Abstract
The purpose of this study was to conduct a systematic review on the association of food patterns (FPs) and endothelial biomarkers. An electronic literature search from 1990 to 2012 was conducted and reference lists and experts were consulted. Studies without dietary intervention and without language restrictions were considered. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines were employed. Methodological quality was assessed by Strengthening the Reporting of Observational Studies in Epidemiology guidelines. A total of 546 references were identified, of which 8 were finally included. Several FPs were identified. Healthy FPs (abundant in fruits and vegetables) had a beneficial impact on endothelial function as estimated by circulating levels of biomarkers such as C‐reactive protein, soluble intercellular adhesion molecule 1, soluble vascular adhesion molecule 1, and E‐selectin molecules. Westernized patterns (higher intakes of processed meats, sweets, fried foods, and refined grains) were positively associated with inflammation molecules and atherogenic promoters. The study of FPs in relation to endothelial function contributes to the development of dietary recommendations for improved cardiovascular health and therefore a better lifestyle.
According to the Global Burden of Disease Study 2010, the combined global burden of poor diet and physical inactivity is estimated at 10% (ranging from 1.9% in Western Saharan Africa to 27.3% in Eastern Europe). Within the Southern Cone of Latin America (made up of Argentina, Chile, and Uruguay), high body mass index (BMI) was the leading risk factor, accounting for almost 10% of overall disease burden, and physical inactivity and diets low in fruits and vegetables ranked in the top 10 risk factors by attributable burden of disease.1 The nutritional transition, especially in South America, has been accompanied by changes in lifestyle related to increased prevalence of noncommunicable diseases.2, 3 The burden imposed by these more proximal risk factors strongly impacts the growing epidemics of cardiovascular diseases (CVDs) that cause 16.7 million deaths globally each year, 80% of which occur in low‐ and middle‐income countries.4, 5
Diet has been one the most studied factors in relation to the pathogenesis and progression of CVD. Several epidemiological studies conducted in different parts of the world have provided the necessary evidence on the important role of food and have contributed to establishing dietary recommendations for the prevention and treatment of CVD.6, 7, 8, 9 However, food is not only a biological activity, it is a social and cultural phenomenon with multiple determinants: biological, economic, cultural, ecological, technological, social, political, ideological, and religious.10, 11 In this context, this study of alimentary, dietary, or food patterns includes measurement and analysis of the usual consumption of food combinations in individuals and groups, analyzes the diet in a more relevant manner, and replaces the traditional study of nutrients separately.12 In addition, the study of dietary patterns could help to identify the synergy between food and nutrients, which can contribute to detecting associations that studies of a single nutrient cannot provide.
The endothelial system plays an important role in the pathogenesis of CVD. It consists of a series of strong vasoconstrictor peptides and their receptors, as well as different bioactive substances that are released from the endothelial cell. For many years, vascular endothelium has been considered a passive barrier between blood flow and vascular wall. However, it has multiple functions related to blood flow regulation with hormonal and tissue homeostasis.13
Endothelial cells act in response to different physical and chemical stimuli, modifying their morphology or producing substances to keep the vascular homeostasis. Nevertheless, when the normal homeostatic function is altered, endothelial dysfunction occurs, characterized by higher vasoconstriction, reduced vasodilation, and prothrombotic and inflammatory activity.14, 15 Therefore, CVD risk factors such as hypertension, atherosclerosis, and arterial stiffness have endothelial dysfunction as a common denominator. The evidence suggests a causal role for oxidative stress in dysfunction of the endothelium. Reactive oxygen species can directly inactivate nitric oxide, modulate peptide function, and act as cellular signaling molecules, such as nuclear factor kappa‐light‐chain‐enhancer of activated B cells activity, which is involved in the regulation and transcription of a number of genes associated with inflammatory and immune pathways.16 These events contribute to the initiation and progression of endothelial damage.17 Figure 1 shows the relationship between environmental and genetic factors, metabolic markers, and their influence on endothelial function.
Figure 1.
