Abstract
One of the frequent clinical complications that results in billions of dollars in health care costs annually in the United States is acute kidney injury (AKI). Ischemia reperfusion (IR) injury is a major cause AKI. Unfortunately, no effective treatment or preventive measure for AKI exists. With increased surgical complexity coupled with increasing number of elderly, the incidence of AKI is becoming more frequent. Adenosine is a metabolic breakdown product of adenosine triphosphate (ATP) and contributes to the regulation of multiple physiological events. Extracellular adenosine activates 4 subtypes of adenosine receptors (AR) including A1AR, A2AAR, A2BAR and A3AR. In the kidney, adenosine regulates glomerular filtration rate, vascular tone, renin release and is an integrative part of tubular glomerular feedback signal to the afferent arterioles. In addition, each AR subtype powerfully modulates renal IR injury. The A1AR activation protects against ischemic insult by reducing apoptosis, necrosis, and inflammation. Activation of A2AAR protects against renal injury by modulating leukocyte-mediated inflammation as well as directly reducing renal tubular inflammation. Activation of A2BAR acts via direct activation of renal parenchymal as well as renovascular receptors and is important in kidney preconditioning. Finally, activation of A3AR exacerbates renal damage following renal IR injury while A3AR antagonism attenuates renal damage following ischemic insult. Latest body of research suggests that kidney AR modulation may be a promising approach to treat ischemic AKI. This brief review focuses on the signaling pathways of adenosine in the kidney followed by the role for various AR modulations in protecting against ischemic AKI.
Keywords: Acute kidney injury, acute renal failure, apoptosis, inflammation, necrosis
Renal ischemia reperfusion injury –clinical significance and pathobiology
Acute kidney injury (AKI) involves a complex series of events that are associated with high health care costs and as well as very high mortality and morbidity rates in hospitalized patients (Chertow et al. 2005). Outcomes from AKI are poor without any significant improvements in the past 50 years (Jones & Lee 2008). The incidence of AKI in critically ill patients is increasing in the US with mortality rates approaching 60% (Srisawat et al. 2010). For over a decade, the incidence of kidney injury due to ischemia has been increasing in the United States due to the rise in major surgical procedures in conjunction with an increasing elderly population (Hoste & Kellum 2007; Kellum & Hoste 2008; Hoste et al. 2010). Prognosis from AKI continues to be poor with mortality rates of patients requiring hemodialysis approaching 60% (Hoste & Kellum 2007; Jones & Lee 2008; Kellum & Hoste 2008; Srisawat et al. 2010; Hoste et al. 2010). AKI usually develops into chronic kidney disease and often correlated with severe extrarenal complications including multi-organ dysfunction and sepsis (Jones & Lee 2008; Kellum & Hoste 2008; Hoste et al. 2010). Presently, there are no drugs or therapies for the treatment of AKI. Therefore, therapies to prevent or accelerate renal recovery during and after ischemic AKI are critically needed.
Renal ischemia reperfusion (IR) injury is a leading cause of perioperative AKI (Jones & Lee 2008). Renal IR injury leading to AKI involves a complex series of events orchestrated by renal tubular cells, endothelial cells and leukocytes. The pathophysiology of IR injury involves initial cellular and tissue damage caused by compromised blood flow (ischemia) resulting in tubular and endothelial necrosis and apoptosis (Kusch et al. 2013). Restoration of blood flow (reperfusion) exacerbates renal injury due to the inflammatory response accompanied by chemokines and cytokines derived from renal tubular proximal tubule cells, resident intrarenal leukocytes (e.g., dendritic cells, macrophages) as well as infiltration bone-marrow derived-leukocytes (e.g., neutrophils, lymphocytes) (Kinsey et al. 2008).
Directly related to protection against renal IR injury, studies over the past 2 decades revealed that adenosine via activation of cell surface adenosine receptors (ARs) plays a major role in protection against ischemic AKI (Day et al. 2004; Lee et al. 2004a; Gallos et al. 2005; Kinsey et al. 2012).
Adenosine generation and function in the kidney
Adenosine, a nucleoside comprised of a ribose sugar and adenine base, is generated by all mammalian cells (Vallon & Osswald 2009). Renal adenosine levels accumulate due to increased consumption of renal ATP, hypoxia and impairment of kidney perfusion. During hypoxia or ischemia, high levels of intracellular ATP or adenosine diphosphate (ADP) are released, typically from apoptotic or necrotic cells, into the extracellular milieu (Trautmann 2009). In addition, with increased renal tubular solute transport activity and increased ATP consumption due to increased ATPase activity, adenosine generation is accelerated (Vallon et al. 2006; Vallon & Osswald 2009).
