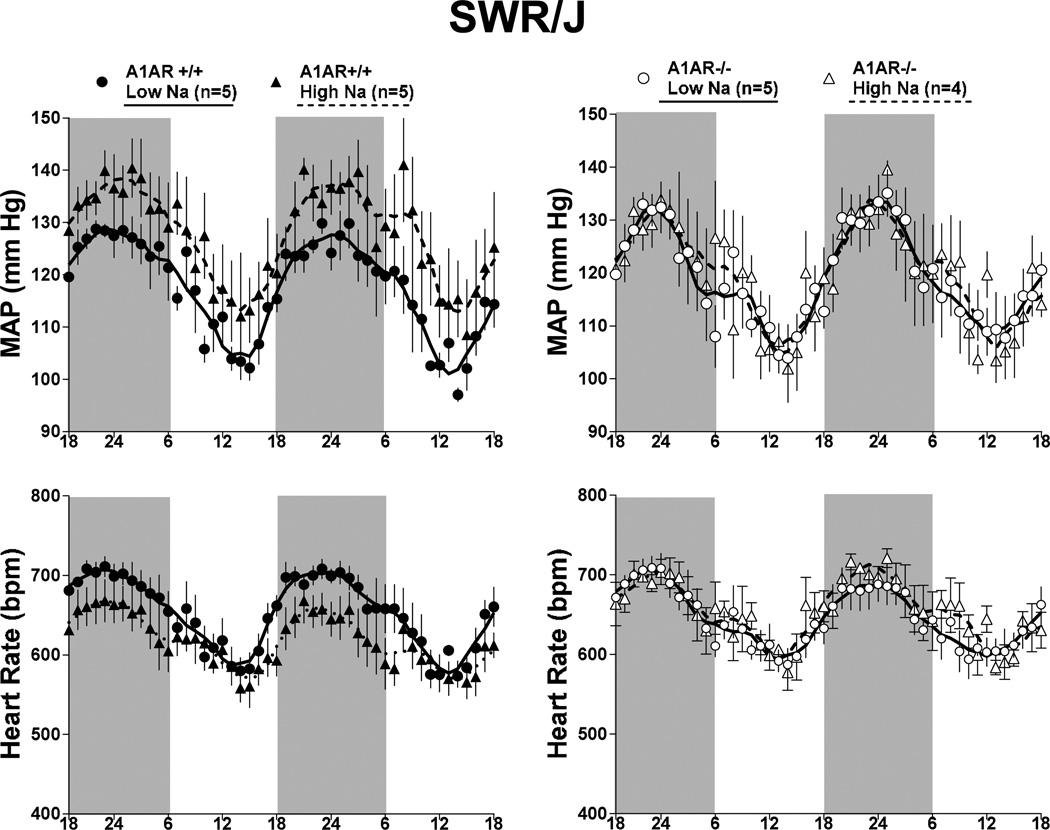
The effects of a one week treatment with low or high Na intake on arterial blood pressure in wild type and A1AR−/− mice with C57Bl/6 or SWR/J background are summarized in Fig. 4. Compared to mice on standard diet (Figs. 1 and 3), reducing salt intake had no effect on MAP and HR in any of the mice studied. Elevating salt intake was associated with a tendency for MAP to increase in both C57Bl/6 and SWR/J wild type mice that did not reach statistical significance. However, a high salt intake increased MAP significantly in the A1AR-deficient mice with C57Bl/6 background, whereas A1AR-deficient mice in the SWR/J background did not show any salt-dependence of blood pressure. Circadian rhythmicity of blood pressure and heart rate in A1AR+/+ and A1AR−/− mice subjected to changes in salt intake are shown in Fig. 5 for C57Bl/6 mice and in Fig. 6 for SWR/J mice. Salt-induced increases in blood pressure and heart rate in the C57Bl/6 strain are apparent in A1AR+/+ at night and in A1AR−/− mice during both day and night. In contrast, in the SWR/J background, the A1AR−/− genotype was not associated with differences in blood pressure or heart rate related to low or high salt treatment.