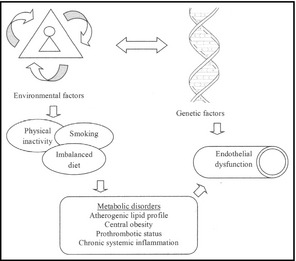
Principal factors involved in endothelial dysfunction.
Despite the advances in nutritional epidemiology during the past decades, the role of diet in the etiology of endothelial function is still poorly understood. Moreover, although several reports have addressed the association of dietary patterns with inflammation,18, 19, 20 few have examined the two related issues at the same time. This systematic review summarizes the findings on the associations of food patterns (FPs) and biomarkers of endothelial function.
Methods
Search Strategy
We conducted a systematic search including data from January 1990 to December 2012 using electronic databases included in MEDLINE, EMBASE, and LILACS. We also used Internet search engines such as Google Scholar and Trip Database. In the computer‐based searches, we combined search terms related to the exposure (eg, food pattern OR diet OR dietary pattern OR dietary habits OR food preferences OR habits) and outcomes of interest (eg, endothelial markers OR endothelium OR cardiovascular diseases OR vascular endothelium OR biomarkers OR endothelial dysfunction OR inflammation OR biological markers). An annotated search strategy for non‐indexed “grey literature” was included to obtain information from relevant sources, such as reports from the Ministries of Health, the World Health Organization, congresses' annals, reference lists of included studies, and consulting experts and associations related to the topic according to a protocol based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement.21
Selection Criteria
We included observational studies, such as cohort studies, case‐control studies, case series, surveillance, and cross‐sectional studies without language restrictions. FP was defined as a comprehensive variable that integrates the consumption of several foods or food groups. The articles reporting food consumption as FPs by the application of multivariate techniques, ie, factor analysis and analysis of multiple correspondences, were included.22, 23 The authors of select articles were contacted to obtain missing or additional information when needed.
Studies with patient enrollment prior to 1980 were excluded, as well as duplicated publications and articles evaluating dietary interventions.
We included studies that reported the association between a posteriori dietary patterns and at least one circulating endothelial dysfunction biomarker. Analytical methodologies were also explored.
Screening and Data Abstraction
Two independent reviewers used a predesigned data abstraction form to extract relevant information from the selected studies. First, they prescreened all identified references and selected potentially eligible studies from their title and abstract that appeared to be eligible for the review. Then, two reviewers independently evaluated full‐text versions of all potentially eligible articles to evaluate whether they met inclusion criteria. Discrepancies were resolved through consensus.
Quality Evaluation Assessment and Risk of Bias
Two independent reviewers evaluated the quality of the included studies. The risk of bias was assessed by using a modified checklist of essential items based on Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.24 This allowed a total score from 0 to 5 points (5 representing the highest quality), with 1 point allocated if a study provided a study rationale; used appropriate selection criteria; employed a validated food questionnaire and assay method; and reported findings adjusted for age, sex, BMI, and smoking status and for other important covariates.
The risk of bias was evaluated considering six criteria (methods for selecting study participants, methods for measuring exposure and outcome variables, and methods to control for confounding, design‐specific sources of bias and comparability among groups, statistical methods, and declaration of conflict interests). Disagreements were solved by consensus.
Because of the observed heterogeneity in methodologies and subject selection from the observational studies, it was not possible to report a meta‐analysis.
Results
The search strategy identified 532 unique references from databases and 14 additional citations from grey literature. After the initial screening, based on titles and abstracts, 144 articles remained for further evaluation. Following detailed assessments, 134 articles were excluded (Figure 2). All pertinent studies identified were published in the English language.
Figure 2.
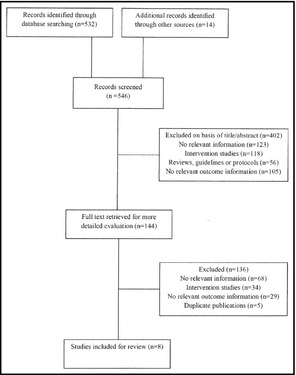
Flow of studies in review.
Eight articles were finally included. Three articles were cross‐sectional cohort studies, three were cohort studies, and two were cross‐sectional studies.