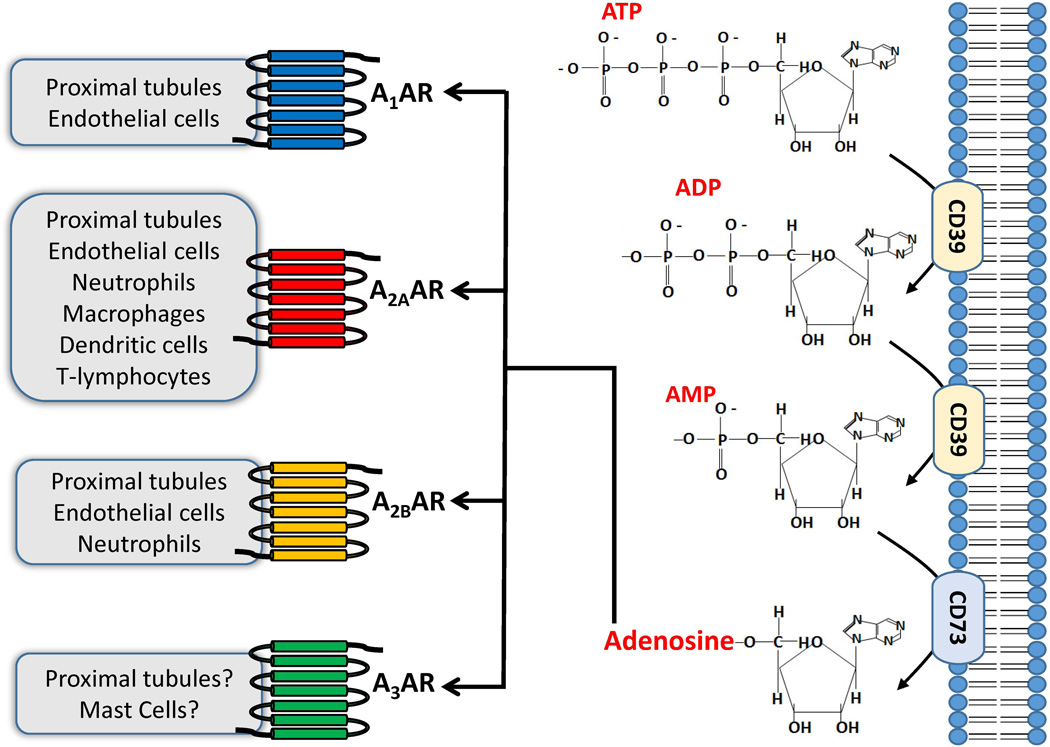
In the extracellular milieu, adenosine is derived from the phosphohydrolysis of adenosine triphosphate (ATP) and adenosine monophosphate (AMP) (Figure 1). Metabolism of ATP and ADP and subsequent adenosine generation is achieved via a 2 step enzymatic reaction. The first step encompasses the enzymatic phosphohydrolysis of ATP and/or ADP by ectonucleoside-triphosphate-diphosphohydrolase-1 (also known as ectopyrase or CD39), which can either elicit the conversion of ATP to ADP and consequently yields adenosine monophosphate (AMP) (Colgan et al. 2006; Grenz et al. 2007). Subsequently, the conversion of AMP to adenosine is accomplished by the surface enzyme ecto-5’-nucleotidase (CD73). CD73 is present in virtually all cell types and particularly in high concentrations of CD73 are present in the kidney. CD73 and adenosine generation serves to protect against inflammatory and ischemic tissue injury (Colgan et al. 2006). Furthermore, activation of CD73 reduces ATP in the extracellular space – a recently recognized danger signal molecule that accelerates cell death and tissue injury (Chen et al. 2006; Trautmann 2009; McDonald et al. 2010). Indeed, recent studies show that ATP not only serves as a robust pro-inflammatory stimulus but also attracts leukocytes to the site of injury (Stagg & Smyth 2010). Consequently, the activation of CD73 may indirectly function as a protective mechanism via the utilization or removal of extracellular ATP in exchange for generating cytoprotective adenosine.
Figure 1.
Renal adenosine production is initiated by cleaving ATP and ADP molecules into AMP by the enzyme ecto-nucleoside-triphosphate-disphosphohydrolase1 (E-NTPDase1 or CD39). Following the cleavage to AMP, dephosphorylation occurs to yield adenosine by ecto-5’-nucleotidase (CD73) which is the rate-limiting step in adenosine synthesis. Adenosine generated then activates 4 distinct purinergic adenosine receptor subtypes (A1AR, A2AAR, A2BAR and A3AR). The A1AR has been demonstrated to protect against renal IR injury by targeting kidney proximal tubules and endothelial cells. The A2AAR modulates ischemic AKI by targeting both renal parenchymal cells as well as multiples subtypes of leukocytes including neutrophils, T-lymphocytes including regulatory T-cells, dendritic cells and macrophages. The A2BAR reduces renal IR injury by targeting proximal tubules and endothelial cells as well as modulating TNF-α synthesis from kidney infiltrating neutrophils. The specific cell types(s) targeted by A3AR activation in the kidney is unclear. However, a selective A3AR agonist induces epithelial apoptosis most likely via calcium overload and also induces mast cell degranulation and histamine release to promote the inflammatory response.
Adenosine plays a major physiological role in the kidney by regulating the release of renin, glomerular filtration rate (GFR) and renal vascular tone (Vallon & Osswald 2009). Additionally, adenosine plays a major role in tubular glomerular feedback (TGF). In the kidney, TGF is triggered by changes in NaCl concentration that are detected by the macula densa cells at the end of the thick ascending limb of Henle's loop which in turn triggers a change in the vascular tone of the afferent arteriole of the juxtaglomerular apparatus resulting in subsequent changes in GFR and renin secretion (Osswald et al. 1997). Adenosine is the factor that mediates the TGF response in the macula densa and afferent arterioles and TGF can be inhibited by selective AR antagonists (Osswald et al. 1996; Osswald et al. 1997; Vallon et al. 2006; Vallon & Osswald 2009).
Adenosine receptor signaling and renal IR injury
Extracellular adenosine signals through 4 G-protein coupled purinergic adenosine receptors (ARs) and includes A1AR, A2AAR, A2BAR and A3AR (Bauerle et al. 2011; Yap & Lee 2012) (Figure 1). These G protein-coupled receptors comprise a seven transmembrane domain in which the A1AR and A3AR receptors are coupled with the Gi subunit to inhibit adenylyl cyclase and the cyclic AMP (cAMP) pathway. In contrast, the A2AAR and A2BAR stimulate adenylyl cyclase and cAMP production by coupling with the Gs subunit (Hasko et al. 2008; Vallon & Osswald 2009). Physiological levels of adenosine (10–100nM) can activate the high affinity receptors A1AR, A2AAR and A3AR. In contrast, the low affinity A2BAR receptor is activated upon pathophysiological conditions (e.g., ischemia or hypoxia) where higher adenosine concentrations (>1µM) are generated (Hasko et al. 2008; Vallon & Osswald 2009). During pathophysiological conditions such as ischemia, inflammation and hypoxia, AR expression changes in various areas of the kidney. Thus, recent studies show that adenosine generation and AR modulation have the potential to attenuate renal IR injury, which will be discussed further in this review.