Figure 4.
Effect of a 1 week treatment with low (0.03%; shaded bars) and high (8%; black bars) NaCl containing diet on mean arterial blood pressure (MAP) averaged for 12 h day time and 12 h night time periods in A1AR+/+ and A1AR−/− mice on C57Bl/6 (left) and SWR/J (right) genetic backgrounds. Numbers indicate animals used and values are means ± S.E.
* p<0.05 comparing low and high Na diets
Figure 5.
Telemetric recordings of mean arterial pressure (MAP, top) and heart rates (bottom) in A1AR+/+ (left, closed symbols) and A1AR−/− mice (right, open symbols) on C57Bl/6 genetic background. Mice received either a low (circles) or high Na diet (triangles). Data are shown for two consecutive days on 12:12-h light/dark cycles (dark periods from 18 h to 6 h indicated by shaded areas). Lines represent smoothed data using the weighted average of the nearest 9 points.
Figure 6.
Telemetric recordings of mean arterial pressure (MAP, top) and heart rates (bottom) in A1AR+/+ (left, closed symbols) and A1AR−/− mice (right, open symbols) on SWR/J genetic background. Mice received either a low (circles) or high Na diet (triangles). Data are shown for two consecutive days on 12:12-h light/dark cycles (dark periods from 18 h to 6 h indicated by shaded areas). Lines represent smoothed data using the weighted average of the nearest 9 points.
Renin
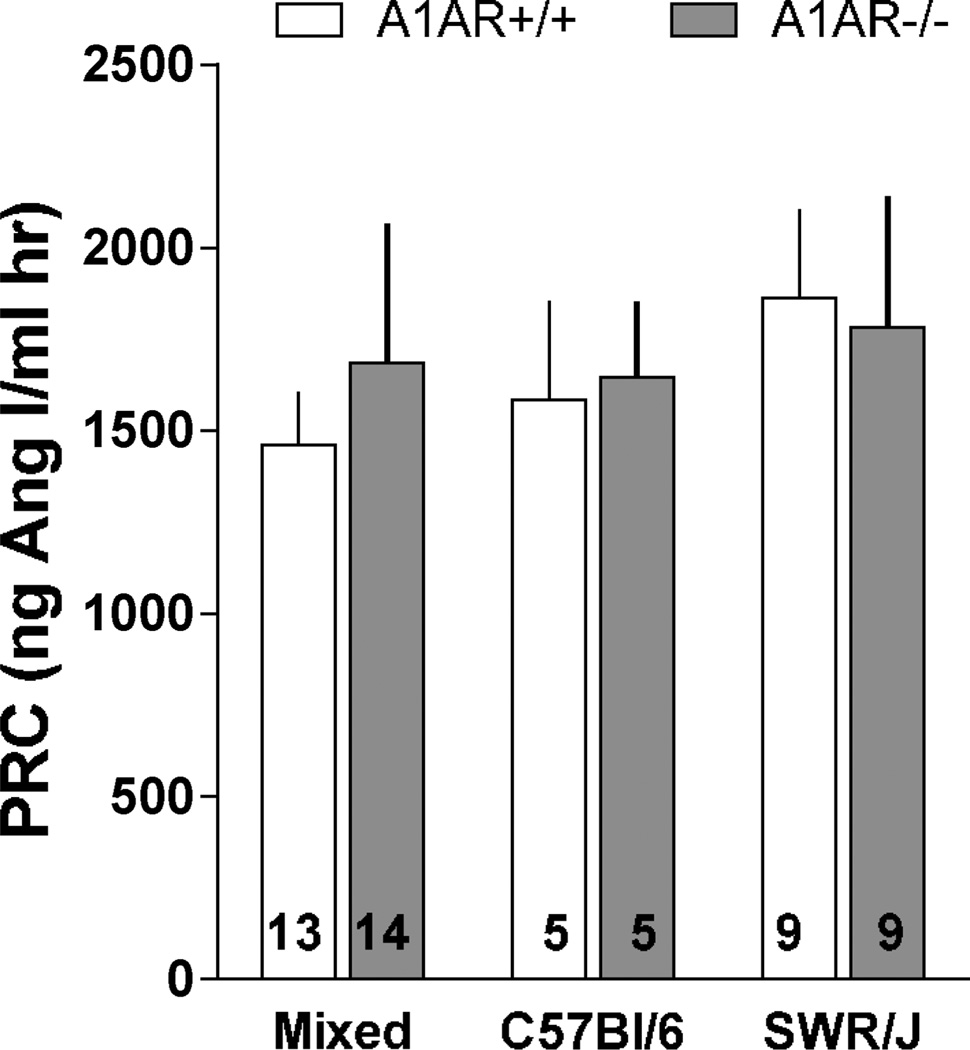
As shown in the data summary of Fig. 7, no significant differences were detectable in average levels of plasma renin concentration, either between genetic backgrounds or A1AR genotypes. Large inter-individual variability, especially in A1AR−/− mice, would require larger numbers of observations to achieve significance. In addition, all of the C57Bl/6 mice and 7 out of the 9 animals in the SWR/J strain were female. Thus, gender dimorphism cannot be excluded although no significant PRC difference between male and female mice were observed in the mixed background animals in which about of the mice were female and half were male.
Figure 7.
Plasma renin concentration (PRC) in A1AR+/+ (open bars) and A1AR−/− mice (closed bars) on mixed (left; 6 female and 7 or 8 male), C57Bl/6 (middle; all female), and SWR/J (right; 7 female and 2 male) genetic backgrounds. Numbers indicate numbers of animals. Values are means ± S.E.