FPs Identification and Endothelial Markers
FP was defined as usual consumption of food combinations in individuals and groups. Dietary information was collected via food frequency questionnaires in seven of eight articles. In addition, factor analysis or principal component analysis were most often used to derive FPs (seven articles). The reported results were adjusted in all cases for potential confounders.
Several FPs were identified. All articles presented some degree of association between endothelial markers and FPs. The Table summarizes the principal characteristics of included studies and the statistical associations. Among 466 men in the Health Professionals Follow‐Up Study (HPFS), the Western Pattern (higher intake of red meats, high‐fat dairy products, and refined grains) was positively correlated with higher concentrations of fasting insulin, C‐peptide, leptin, tissue‐type plasminogen activator antigen, C‐reactive protein (CRP), and homocysteine.25 In 732 women in the Nurses' Health Study I, the Western Pattern (higher intake of red and processed meats, sweets, desserts, French fries, and refined grains) was positively associated with higher levels of CRP, E‐selectin (sSELE), intercellular adhesion molecule 1 (sICAM‐1), vascular cell adhesion molecule 1 (sVCAM‐1), and interleukin 6 (IL‐6). On the other hand, the Prudent Pattern (higher intake of fruit, vegetables, legumes, fish, poultry, and whole grains) was inversely associated with plasma concentrations of CRP and sSELE.26
Table 1.
Food Patterns and Endothelial System: Summary of Data Included in the Systematic Review
| Author | Design | Country | Participants, No. | Age Range, y | Principal Results |
|---|---|---|---|---|---|
| Fung et al25 |
Cross‐sectional cohort Health Professionals Follow‐up Study (HPFS) |
United States | 466 | 40–75 |
Western pattern Positively correlated with fasting insulin (r=0.32, P<.01), C‐peptide (r=0.31, P<.01), leptin (r=0.28, P<.0001), tPA (r=0.19, P<.01), CRP (r=0.22, P<.0001), homocysteine (r=0.23, P<.01) Inversely correlated with plasma folate (r=−0.39, P<.0001) Prudent pattern Positively correlated with lipoprotein(a) (r=0.10, P<.05), plasma folate (r=0.28, P<.0001) Inversely correlated with fasting insulin (r=−0.25, P<.05), homocysteine (r=−0.20, P<.01) Values adjusted by age, smoking status, physical activity, total energy, television watching, and total alcohol |
| Lopez‐Garcia et al26 |
Cross‐sectional cohort Nurses' Health Study I |
United States | 732 | 43–69 |
Western pattern Positively associated with CRP (β=0.10, P<.02), sSELE (β=0.06, P<.001), sICAM‐1 (β=0.03, P<.002), sVCAM‐1 (β=0.02, P=.02), and IL‐6 (β=0.08, P=.006) Prudent pattern Inversely associated with CRP (β=−0.10, P<.02), sSELE (β=−0.05, P=.001) Values were adjusted by age, BMI, physical activity, smoking status, and average alcohol consumption |
| Nettleton et al27 |
Cross‐sectional cohort Multi‐Ethnic Study of Atherosclerosis (MESA) |
United States | 5089 | 45–84 |
Fats and processed meats pattern Positively associated with CRP (β=0.048, P<.05), homocysteine (β=0.016, P<.05), IL‐6 (β=0.050, P<.05) Beans, tomatoes, and refined grains Positively associated with: sICAM‐1 (β=0.021, P<.05) Whole grains and fruit pattern Inversely associated with: CRP (β=−0.060, P<.05), IL‐6 (β=−0.036, P<.05), homocysteine (β=−0.015, P<.05), and sICAM‐1 (β=−0.013, P<.05) Vegetables and fish pattern Inversely related to IL‐6 (β=−0.025, P<.05) Values adjusted by study center, age, sex, race, education, energy, active leisure activity, inactive leisure activity, smoking, and supplement use |