A1AR and renal IR injury
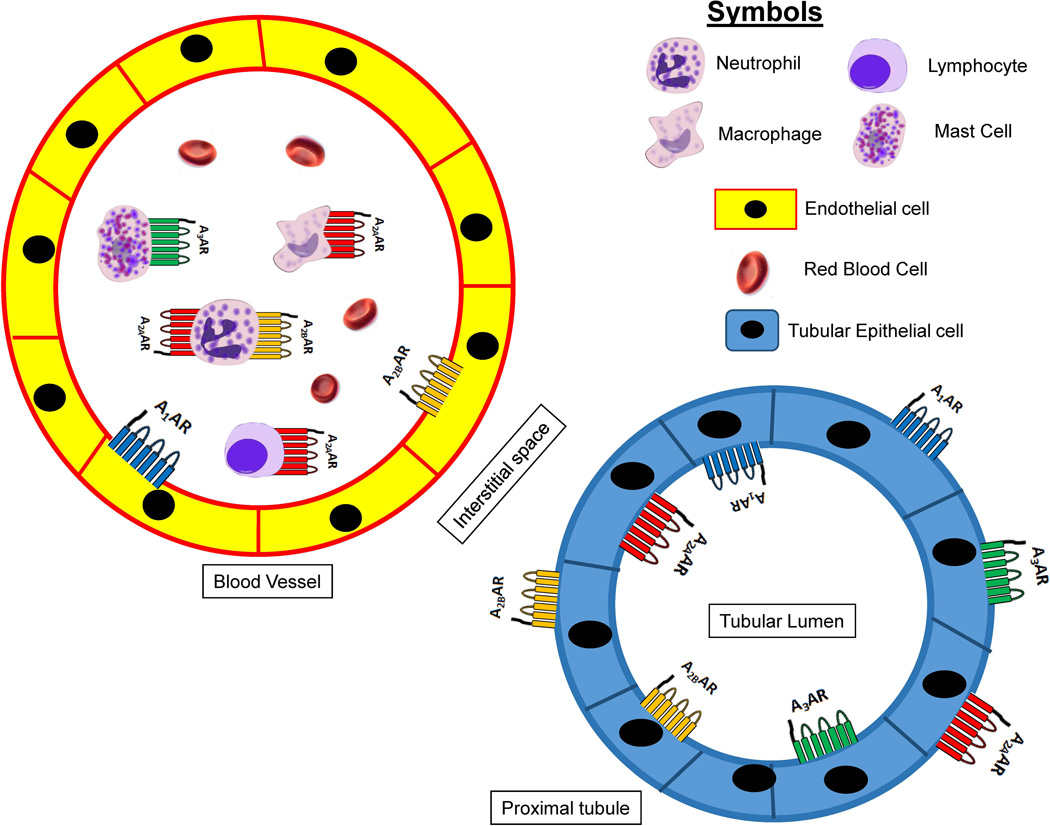
In the kidney, the A1AR is located in the distal afferent arteriole, glomerulus, proximal tubules, mesangial cells and collecting ducts (Freissmuth et al. 1987; Weaver & Reppert 1992; Jackson et al. 2002; Vallon et al. 2006; Vallon & Osswald 2009) (Figure 2). Facilitation of A1AR signaling is achieved via the pertussis toxin-sensitive G-proteins and is coupled to protein kinase C, extracellular signal-regulated protein kinase mitogen-activated protein kinase (ERK MAPK) and Akt pathways (Figure 3) (Joo et al. 2007). The A1AR activation regulates kidney vascular tone, TGF and renin secretion by reducing cAMP levels and by decreasing the activity of protein kinase A (PKA) with resultant increase in intracellular calcium concentration due to decrease in its sequestration (Vallon et al. 2006; Vallon & Osswald 2009). Renal A1AR activation lowers GFR by directly causing arteriolar vasoconstriction and stimulates NaCl, HCO3−, phosphate and fluid reabsorption (Vallon & Osswald 2009; Bauerle et al. 2011).
Figure 2.
Adenosine receptors (ARs) in the kidney mediating cytoprotection. A1AR activation in endothelial and renal tubular cells produces cytoprotection. Neutrophil and lymphocyte A2AAR activation protect against renal injury by reducing inflammation. Renal tubular and endothelial A2BARs protect from ischemic insult by attenuating inflammation and improving blood flow to the kidney, respectively. A2BAR activation in neutrophils also decreases kidney injury after IR by downreguating neutrophil TNF-α synthesis. A3ARs are expressed in various cell types (e.g., epithelial and endothelial cells) in the kidney as well as in mast cells. Selective antagonists of A3AR protect against ischemic AKI.
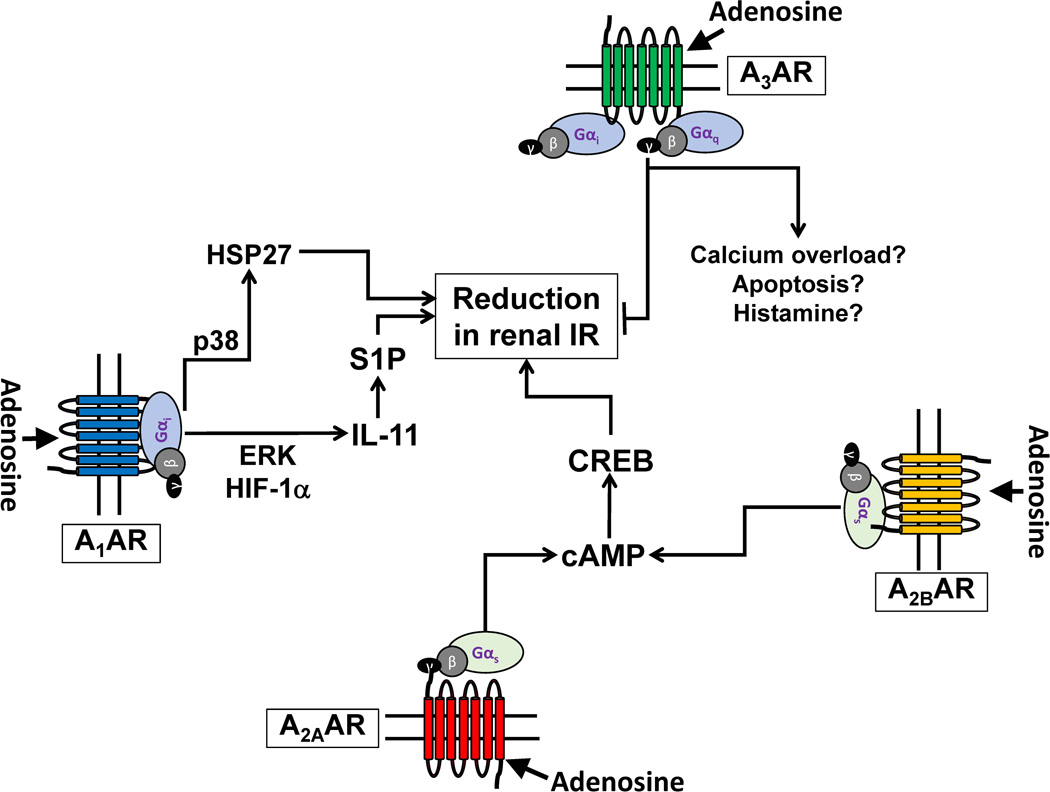
Figure 3.