TGF
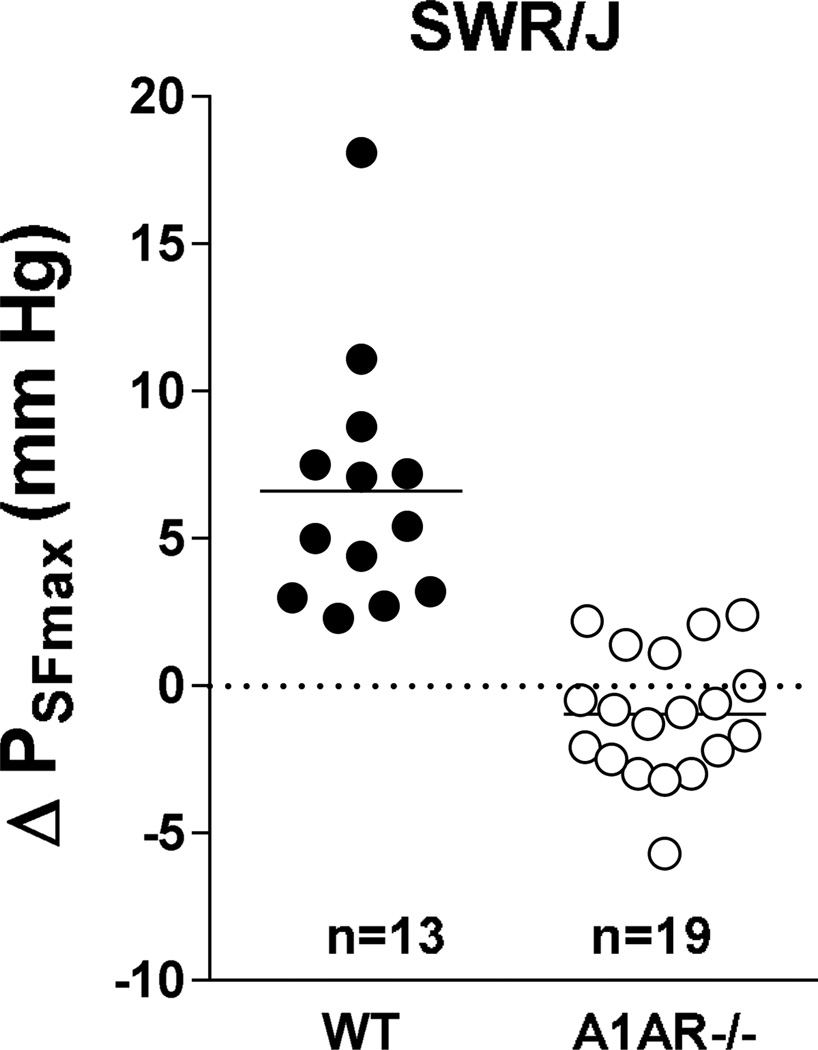
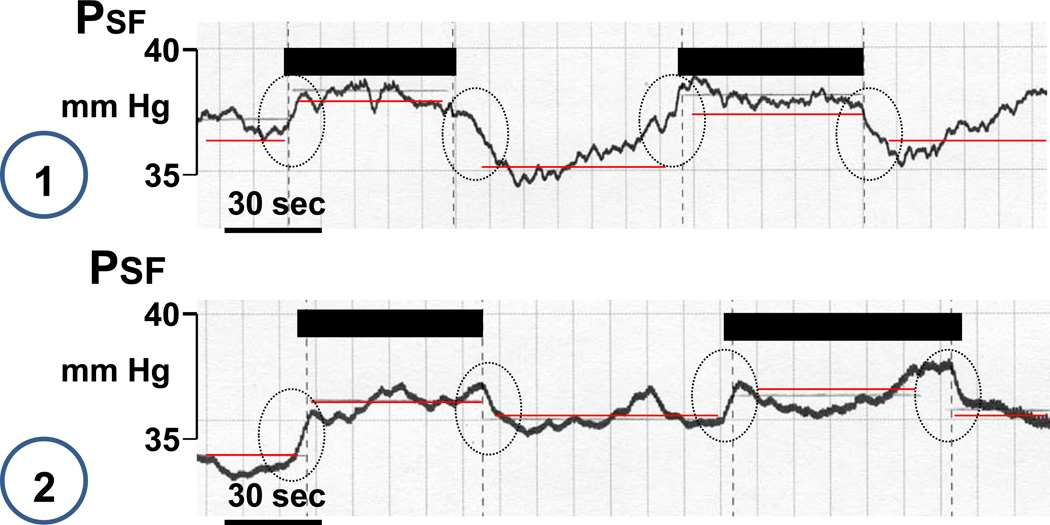
Measurements of changes in stop flow pressure (PSF) in response to an increase in loop of Henle flow rate from 0 to 30 nl/min were done in male SWR/J mice and data are summarized in Fig. 8. PSF fell by 6.6 ± 1.2 nl/min from 38.8 ±1.35 mm Hg to 32.2 ± 0.9 mm Hg in wild type animals (p=0.00014; n=13 tubules, 3 mice). In contrast, there was no significant change in A1AR−/− animals with PSF being 31.03 ± 1.3 mm Hg at low and 31.99 ± 1.3 mm Hg at high flow (p=0.067; n=19 tubules, 5 mice). In 13 of 19 tubules studied PSF actually increased with increases in flow. Two examples of such reversed TGF responses are shown in Fig. 9. Mean arterial blood pressure during micropuncture was lower in the A1AR-deficient (71.8 ± 2.8 mm Hg) than in wild type mice (94.7 ± 4.1 mm Hg; p < 0.001).
Figure 8.
Change in tubular stop flow pressure (PSF) in A1AR+/+ (WT) and A1AR−/− mice on SWR/J genetic background in response to a saturating increase in loop of Henle flow rate. Numbers are numbers of tubules.
Figure 9.