| Esmaillzadeh et al28 | Cross‐sectional | Iran | 486 | 40–60 |
Healthy pattern Inversely associated with CRP (β=−0.05, P=.011) and sVCAM‐1 (β=−0.04, P=.027) Western pattern Positively related to SAA (β=0.06, P=.039) and IL‐6 (β=0.07, P<.001) Traditional pattern No associations were observed Values adjusted by smoking, physical activity, current estrogen use, menopausal status, family history of diabetes and stroke, and energy intake |
| Nanri et al29 | Cohort | Japan | 7802 | 50–74 |
Healthy pattern Inversely related to CRP in women (β=0.33, P=.0004) and men (β=0.39, P=.0006) High‐fat, seafood, and Westernized breakfast patterns No associations were observed Values adjusted by age, BMI, smoking, alcohol consumption, and physical activity |
| Eilat‐Adar et al30 |
Cross‐sectional Genetics of Coronary Artery Disease in Alaska Natives (GODACAN) |
United States | 1066 | 18–92 |
Traditional dietary pattern Positively associated with systolic blood pressure (P=.04) and triglycerides (P<.01) Purchased healthy food pattern Inversely associated with homocysteine (P=.01) and fibrinogen (P=.01) Beverages and sweets food pattern Inversely associated with CRP (P=.04) Western food pattern Positively associated with homocysteine (P=.02) Valued adjusted by age, sex, BMI, MET, smoking and drinking status, education level, total energy intake, cancer status, baseline CVD, hypertension, and cholesterol medication use |
| Hlebowicz et al31 |
Cohort Malmo Diet and Cancer Cohort Study (MDC) |
Sweden | 4999 | 45–73 |
Low fat and high fiber pattern Inversely associated with Lp‐PLA(2) activity in men (OR, 0.62; P=.036) and in women (OR, 0.69; P=.002) Sweets and cakes dietary pattern Positively associated with Lp‐PLA(2) in women (OR, 1.29; P=.030) Milk fat pattern Positively associated with Lp‐PLA(2) mass in men (OR, 1.50; P=.011) Many foods and drinks, fiber bread, and white bread dietary patterns No associations were observed Values adjusted by age, sex, BMI, physical activity, smoking and drinking status, education level, total energy intake, cancer status, baseline CVD, hypertension, and cholesterol medication use |
| Nanri et al32 |
Cohort Japan Multi‐Institutional Collaborative Cohort Study (J‐MICC) |
Japan | 9545 | 40–69 |
Healthy pattern Inversely associated with CRP in men (P=.01) and women (P<.01) Bread pattern Inversely associated with CRP in men (P=.04) and women (P<.01) Dessert pattern Inversely associated with CRP in men (P<.01) Seafood pattern Positively associated with CRP in men (P=.02) Western pattern Positively associated with CRP in women (P<.01) Values adjusted by age, alcohol consumption, smoking, and physical activity level |
Abbreviations: CRP, C‐reactive protein; IL‐6, interleukin 6; Lp‐PLA(2), lipoprotein‐associated phospholipase A2; MET, metabolic equivalent; OR, odds ratio; SAA, serum amyloid A; sICAM‐1, intercellular adhesion molecule 1; sSELE, E‐selectin; sVCAM‐1, vascular cell adhesion molecule 1; TNF‐αRII, tumor necrosis factor‐α receptor II; tPA, tissue‐type plasminogen activator antigen; BMI, Body‐Mass‐Index; CVD, cardiovascular disease.