Summary of mechanisms of adenosine receptor (AR)-mediated modulation of renal IR injury. Activation of A1AR yields Gi-mediated activation of ERK and hypoxia inducible factor 1-alpha to synthesize a cytoprotective cytokine interleukin-11 (IL-11). IL-11 then subsequently induces the synthesis of another cytoprotective molecule sphingosine 1-phosphate (S1P) via upregulation of sphingosine kinase-1 synthesis. The A1AR also phosphorylates and induces cytoprotective heat shock protein 27 (HSP27) synthesis via p38 MAPK activation resulting in decreased renal tubular apoptosis and inflammation. The A2AAR and the A2BAR couple with cholera toxin-sensitive Gs and stimulates adenylyl cyclase, raising cAMP and activating Protein Kinase A (PKA). Following PKA activation, nuclear translocation of cAMP Response-Element Binding (CREB) protein occurs to generate renal protection against IR injury by targeting renal tubules as well as leukocytes. The mechanisms of A3AR activation leading to exacerbation of renal IR injury are still unknown. The A3AR activation may stimulate apoptosis, calcium overload and degranulate mast cells leading to enhanced ischemic AKI.
In addition to its important role in renal physiology by modulating renal hemodynamics and TGF, renal A1AR activation with a selective A1AR agonist 2-chlolo-cyclopentyladenosine (CCPA) protects against renal IR injury by decreasing renal tubular necrosis, apoptosis and the inflammatory response while global genetic deletion of this receptor increases renal injury following IR (Lee et al. 2004a; Lee et al. 2004c). Moreover, pretreatment with a selective A1AR antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX) after renal IR resulted in increased renal dysfunction with marked renal tubular necrosis, inflammation as well as apoptosis.
Previous work also showed that A1AR activation directly protects cultured renal tubules against necrosis as well as apoptosis (Lee et al. 2007a). Furthermore, overexpression of the A1AR in renal tubule cell line protected against peroxide induced necrosis and apoptosis induced by tumor necrosis factor-α (TNF-α) and cycloheximide, via upregulation of total and phosphorylated heat-shock protein 27 (HSP27) expression via p38 MAPK activation. Moreover, proximal tubule cells obtained from A1AR deficient mice exhibited enhanced hydrogen peroxide-induced necrosis and apoptosis compared to proximal tubule cells obtained from wild-type mice (Lee et al. 2007a).
Proximal tubule specific genetic ablation of the A1AR increased renal IR injury suggesting that endogenous activation of the renal proximal tubular A1AR is renoprotective (Kim et al. 2013a). Furthermore, reconstitution of renal A1AR with intrarenal injection of A1AR lentivirus injection resulted in a lower plasma creatinine, indicative of improved renal function, and reduced tubular inflammation shown by reduced leukocyte infiltration and pro-inflammatory cytokine production following renal IR (Kim et al. 2009). Thus, renal tubular A1AR plays a major role in mediating the cytoprotective effects of adenosine in the kidney.
Recent studies suggest a critical role for activation of additional cytoprotective pathways in A1AR-mediated protection against ischemic AKI (Park et al. 2012; Kim et al. 2013a). The A1AR activation results in increased renal tubular interleukin-11 (IL-11) synthesis via ERK MAPK activation (Kim et al. 2013a). Furthermore, renal tubular A1AR activation protects against ischemic AKI via induction of sphingosine kinase-1 (SK-1) synthesis and sphingosine 1-phosphate generation (Park et al. 2012). IL-11 as well as SK1 synthesis was critical for A1AR-mediated renal tubular protection as mice deficient in IL-11 or SK-1 were not protected against ischemic AKI with a specific A1AR agonist. Consistent with these findings, recent studies have shown that IL-11 receptor activation directly induces new SK-1 synthesis to increase cytoprotective S1P generation (Kim et al. 2013a).
Activation of renal A1ARs leads to both acute and delayed protection from renal ischemic insult via separate signaling pathways (Joo et al. 2007). In particular, acute activation of A1AR leads to the phosphorylation of HSP27, Akt, and ERK MAPK to protect against renal IR injury whereas the delayed phase of renal protection with A1AR activation occurs several hours later via induction of new HSP27 synthesis (Joo et al. 2007).
In summary, the A1AR activation protects against ischemic insult by decreasing necrosis, apoptosis and inflammation making it a promising candidate in improving renal function after renal IR injury. Intense efforts have been made to investigate several A1AR agonists and antagonists to demonstrate renoprotective effects in both animal models and in human subjects. Challenge remains; however, as A1AR activation produces wide-ranging pharmacological effects on different organs (bradycardia, sedation, hypotension) potentially limiting their use systemically.