Original recordings of stop flow pressure (PSF) showing two examples of inverted responses to a saturating increase in loop of Henle perfusion rate. Periods of perfusion are indicated by black bars and stippled vertical lines. Horizontal red lines indicate mean PSF for periods without and with perfusion.
Discussion
The present study was performed to assess the effects of A1AR gene deletion on blood pressure and heart rate in mice with different genetic backgrounds. While we observed differences between C57Bl/6 and SWR/J mice, we were unable to replicate A1AR-specific cardiovascular phenotypes in any of our knockout strains. The absence of the tubuloglomerular regulatory system in A1AR-deficient mice was confirmed in SWR/J congenic animals.
The present results are largely in agreement with some earlier studies in mixed background mice in which absence of A1AR did not affect arterial blood pressure to a detectable extent (Kim et al., 2006, Lee et al., 2012). This observation is also concordant with earlier results showing that acute inhibition of A1AR by receptor-specific drugs did not alter blood pressure in anesthetized rats (Munger and Jackson, 1994, Wilcox et al., 1999, Patinha et al., 2013). It is noteworthy that no significant differences in heart rates could be detected between wild type and A1AR-deficient mice. This finding is somewhat unexpected because A1AR activation by acute administration of agonists exerts clear negative chronotropic and dromotropic effects resulting in bradycardia (Mustafa et al., 2009). The lack of the expected acceleration of heart rates in any of our A1AR-deficient strains indicates that A1AR in pacemaker cells and cardiac conductive tissue do not contribute measurably to heart rate under baseline conditions in awake mice. This is consonant with the notion that clear adenosine effects are most commonly seen in conditions of tissue damage and distress in which endogenous adenosine levels are increased (Fredholm, 2007). In contrast to the present results, A1AR-deficiency was associated with an increase in blood pressure by 7–10 mm Hg in an independently generated strain of targeted A1AR null mice with both mixed and C57Bl/6 backgrounds (Brown et al., 2006, Gao et al., 2011). The use of a different construct for recombination may have led to a different set of flanking genes with blood pressure modifying effects. While this cannot be excluded, we believe that the detection of a blood pressure difference as small as 10 mm Hg or less will always be a challenge because blood pressure in mice of the same genotype shows considerable individual differences. For example, statistical comparison of the hourly values from 3 days (n=72 per mouse) by analysis of variance revealed that blood pressure in four of the five C57Bl/6 wild type mice was significantly different from two others while one of the mice was different from all others. In order to obtain sufficient statistical power a larger number of animals than used in our and most other studies would be required. In contrast to the A1AR genotype clear differences in MAP and heart rate exist between C57Bl/6 and SWR/J mice. The reasons for this are not known, but our data agree with earlier observations of higher blood pressures in mice with SWR/J background (Tsukahara et al., 2004, DiPetrillo et al., 2004).
Our studies of the effect of salt intake on blood pressure are not entirely conclusive. Although wild type mice in both C57Bl/6 and SWR/J backgrounds show an elevation of MAP by about 6 mm Hg when comparing low and high salt intakes, this tendency did not satisfy accepted levels of statistical probability. A similar ambiguity can be seen in previous studies. For example, an elevation in salt intake in C57Bl/6 mice has been reported in some studies (Sugiyama et al., 2001, Escano et al., 2009, Staehr et al., 2013, Heringer-Walther et al., 2012) whereas no effect was seen in others (Mangrum et al., 2002, Tallam et al., 2005, Kopkan et al.). We believe that sample sizes in these studies may too small in general to establish true differences when the effect sizes are relatively minor. In A1AR-deficient mice, elevating salt intake caused a significant increase of MAP by about 10 mm Hg in the C57Bl/6 background, but it had no effect in A1AR−/− mice on SWR/J background. One might have expected A1AR deficiency to facilitate salt excretion and thereby to reduce salt sensitivity, both because the absence of A1AR reduces Na reabsorption in the proximal tubule and because it inhibits tubuloglomerular feedback. A less pronounced effect of salt intake on blood pressure changes in A1AR-deficient than wild type mice has indeed been observed by Brown et al. (Brown et al., 2006). Nevertheless, the mechanisms that override the excretion-promoting effects of A1AR deficiency in the C57Bl/6 mice are unclear. Because there is no consistent relation between the A1AR genotype and salt effects on blood pressure, one suspects that some indirect and strain-dependent consequence of the A1AR deficiency creates a salt sensitivity phenotype in the C57Bl/6 background.