In 5089 participants in the Multi‐Ethnic Study of Atherosclerosis (MESA) trial, a Fats and Processed Meats Pattern (fats, oils, processed meats, fried potatoes, salty snacks, and desserts) was positively associated with higher concentrations of CRP, homocysteine, and IL‐6. In the same study, the Beans, Tomatoes, and Refined Grains Pattern (beans, tomatoes, refined grains, and high‐fat dairy products) was positively associated with sICAM‐1.27 Moreover, the Whole Grains and Fruit Pattern (whole grains, fruit, nuts, and green leafy vegetables) showed an inverse association with circulating levels of CRP, IL‐6, homocysteine, and sICAM‐1, and the Vegetables and Fish Pattern (fish and dark yellow, cruciferous, and other vegetables) was inversely related to CRP and sSELE. In one study carried out in 486 Iranian women, a Healthy Pattern (high in fruits, vegetables, tomato, poultry, legumes, tea, fruit juices, and whole grains) was inversely associated with CRP and sVCAM‐1 concentrations, whereas the Western Pattern showed a positive association with serum amyloid A and IL‐6.28 In another study, conducted in 7802 Japanese individuals, an inverse association with CRP levels in participants with Healthy Pattern (high intakes of vegetables, fruit, soy products, and fish) was observed.29
Among 1066 participants in the Genetics of Coronary Artery Disease in Alaska Natives (GOCADAN) study, homocysteine levels were inversely associated with the Purchased Healthy Food Pattern (dry beans, store‐bought fruits, vegetables, lettuce salad, dark bread, hot cereal, store‐bought nonhydrogenated vegetable fats, peanut butter, milk, cheese, ice cream, nondairy creamer, and lower‐fat milks). On the other hand, homocysteine values were positive associated with the Western Food Pattern (scored high in store‐bought meats, chicken, fatty meats, snack chips, pizza, fried potatoes, soda pop, milk, cheese, ice cream, nondairy creamer, stew with mostly meat, and stew with mostly rice or noodles). In the same study, participants with Beverages and Sweets Food Pattern (coffee, tea, candy bars, sugar, syrup, eggs, store‐bought animal fats, and pancakes) showed an inverse association with CRP concentrations.30
In 4999 participants in the Malmo Diet and Cancer Cohort Study, the Low‐Fat and High‐Fiber Pattern (fruit, low‐fat milk, high‐fat and low‐fat meats, sweets, fiber‐rich bread) was inversely associated with lipoprotein‐associated phospholipase A2. In contrast, the Sweets and Cakes Dietary Pattern (sugar, sweets, jam, cakes, biscuits, and soft drinks) and Milk Fat Pattern (butter, cheese, and whole milk) were positively associated with higher levels of this marker.31
The last included study was carried out in 9545 participants in the Japan Multi‐Institutional Collaborative Cohort Study. The Healthy Pattern (high in vegetables and fruit), Bread Pattern (high in bread and low in rice), and Dessert Pattern (confections and fruit) showed an inverse association with CRP levels. Otherwise, the Seafood Pattern (high in shellfish, squid, and fish) and Western Pattern (high in meat and fried foods) were related to higher concentrations of CRP.32
Table SI reports the scientific evidence of the analyzed biomarkers and endothelial function.
Discussion
This study represents the first systematic review of FPs and endothelial function, synthesizing data from observational studies from 1990 to 2012. The review provides convincing evidence of the beneficial or deleterious influence of FPs on endothelial damage evaluated via the analysis of several well‐known serum inflammatory‐related markers.
In the past decades, nutritional epidemiology has shown a growing interest in studies of FPs, and a vast source of literature has been published on the association between diet and chronic disease risk as a relevant area of research to understand the role of diet in modifying disease risk.12, 33 FP analysis has emerged as an alternative and complementary approach to examining the relationship between diet and the risk of chronic diseases. As has been previously mentioned, FPs have shown a relationship with CVD risk and with several markers of endothelial function. However, few studies have examined the association between global diet, assessed through FPs, and the endothelial system.
Clearly, a healthy type of diet characteristically abundant in fruits and vegetables had a beneficial impact on endothelial function as estimated by a decrease in circulating levels of soluble adhesion molecules, such as sSELE, sICAM‐1, and sVCAM‐1; inflammation markers, such as IL‐6 and CRP; and molecular biomarkers of endothelial dysfunction released into the circulation during endothelial injury. On the other hand, Westernized Patterns characterized by higher intakes of red and processed meats, sweets, desserts, fried foods, and refined grains, was positively related to an increase in inflammation molecules, adhesion endothelial cells, and atherogenic promoters.26, 27 With regards to the Seafood Pattern, other studies have described an association between fish intake (rich in omega‐3 fatty acids, considered anti‐inflammatory) as part of the traditional Japanese diet with reduced risk of coronary heart disease and primarily nonfatal cardiac events.34 Therefore, future studies should focus on identifying synergistic, additive, or antagonistic effects of food and food combination and endothelial health.