A2AAR and renal IR injury
The location of A2AAR in the kidney includes the vasculature and glomerular epithelium (Bauerle et al. 2011) as well as proximal tubules (Lee & Emala 2002a; Wengert et al. 2005; Jackson et al. 2006). In contrast to the A1AR, A2AAR activation dilates blood vessels in the kidney and stimulates renin release (Okusa et al. 2000; Okusa 2002a) (Vallon & Osswald 2009). In particular, A2AAR activation increases blood flow and oxygenation to the renal medulla while reducing solute transport activity in the medulla (Vallon & Osswald 2009). Consistent with these physiological effects, previous studies have demonstrated that A2AAR activation ameliorates hypoxia or hypoperfusion after ischemic renal injury (Okusa et al. 2000; Day et al. 2003).
The A2AARs are well recognized for their ability to modulate the inflammatory response after renal IR injury. Indeed, A2AAR activation reduces cytokine and chemokine expression in renal tubules cells as well as in leukocytes including macrophages, lymphocytes as well neutrophils (Okusa 2002a; Day et al. 2003; Day et al. 2005). The anti-inflammatory effects of A2AAR activation is a critical component of renal protection with selective A2AAR agonists (Linden 2006; Hasko et al. 2008). In terms of signal transduction, the A2AAR-coupled Gs-mediated stimulation of adenylyl cyclase and PKA results in cAMP response element-binding protein (CREB)-mediated cytoprotection against renal IR injury (Figure 3) (Lee & Emala 2001; Okusa 2002a; Lee & Emala 2002b). Indeed, A2AAR-mediated cAMP induction and PKA activation powerfully reduces renal inflammation as well as promotes medullary vasorelaxation after IR (Okusa et al. 2001; Linden 2006).
Adenosine may powerfully suppress inflammation via A2AAR activation in regulatory T-cells (Tregs) (Kinsey et al. 2012). Adoptive transfer of Tregs deficient in CD73, the enzyme that produces extracellular adenosine from AMP, or transfer of A2AAR deficient Tregs led to inhibition of Treg function. Therefore, these studies show that simultaneous ability to generate and respond to adenosine is required for Tregs to suppress innate immune responses in renal IR injury. Recent studies further demonstrate that A2AAR activation in dendritic cells plays a critical role in protection against ischemic AKI (Li et al. 2012). Indeed, mice with specific deletion of A2AARs in dendritic cells had exacerbated renal injury after IR. Furthermore, dendritic cell A2AAR deficient mice were not protected against renal IR injury with specific A2AAR agonist treatment. Furthermore, exogenous administration of dendritic cells treated ex vivo with A2AAR agonist was protective against renal IR injury by suppressing natural killer T-cell mediated inflammation.
In summary, recent studies have shown that selective A2AAR agonists attenuate inflammation and protect against kidney IR injury by PKA activation. However, additional investigations are necessary to increase the understanding of mechanisms of A2AAR agonist-mediated reduction in inflammation and tissue damage.
A2BAR and renal IR injury
The A2BAR receptors are located in renal vasculature as well as in the renal epithelia (Lee & Emala 2002a; Wengert et al. 2005; Linden 2006; Jackson et al. 2006; Eckle et al. 2008) (Figure 2). Similar to the A2AARs, the A2BARs cause renovascular dilatation and increased renin secretion and decreased tissue inflammation via Gs and cAMP signaling pathways (Figure 3) (Vallon & Osswald 2009). In a murine model, the - renoprotective effects of ischemic preconditioning against ischemic AKI (intermittent ischemia and reperfusion before more prolonged ischemic insult) was lost in A2BAR deficient mice (Grenz et al. 2008). On the contrary, ischemic preconditioning was preserved in animals lacking A1AR, A2aAR or A3AR. Moreover, wild type animals given BAY 60–6586 (a selective A2BAR agonist) were protected from AKI induced by warm renal IR injury with reduced renal tubular necrosis and inflammation. Consistent with the renoprotective effects of A2BAR in renal ischemic preconditioning, PSB-1115 (a selective A2BAR antagonist) abolished the renoprotective effects of kidney ischemic preconditioning. Bone marrow chimera studies conducted in mice also showed that bone marrow-derived leukocyte A2BARs do not play an important role in renal protection against IR injury. Therefore, unlike the A2AARs that regulate infiltrating pro-inflammatory leukocytes including Tregs and dendritic cells, the A2BARs target renal parenchymal (renal tubular cells and/or renal endothelial cells) cells to attenuate renal IR injury.
TNF-α plays a major role in renal IR injury as mice treated with TNF-α neutralizing antibody or mice deficient in TNF-α are protected against ischemic AKI (Donnahoo et al. 1999; Grenz et al. 2012b). The A2BAR activation also plays a critical role in modulating neutrophil production of TNF-α during and after renal IR (Grenz et al. 2012b). The A2BAR deficient mice generated significantly increased renal TNF-α after IR injury. Neutrophils are the source of exacerbated TNF-a generation after renal IR as neutrophil depletion or reconstituting A2BAR deficient mice with TNF-α deficient neutrophils significantly attenuated renal injury.
Endothelial A2BAR activation also plays a critical role in renal protection against IR injury by improving post-ischemic renal peritubular capillary blood flow (Grenz et al. 2012a). Adenosine generated during renal ischemia is rapidly removed through equilibrative nucleoside transporters (ENT). Indeed, Pharmacological ENT blockade or genetic deletion significantly increased renal adenosine levels and profoundly protected against renal IR injury in mice. The renal protection with ENT blockade mediated by activation of vascular endothelial A2BARs as mice deficient in vascular endothelial A2BARs were not protected against renal IR injury with ENT blockade. Vascular endothelial A1AR, A2AAR and A3ARs do not appear to play a role in improved post-ischemic renal blood flow after IR injury. Therefore, crosstalk between renal ENTs and the A2BAR in vascular endothelia is critical in regulating post-ischemic no-reflow phenomenon.