Previous experiments have documented that activation of A1AR by adenosine exerts clear inhibitory effects on renin secretion, probably reflecting a direct effect on renal JG cells (Vallon et al., 2006). More recent studies indicate that A1AR contribute critically to the inhibition of renin release caused by elevated arterial pressure and elevated NaCl at the macula densa while the stimulatory pathways activated by reductions of blood pressure or macula densa NaCl were not affected (Kim et al., 2006, Schweda et al., 2005). In general agreement with these observations, A1AR-deficient mice have been reported to have an elevated plasma renin concentration as well as an increased renal renin content (Brown et al., 2006, Schweda et al., 2003). This is a plausible result both because of the lack of A1AR inhibition, and because the natriuretic effect of A1AR deficiency could cause volume depletion and therefore indirectly enhance renin secretion. It is unclear why the present studies have failed to replicate these previous observations. This may be due to discrete differences in experimental and analytical approaches and it emphasizes the complex nature of renin secretion. Nevertheless, absence of an elevation of plasma renin in our studies is consistent with lack of a blood pressure effect since increased plasma renin has been proposed to contribute to the elevated blood pressure in A1AR-deficient mice (Brown et al., 2006). It has been shown previously that A1AR−/− mice with mixed genetic background always carry two renin genes while wild type mice have only one (Schweda et al., 2003). Due to the proximity of the ren and adora1 gene loci and the use of 129J stem cells for recombination, the duplicated renin genes in the 129J DNA are linked to the A1AR null genotype so that A1AR null homozygosity is associated with two renin genes even after multiple crossings into the one renin gene C57Bl/6 background (Schweda et al., 2003, Brown et al., 2006). This is a concrete example of the flanking gene problem that cannot be solved by the congenic breeding strategy. On the other hand, since SWR/J mice have two renin genes, both wild type and A1AR-deficient mice on the SWR/J background possess the duplicated renin gene. It is noteworthy that these differences in the number of renin genes do not seem to affect levels of plasma renin as has been shown earlier (Hansen et al., 2004).
Near complete abolishment of TGF-mediated responses of GFR or glomerular capillary pressure has been a striking observation in A1AR-deficient mice with mixed genetic background (Brown et al., 2001, Sun et al., 2001). The present results confirm this finding in an A1AR-deficient strain congenic for the SWR/J background. Thus, activation of A1AR accompanies the vasoconstriction elicited by elevating NaCl concentration at the macula densa. Recent data suggest that there is also an activation of A2a adenosine receptors during TGF and that this counteracts A1AR-mediated constriction by a concurrent component of afferent arteriolar vasodilatation (Carlstrom et al., 2010). The presence of such an effect could explain the inverted TGF responses observed in the majority of nephrons in the A1AR-deficient strain in the present study since A2a-mediated dilatation would be superimposed on an inhibited constrictor response. Overall, the present and earlier studies from our laboratory indicate that the effects of the TGF-less state created by A1AR deletion on blood pressure regulation and renal salt handling are relatively subtle.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (DK-043408-12).
Footnotes
Conflict of Interest
None of the authors has any conflict of interest to declare.