Strategically located at the interface between tissue and blood, endothelial cells produce numerous substances that regulate the role of vascular smooth muscle and circulating blood cells. At the early stages of atherosclerosis, endothelial cell activation by several inflammatory stimuli results in the synthesis of adhesion molecules and increases the adherence of monocytes.35 Increased levels of soluble adhesion molecules such as sVCAM‐1 and sSELE, biomarkers of endothelial dysfunction, have been observed in human atherosclerotic lesions and hypertensive patients.36, 37, 38
Cluster and factor analysis use distinct statistical approaches to approximate FPs. In addition, there is growing interest in using dietary quality indices to evaluate the adherence to certain FPs. The findings can vary depending on the methods used to elucidate FPs, because each method is designed to answer a different question. For example, cluster and factor analyses are focused on intake variation and its relationship with risk, whereas index analyses explore the variation of a predefined diet and how it relates to risk.39
Study Limitations
This review has some limitations. First, because of the use of different statistical methodologies, the delimitation of FPs depends on food intake or individuals' characteristics for the population under research. Thus, comparison between studies is hindered by heterogeneity. Second, observational studies are more vulnerable to methodological problems and causality cannot be established by this design. Third, the possibility of change in biomarker values within an individual is relative to the physiological intraindividual fluctuation and not directly related to endothelial function.
Endothelial dysfunction is a common and powerful contributor to CVD. However, lifestyle changes, mainly those associated with dietary changes and increased physical activity may improve endothelial function and produce blood pressure–lowering effects, as those seen with drug treatment. FPs also have the advantage of being practical tools for the dissemination of food guidelines for the population recommendations. Thus, the study of FPs may be considered a valuable alternative to analyze food intake, contributing to the identification and prevention of CVD risk factors.
Conclusions
Major FPs are associated with markers of endothelial dysfunction and inflammation. Furthermore, new dietary studies to pinpoint the causal relationship with endothelial health are warranted.
Acknowledgments and disclosures
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) grant number HHSN268200900029C and SeCyT‐UNC (Universidad Nacional de Córdoba). The authors report no conflicts of interest to disclose.
Supporting information
Table SI. Relationship between analyzed biomarkers and endothelial function.
J Clin Hypertens (Greenwich). 2014;16:907–913. DOI: 10.1111/jch.12431. © 2014 Wiley Periodicals, Inc.
References
- 1. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pou SA, Niclis C, Eynard AR, Díaz Mdel P. Dietary patterns and risk of urinary tract tumors: a multilevel analysis of individuals in rural and urban contexts. Eur J Nutr. 2014;53:1247–1253. [DOI] [PubMed] [Google Scholar]
- 3. Aballay LR, Eynard AR, Díaz Mdel P, et al. Overweight and obesity: a review of their relationship to metabolic syndrome, cardiovascular disease, and cancer in South America. Nutr Rev. 2013;71:168–179. [DOI] [PubMed] [Google Scholar]
- 4. Gersh BJ, Sliwa K, Mayosi BM, Yusuf S. Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J. 2010;31:642–648. [DOI] [PubMed] [Google Scholar]
- 5. Abegunde DO, Mathers CD, Adam T, et al. The burden and costs of chronic diseases in low‐income and middle‐income countries. Lancet. 2007;370:1929–1938. [DOI] [PubMed] [Google Scholar]
- 6. Tzoulaki I, Siontis KC, Ioannidis JP. Prognostic effect size of cardiovascular biomarkers in datasets from observational studies versus randomized trials: meta‐epidemiology study. BMJ. 2011;343:d6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. EUROCISS Working Group . Coronary and cerebrovascular population‐based registers in Europe: are morbidity indicators comparable? Results from the EUROCISS Project. Eur J Public Health. 2003;13(3 Suppl):S55–S60. [PubMed] [Google Scholar]