In summary, A2BAR is drastically induced during and after inflammation and ischemia. A2BAR activation protects against renal ischemic injury with decreased renal tubular necrosis and inflammation as well as by modulating neutrophil TNF-α signaling. Thus, potentiating A2BAR activation could be show promise in improving renal function after IR injury.
A3AR and renal IR injury
The A3AR is the least studied AR subtype in the kidney (Linden 2001). Although transcripts of A3AR can be detected throughout the kidney, the specific function of A3ARs in the kidney is still unknown as A3AR activation does not affect solute excretion, TGF or GFR (Mozaffari et al. 2000; Vallon & Osswald 2009). In several cell lines, A3ARs couple to both Gi and Gq for signal transduction (Fredholm et al. 2011). Interestingly, selective A3AR activation increases renal tubular necrosis, apoptosis and inflammation after renal IR injury (Lee et al. 2003; Mabley et al. 2003; Young et al. 2004). Conversely, mice genetically deficient in A3ARs or wild type mice treated with a specific A3AR antagonist had improved kidney function and reduced renal injury after IR (Lee & Emala 2000; Lee et al. 2003).
The mechanism of A3AR-mediated modulation of ischemic renal injury is unclear. The A3AR activation has been shown to degranulate mast cells and increases the release of several inflammatory mediators including proteolytic enzymes and histamine (Fozard et al. 1996; Reeves et al. 1997), consistent with our findings that administration of a selective A3AR agonist (IB-MECA) significantly increased the plasma histamine levels in mice, worsening renal function after renal IR injury (Lee et al. 2003). Therefore, mast cell activation may play a role in A3AR-mediated exacerbation of renal IR injury. Furthermore, A3AR activation increases calcium influx and induces apoptosis in several cell types including human proximal tubule cells, cardiomyocytes and leukocyte cell lines (HK-2) cells (Kohno et al. 1996; Shneyvays et al. 1998; Jacobson 1998). Finally, A3AR overexpression is embryonically lethal with increased DNA fragmentation and chronic activation is detrimental to cell survival (Zhao et al. 2002). These mechanisms may also play a role in A3AR-mediated modulation of renal IR injury.
Although substantial progression has been made in investigating the physiological significance of AR subtypes, the role for renal A3ARs remains unclear. Studies utilizing knockout A3AR mice as well as pharmacological agonists and antagonists have identified this receptor to potentiate ischemic injury by promoting necrosis and apoptosis.
Role of adenosine receptors in volatile anesthetic-mediated protection against ischemic AKI
Inhalation volatile anesthetics are one of the most widely used drugs during the perioperative period. Several clinically utilized volatile anesthetics including sevoflurane and isoflurane protects against renal IR injury via directly reducing renal tubular necrosis, inflammation and apoptosis (Lee et al. 2004b; Lee et al. 2007b). Recent studies suggest that adenosine plays a critical role in isoflurane-mediated protection against renal IR injury (Kim et al. 2013b). Isoflurane treatment induced CD73 induction in cultured proximal tubule cells as well as in mouse kidney with significantly increased renal tubular adenosine generation. Furthermore, isoflurane-mediated induction of CD73 and adenosine generation was critical for protection against ischemic AKI in mice as mice treated with a selective CD73 inhibitor or mice deficient in CD73 were not protected against renal injury after IR with isoflurane anesthesia. Therefore, these studies imply that isoflurane-mediated modulation of renal epithelial CD73 induction and adenosine generation may have important therapeutic implications to protect against renal IR injury.
Conclusions
Ischemic AKI continues to be a major clinical problem in hospitalized patients. Ischemic AKI is characterized by renal tubular cell death due to necrosis and apoptosis. Renal injury is further compounded by massive inflammatory response due to renal tubular cytokine and chemokine generation followed by infiltration of several pro-inflammatory leukocytes including T-lymphocytes, neutrophils and macrophages (Okusa 2002b; Kinsey et al. 2008). Modulation of renal AR activation has exciting potential to protect against renal injury by targeting various aspects of pathophysiology of ischemic AKI by reducing renal tubular necrosis and apoptosis and by dampening leukocyte-mediated inflammation. Specifically, renal tubular A1AR activation reduces necrosis and apoptosis after renal IR (Lee et al. 2004a). Several leukocytes are targeted by A2AAR activation including neutrophils, Tregs and dendritic cells (Kinsey et al. 2008; Li et al. 2012; Kinsey et al. 2012; Grenz et al. 2012b). Renal vascular A2BAR also play a major role in improving post-ischemic kidney blood flow (Grenz et al. 2012a). The A3AR activation, in contrast exacerbates renal IR injury and therefore, A3AR antagonist therapy may be useful to protect against ischemic AKI (Lee & Emala 2000; Lee et al. 2003). Furthermore, renal AR activation appears to play a role in anesthetic-induced reduction in renal tubular inflammation and necrosis after IR (Kim et al. 2013b). More studies are required to better understand the mechanisms and distal signaling molecules generated with renal AR activation to translate these experimental studies to clinical setting.
Acknowledgements
This work was supported by Department of Anesthesiology, College of Physicians and Surgeons of Columbia University and by the National Institutes of Health Grants GM-067081 and DK-058547.
Footnotes
Conflict of Interest: No conflict of interest exists for each author.