References
- Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, et al. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1362–R1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- Brown RD, Thoren P, Steege A, Mrowka R, Sallstrom J, Skott O, et al. Influence of the adenosine A1 receptor on blood pressure regulation and renin release. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1324–R1329. doi: 10.1152/ajpregu.00313.2005. [DOI] [PubMed] [Google Scholar]
- Carlstrom M, Wilcox CS, Welch WJ. Adenosine A(2) receptors modulate tubuloglomerular feedback. Am J Physiol Renal Physiol. 2010;299:F412–F417. doi: 10.1152/ajprenal.00211.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPetrillo K, Tsaih SW, Sheehan S, Johns C, Kelmenson P, Gavras H, et al. Genetic analysis of blood pressure in C3H/HeJ and SWR/J mice. Physiol Genomics. 2004;17:215–220. doi: 10.1152/physiolgenomics.00212.2003. [DOI] [PubMed] [Google Scholar]
- Escano CS, Armando I, Wang X, Asico LD, Pascua A, Yang Y, et al. Renal dopaminergic defect in C57Bl/6J mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1660–R1669. doi: 10.1152/ajpregu.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- Gao X, Patzak A, Sendeski M, Scheffer PG, Teerlink T, Sallstrom J, et al. Adenosine A(1)-receptor deficiency diminishes afferent arteriolar and blood pressure responses during nitric oxide inhibition and angiotensin II treatment. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1669–R1681. doi: 10.1152/ajpregu.00268.2011. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- Hackbarth H, Hackbarth D. Genetic analysis of renal function in mice. 1. Glomerular filtration rate and its correlation with body and kidney weight. Lab Anim. 1981;15:267–272. doi: 10.1258/002367781780893731. [DOI] [PubMed] [Google Scholar]
- Hackbarth H, Hackbarth D. Genetic analysis of renal function in mice. 2. Strain differences in clearances of sodium, potassium, osmolar and free water, and their correlations with body and kidney weight. Lab Anim. 1982;16:27–32. doi: 10.1258/002367782780908733. [DOI] [PubMed] [Google Scholar]
- Hansen PB, Yang T, Huang Y, Mizel D, Briggs J, Schnermann J. Plasma renin in mice with one or two renin genes. Acta Physiol Scand. 2004;181:431–437. doi: 10.1111/j.1365-201X.2004.01315.x. [DOI] [PubMed] [Google Scholar]
- Heringer-Walther S, Gembardt F, Perschel FH, Katz N, Schultheiss HP, Walther T. The genetic deletion of Mas abolishes salt induced hypertension in mice. Eur J Pharmacol. 2012;689:147–153. doi: 10.1016/j.ejphar.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc. Nat. Acad. Sci. U.SA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Chen L, Mizel D, Huang YG, Briggs JP, Schnermann J. Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol. 2007;292:F415–F422. doi: 10.1152/ajprenal.00317.2006. [DOI] [PubMed] [Google Scholar]
- Kim SM, Huang Y, Qin Y, Mizel D, Schnermann J, Briggs JP. Persistence of circadian variation in arterial blood pressure in beta1/beta2-adrenergic receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1427–R1434. doi: 10.1152/ajpregu.00074.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Mizel D, Huang YG, Briggs JP, Schnermann J. Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol. 2006;290:F1016–F1023. doi: 10.1152/ajprenal.00367.2005. [DOI] [PubMed] [Google Scholar]