- 8. Hinderliter AL, Babyak MA, Sherwood A, Blumenthal JA. The DASH diet and insulin sensitivity. Curr Hypertens Rep. 2011;13:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Savica V, Bellinghieri G, Kopple JD. The effect of nutrition on blood pressure. Annu Rev Nutr. 2010;30:365–401. [DOI] [PubMed] [Google Scholar]
- 10. Pérez‐Escamilla R. Acculturation, nutrition, and health disparities in Latinos. Am J Clin Nutr. 2011;93(Suppl):1163S–1167S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luca F, Perry GH, Di Rienzo A. Evolutionary adaptations to dietary changes. Annu Rev Nutr. 2010;30:291–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tucker KL. Dietary patterns, approaches, and multicultural perspective. Appl Physiol Nutr Metab. 2010;35:211–218. [DOI] [PubMed] [Google Scholar]
- 13. Taddei S, Salvetti A. Pathogenetic factors in hypertension. Endothelial factors. Clin Exp Hypertens. 1996;18:323–335. [DOI] [PubMed] [Google Scholar]
- 14. Lurie A. Endothelial dysfunction in adults with obstructive sleep apnea. Adv Cardiol. 2011;46:139–170. [DOI] [PubMed] [Google Scholar]
- 15. Thompson AM, Zhang Y, Tong W, et al. Association of inflammation and endothelial dysfunction with metabolic syndrome, prediabetes and diabetes in adults from Inner Mongolia, China. BMC Endocr Disord. 2011;11:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Defagó MD, Soria EA. Biomarker assessment in nutritional modulation of oxidative stress‐induced cancer development by lipid‐related bioactive molecules. Recent Pat Anticancer Drug Discov. 2010;5:188–196. [DOI] [PubMed] [Google Scholar]
- 17. Briones AM, Touyz RM. Oxidative stress and hypertension: current concepts. Curr Hypertens Rep. 2010;12:135–142. [DOI] [PubMed] [Google Scholar]
- 18. Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(3 Suppl):S5–S78. [DOI] [PubMed] [Google Scholar]
- 19. Ahluwalia N, Andreeva VA, Kesse‐Guyot E, Hercberg S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. 2013;39:99–110. [DOI] [PubMed] [Google Scholar]
- 20. Oude Griep LM, Wang H, Chan Q. Empirically‐derived dietary patterns, diet quality scores, and markers of inflammation and endothelial dysfunction. Curr Nutr Rep. 2013;2:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;62:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schulze MB, Hoffmann K, Kroke A, Boeing H. An approach to construct simplified measures of dietary patterns from exploratory factor analysis. Br J Nutr. 2003;89:409–418. [DOI] [PubMed] [Google Scholar]
- 23. Panagiotakos DB, Pitsavos C, Skoumas Y, Stefanadis C. The association between food patterns and the metabolic syndrome using principal components analysis: The ATTICA Study. J Am Diet Assoc. 2007;107:979–987. [DOI] [PubMed] [Google Scholar]
- 24. von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 25. Fung TT, Rimm EB, Spiegelman D, et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73:61–67. [DOI] [PubMed] [Google Scholar]
- 26. Lopez‐Garcia E, Schulze MB, Fung TT, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029–1035. [DOI] [PubMed] [Google Scholar]
- 27. Nettleton JA, Steffen LM, Mayer‐Davis EJ, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2006;83:1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Esmaillzadeh A, Kimiagar M, Mehrabi Y, et al. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137:992–998. [DOI] [PubMed] [Google Scholar]
- 29. Nanri A, Yoshida D, Yamaji T, et al. Dietary patterns and C‐reactive protein in Japanese men and women. Am J Clin Nutr. 2008;87:1488–1496. [DOI] [PubMed] [Google Scholar]
- 30. Eilat‐Adar S, Mete M, Nobmann ED, et al. Dietary patterns are linked to cardiovascular risk factors but not to inflammatory markers in Alaska Eskimos. J Nutr. 2009;139:2322–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hlebowicz J, Persson M, Gullberg B, et al. Food patterns, inflammation markers and incidence of cardiovascular disease: the Malmö Diet and Cancer study. J Intern Med. 2011;270:365–376. [DOI] [PubMed] [Google Scholar]