References
- Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK. Adenosine generation and signaling during acute kidney injury. J. Am. Soc. Nephrol. 2011;22:14–20. doi: 10.1681/ASN.2009121217. [DOI] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5'-nucleotidase (CD73) Purinergic. Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J. Clin. Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am. J. Physiol Renal Physiol. 2005;288:F722–F731. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am. J. Physiol Gastrointest. Liver Physiol. 2004;286:G285–G293. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- Donnahoo KK, Meng X, Ayala A, Cain MP, Harken AH, Meldrum DR. Early kidney TNF-alpha expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am. J. Physiol. 1999;277:R922–R929. doi: 10.1152/ajpregu.1999.277.3.R922. [DOI] [PubMed] [Google Scholar]
- Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozard JR, Pfannkuche HJ, Schuurman HJ. Mast cell degranulation following adenosine A3 receptor activation in rats. Eur. J. Pharmacol. 1996;298:293–297. doi: 10.1016/0014-2999(95)00822-5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol. Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M, Hausleithner V, Tuisl E, Nanoff C, Schutz W. Glomeruli and microvessels of the rabbit kidney contain both A1- and A2-adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1987;335:438–444. doi: 10.1007/BF00165560. [DOI] [PubMed] [Google Scholar]
- Gallos G, Ruyle TD, Emala CW, Lee HT. A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. Am. J. Physiol Renal Physiol. 2005;289:F369–F376. doi: 10.1152/ajprenal.00470.2004. [DOI] [PubMed] [Google Scholar]
- Grenz A, Bauerle JD, Dalton JH, Ridyard D, Badulak A, Tak E, McNamee EN, Clambey E, Moldovan R, Reyes G, Klawitter J, Ambler K, Magee K, Christians U, Brodsky KS, Ravid K, Choi DS, Wen J, Lukashev D, Blackburn MR, Osswald H, Coe IR, Nurnberg B, Haase VH, Xia Y, Sitkovsky M, Eltzschig HK. Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin. Invest. 2012a;122:693–710. doi: 10.1172/JCI60214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Grenz A, Kim JH, Bauerle JD, Tak E, Eltzschig HK, Clambey ET. Adora2b adenosine receptor signaling protects during acute kidney injury via inhibition of neutrophil-dependent TNF-alpha release. J. Immunol. 2012b;189:4566–4573. doi: 10.4049/jimmunol.1201651. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS. Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Muller CE, Robson SC, Osswald H, Eltzschig HK. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007 doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoste EA, Kellum JA. Incidence, classification, and outcomes of acute kidney injury. Contrib. Nephrol. 2007;156:32–38. doi: 10.1159/000102013. [DOI] [PubMed] [Google Scholar]
- Hoste EA, Kellum JA, Katz NM, Rosner MH, Haase M, Ronco C. Epidemiology of acute kidney injury. Contrib. Nephrol. 2010;165:1–8. doi: 10.1159/000313737. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Zacharia LC, Zhang M, Gillespie DG, Zhu C, Dubey RK. cAMP-adenosine pathway in the proximal tubule. J. Pharmacol. Exp. Ther. 2006;317:1219–1229. doi: 10.1124/jpet.106.101360. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Zhu C, Tofovic SP. Expression of adenosine receptors in the preglomerular microcirculation. Am. J. Physiol Renal Physiol. 2002;283:F41–F51. doi: 10.1152/ajprenal.00232.2001. [DOI] [PubMed] [Google Scholar]
- Jacobson KA. Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol. Sci. 1998;19:184–191. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DR, Lee HT. Perioperative renal protection. Best. Pract. Res. Clin. Anaesthesiol. 2008;22:193–208. doi: 10.1016/j.bpa.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Joo JD, Kim M, Horst P, Kim J, D'Agati VD, Emala CW, Sr, Lee HT. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol. 2007;293:F1847–F1857. doi: 10.1152/ajprenal.00336.2007. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Hoste EA. Acute kidney injury: epidemiology and assessment. Scand. J. Clin. Lab Invest Suppl. 2008;241:6–11. doi: 10.1080/00365510802144813. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim M, Ham A, Brown KM, Greene RW, D'Agati VD, Lee HT. IL-11 Is Required for A1 Adenosine Receptor-Mediated Protection against Ischemic AKI. J Am Soc. Nephrol. 2013a doi: 10.1681/ASN.2013010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Chen SW, Park SW, Kim M, D'Agati VD, Yang J, Lee HT. Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int. 2009;75:809–823. doi: 10.1038/ki.2008.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Ham A, Kim JY, Brown KM, D'Agati VD, Lee HT. The volatile anesthetic isoflurane induces ecto-5'-nucleotidase (CD73) to protect against renal ischemia and reperfusion injury. Kidney Int. 2013b;84:90–103. doi: 10.1038/ki.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey GR, Huang L, Jaworska K, Khutsishvili K, Becker DA, Ye H, Lobo PI, Okusa MD. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J. Am. Soc. Nephrol. 2012;23:1528–1537. doi: 10.1681/ASN.2012010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109:e102–e107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno Y, Sei Y, Koshiba M, Kim HO, Jacobson KA. Induction of apoptosis in HL-60 human promyelocytic leukemia cells by adenosine A3 receptor agonists. Biochem Biophys Res Comm. 1996;232:904–910. doi: 10.1006/bbrc.1996.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch A, Hoff U, Bubalo G, Zhu Y, Fechner M, Schmidt-Ullrich R, Marko L, Muller DN, Schmidt-Ott KM, Gurgen D, Blum M, Schunck WH, Dragun D. Novel signalling mechanisms and targets in renal ischaemia and reperfusion injury. Acta Physiol (Oxf) 2013;208:25–40. doi: 10.1111/apha.12089. [DOI] [PubMed] [Google Scholar]
- Lee HT, Emala CW. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A(1) and A(3) receptors. Am. J. Physiol Renal Physiol. 2000;278:F380–F387. doi: 10.1152/ajprenal.2000.278.3.F380. [DOI] [PubMed] [Google Scholar]
- Lee HT, Emala CW. Characterization of adenosine receptors in human kidney proximal tubule (HK-2) cells. Exp. Nephrol. 2002a;10:383–392. doi: 10.1159/000065306. [DOI] [PubMed] [Google Scholar]