- Kopkan L, Hess A, Huskova Z, Cervenka L, Navar LG, Majid DS. High-salt intake enhances superoxide activity in eNOS knockout mice leading to the development of salt sensitivity. Am J Physiol Renal Physiol. 299:F656–F663. doi: 10.1152/ajprenal.00047.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DL, Bell TD, Bhupatkar J, Solis G, Welch WJ. Adenosine A1-receptor knockout mice have a decreased blood pressure response to low-dose ANG II infusion. Am J Physiol Regul Integr Comp Physiol. 2012;303:R683–R688. doi: 10.1152/ajpregu.00116.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangrum AJ, Gomez RA, Norwood VF. Effects of AT(1A) receptor deletion on blood pressure and sodium excretion during altered dietary salt intake. Am J Physiol Renal Physiol. 2002;283:F447–F453. doi: 10.1152/ajprenal.00259.2001. [DOI] [PubMed] [Google Scholar]
- Munger KA, Jackson EK. Effects of selective A1 receptor blockade on glomerular hemodynamics: involvement of renin-angiotensin system. Am J Physiol. 1994;267:F783–F790. doi: 10.1152/ajprenal.1994.267.5.F783. [DOI] [PubMed] [Google Scholar]
- Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol. 2009:161–188. doi: 10.1007/978-3-540-89615-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patinha D, Fasching A, Pinho D, Albino-Teixeira A, Morato M, Palm F. Angiotensin II contributes to glomerular hyperfiltration in diabetic rats independently of adenosine type I receptors. Am J Physiol Renal Physiol. 2013;304:F614–F622. doi: 10.1152/ajprenal.00285.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnermann JB, Traynor T, Yang T, Huang YG, Oliverio MI, Coffman T, et al. Absence of tubuloglomerular feedback responses in AT1A receptor-deficient mice. Am J Physiol Renal Physiol. 1997;273:F315–F320. doi: 10.1152/ajprenal.1997.273.2.F315. [DOI] [PubMed] [Google Scholar]
- Schweda F, Segerer F, Castrop H, Schnermann J, Kurtz A. Blood pressure-dependent inhibition of Renin secretion requires A1 adenosine receptors. Hypertension. 2005;46:780–786. doi: 10.1161/01.HYP.0000183963.07801.65. [DOI] [PubMed] [Google Scholar]
- Schweda F, Wagner C, Kramer BK, Schnermann J, Kurtz A. Preserved macula densa-dependent renin secretion in A1 adenosine receptor knockout mice. Am J Physiol Renal Physiol. 2003;284:F770–F777. doi: 10.1152/ajprenal.00280.2002. [DOI] [PubMed] [Google Scholar]
- Sigmund CD. Viewpoint: are studies in genetically altered mice out of control? Arterioscler Thromb Vasc Biol. 2000;20:1425–1429. doi: 10.1161/01.atv.20.6.1425. [DOI] [PubMed] [Google Scholar]
- Staehr M, Hansen PB, Madsen K, Vanhoutte PM, Nusing RM, Jensen BL. Deletion of cyclooxygenase-2 in the mouse increases arterial blood pressure with no impairment in renal NO production in response to chronic high salt intake. Am J Physiol Regul Integr Comp Physiol. 2013;304:R899–R907. doi: 10.1152/ajpregu.00103.2012. [DOI] [PubMed] [Google Scholar]
- Sugiyama F, Churchill GA, Higgins DC, Johns C, Makaritsis KP, Gavras H, et al. Concordance of murine quantitative trait loci for salt-induced hypertension with rat and human loci. Genomics. 2001;71:70–77. doi: 10.1006/geno.2000.6401. [DOI] [PubMed] [Google Scholar]
- Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, et al. Mediation of tubuloglomerular feedback by adenosine: Evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46:326–332. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- Tsukahara C, Sugiyama F, Paigen B, Kunita S, Yagami K. Blood pressure in 15 inbred mouse strains and its lack of relation with obesity and insulin resistance in the progeny of an NZO/HILtJ × C3H/HeJ intercross. Mamm Genome. 2004;15:943–950. doi: 10.1007/s00335-004-2411-3. [DOI] [PubMed] [Google Scholar]
- Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- Wilcox CS, Welch WJ, Schreiner GF, Belardinelli L. Natriuretic and diuretic actions of a highly selective adenosine A1 receptor antagonist. J. Am. Soc. Nephrol. 1999;10:714–720. doi: 10.1681/ASN.V104714. [DOI] [PubMed] [Google Scholar]
- Wright FS, Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest. 1974;53:1695–1708. doi: 10.1172/JCI107721. [DOI] [PMC free article] [PubMed] [Google Scholar]