- 32. Nanri H, Nakamura K, Hara M, et al. Association between dietary pattern and serum C‐reactive protein in Japanese men and women. J Epidemiol. 2011;21:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 34. Iso H, Kobayashi M, Ishihara J, et al. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center‐Based (JPHC) Study Cohort. Circulation. 2006;113:195–202. [DOI] [PubMed] [Google Scholar]
- 35. Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte‐derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. [DOI] [PubMed] [Google Scholar]
- 36. Hagan G, Pepke‐Zaba J. Pulmonary hypertension, nitric oxide and nitric oxide‐releasing compounds. Expert Rev Respir Med. 2011;5:163–171. [DOI] [PubMed] [Google Scholar]
- 37. Lajemi M, Gautier S, Poirier O, et al. Endothelin gene variants and aortic and cardiac structure in never‐treated hypertensives. Am J Hypertens. 2001;14:755–760. [DOI] [PubMed] [Google Scholar]
- 38. Zhang Y, Thompson AM, Tong W, et al. Biomarkers of inflammation and endothelial dysfunction and risk of hypertension among Inner Mongolians in China. J Hypertens. 2010;28:35–40. [DOI] [PubMed] [Google Scholar]
- 39. Reedy J, Wirfält E, Flood A, et al. Comparing 3 dietary pattern methods–cluster analysis, factor analysis, and index analysis–With colorectal cancer risk: The NIH‐AARP Diet and Health Study. Am J Epidemiol. 2010;171:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lozano‐Nuevo JJ, Estrada‐Garcia T, Vargas‐Robles H, et al. Correlation between circulating adhesion molecules and resistin levels in hypertensive type‐2 diabetic patients. Inflamm Allergy Drug Targets. 2011;10:27–31. [DOI] [PubMed] [Google Scholar]
- 41. West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 2011;54:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fouillioux C, Contreras F, Lares M, et al. Metabolic and hemodynamic markers of endothelial dysfunction in patients with hypertension and patients with type 2 diabetes during the cold pressor test. Am J Ther. 2008;15:389–396. [DOI] [PubMed] [Google Scholar]
- 43. Wahren J, Kallas A, Sima AA. The clinical potential of C‐peptide replacement in type 1 diabetes. Diabetes. 2012;61:761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barone Gibbs B, Dobrosielski DA, Bonekamp S, et al. A randomized trial of exercise for blood pressure reduction in type 2 diabetes: effect on flow‐mediated dilation and circulating biomarkers of endothelial function. Atherosclerosis. 2012;224:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lubomirova M, Tzoncheva A, Petrova J, Kiperova B. Homocystein and carotid atherosclerosis in chronic renal failure. Hippokratia. 2007;11:205–209. [PMC free article] [PubMed] [Google Scholar]
- 46. Hoch AZ, Papanek P, Szabo A, et al. Folic acid supplementation improves vascular function in professional dancers with endothelial dysfunction. PM R. 2011;3:1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McDermott MM, Liu K, Ferrucci L, et al. Relation of interleukin‐6 and vascular cellular adhesion molecule‐1 levels to functional decline in patients with lower extremity peripheral arterial disease. Am J Cardiol. 2011;107:1392–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lominadze D, Dean WL, Tyagi SC, Roberts AM. Mechanisms of fibrinogen‐induced microvascular dysfunction during cardiovascular disease. Acta Physiol (Oxf). 2010;198:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang X, Chai H, Wang Z, et al. Serum amyloid A induces endothelial dysfunction in porcine coronary arteries and human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2008;295:2399–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bekci TT, Kayrak M, Kiyici A, et al. The association among lipoprotein‐associated phospholipase A2 levels, total antioxidant capacity and arousal in male patients with OSA. Int J Med Sci. 2011;8:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pircher J, Merkle M, Wörnle M, et al. Prothrombotic effects of tumor necrosis factor alpha in vivo are amplified by the absence of TNF‐alpha receptor subtype 1 and require TNF‐alpha receptor subtype 2. Arthritis Res Ther. 2012;14:R225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Relationship between analyzed biomarkers and endothelial function.