- Lee HT, Emala CW. Adenosine attenuates oxidant injury in human kidney proximal tubular cells via A1 and A2a adenosine receptor activation. Am J Physiol Renal Physiol. 2002b;282(5):F844–F852. doi: 10.1152/ajprenal.00195.2001. [DOI] [PubMed] [Google Scholar]
- Lee HT, Emala CW. Systemic adenosine given after ischemia protects renal function via A2a adenosine receptor activation. Am J Kidney Dis. 2001;38:610–618. doi: 10.1053/ajkd.2001.26888. [DOI] [PubMed] [Google Scholar]
- Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J. Am. Soc. Nephrol. 2004a;15:102–111. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- Lee HT, Kim M, Jan M, Penn RB, Emala CW. Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int. 2007a;71(12):1249–1261. doi: 10.1038/sj.ki.5002227. [DOI] [PubMed] [Google Scholar]
- Lee HT, Kim M, Kim M, Kim N, Billings Iv FT, D'Agati VD, Emala CW., Sr Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am. J. Physiol Renal Physiol. 2007b;293:F713–F722. doi: 10.1152/ajprenal.00161.2007. [DOI] [PubMed] [Google Scholar]
- Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004b;101:1313–1324. doi: 10.1097/00000542-200412000-00011. [DOI] [PubMed] [Google Scholar]
- Lee HT, Ota-Setlik A, Xu H, D'Agati VD, Jacobson MA, Emala CW. A3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am. J. Physiol Renal Physiol. 2003;284:F267–F273. doi: 10.1152/ajprenal.00271.2002. [DOI] [PubMed] [Google Scholar]
- Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am. J. Physiol Renal Physiol. 2004c;286:F298–F306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, Moser EK, Jaworska K, Kinsey GR, Day YJ, Linden J, Lobo PI, Rosin DL, Okusa MD. Dendritic cells tolerized with adenosine A(2)AR agonist attenuate acute kidney injury. J. Clin. Invest. 2012;122:3931–3942. doi: 10.1172/JCI63170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. New insights into the regulation of inflammation by adenosine. J. Clin. Invest. 2006;116:1835–1837. doi: 10.1172/JCI29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- Mabley J, Soriano F, Pacher P, Hasko G, Marton A, Wallace R, Salzman A, Szabo C. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5'-N-methyluronamide, is protective in two murine models of colitis. Eur. J. Pharmacol. 2003;466:323–329. doi: 10.1016/s0014-2999(03)01570-x. [DOI] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- Mozaffari MS, Abebe W, Warren BK. Renal adenosine A3 receptors in the rat: assessment of functional role. Can. J. Physiol Pharmacol. 2000;78:428–432. [PubMed] [Google Scholar]
- Okusa MD. A(2A) adenosine receptor: a novel therapeutic target in renal disease. Am. J. Physiol Renal Physiol. 2002a;282:F10–F18. doi: 10.1152/ajprenal.2002.282.1.F10. [DOI] [PubMed] [Google Scholar]
- Okusa MD. The inflammatory cascade in acute ischemic renal failure. Nephron. 2002b;90:133–138. doi: 10.1159/000049032. [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Huang L, Rieger JM, Macdonald TL, Huynh LP. A(2A) adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am. J. Physiol Renal Physiol. 2000;279:F809–F818. doi: 10.1152/ajprenal.2000.279.5.F809. [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Huang L, Rosin DL, Smith DF, Sullivan G. Enhanced protection from renal ischemia-reperfusion [correction of ischemia:reperfusion] injury with A(2A)-adenosine receptor activation and PDE 4 inhibition. Kidney Int. 2001;59:2114–2125. doi: 10.1046/j.1523-1755.2001.00726.x. [DOI] [PubMed] [Google Scholar]
- Osswald H, Muhlbauer B, Vallon V. Adenosine and tubuloglomerular feedback. Blood Purif. 1997;15:243–252. doi: 10.1159/000170342. [DOI] [PubMed] [Google Scholar]
- Osswald H, Vallon V, Muhlbauer B. Role of adenosine in tubuloglomerular feedback and acute renal failure. J. Auton. Pharmacol. 1996;16:377–380. doi: 10.1111/j.1474-8673.1996.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Park SW, Kim M, Brown KM, Haase VH, Lee HT. Proximal tubule sphingosine kinase-1 has a critical role in A1 adenosine receptor-mediated renal protection from ischemia. Kidney Int. 2012 doi: 10.1038/ki.2012.224. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves JJ, Jones CA, Sheehan MJ, Vardey CJ, Whelan CJ. Adenosine A3 receptors promote degranulation of rat mast cells both in vitro and in vivo. Inflamm. Res. 1997;46:180–184. doi: 10.1007/s000110050169. [DOI] [PubMed] [Google Scholar]
- Shneyvays V, Nawrath H, Jacobson KA, Shainberg A. Induction of apoptosis in cardiac myocytes by an A3 adenosine receptor agonist. Exp. Cell Res. 1998;243:383–397. doi: 10.1006/excr.1998.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisawat N, Hoste EE, Kellum JA. Modern classification of acute kidney injury. Blood Purif. 2010;29:300–307. doi: 10.1159/000280099. [DOI] [PubMed] [Google Scholar]
- Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- Trautmann A. Extracellular ATP in the immune system: more than just a "danger signal". Sci. Signal. 2009;2:e6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- Vallon V, Osswald H. Adenosine receptors and the kidney. Handb. Exp. Pharmacol. 2009:443–470. doi: 10.1007/978-3-540-89615-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DR, Reppert SM. Adenosine receptor gene expression in the kidney. Am J Physiol. 1992;32:F991–F995. doi: 10.1152/ajprenal.1992.263.6.F991. [DOI] [PubMed] [Google Scholar]
- Wengert M, Berto C, Jr, Kaufman J, Leao-Ferreira LR, Paes-de-Carvalho R, Lopes AG, Caruso-Neves C. Stimulation of the proximal tubule Na+-ATPase activity by adenosine A(2A) receptor. Int. J. Biochem. Cell Biol. 2005;37:155–165. doi: 10.1016/j.biocel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yap SC, Lee HT. Adenosine and protection from acute kidney injury. Curr. Opin. Nephrol Hypertens. 2012;21:24–32. doi: 10.1097/MNH.0b013e32834d2ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HW, Molina JG, Dimina D, Zhong H, Jacobson M, Chan LN, Chan TS, Lee JJ, Blackburn MR. A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice. J. Immunol. 2004;173:1380–1389. doi: 10.4049/jimmunol.173.2.1380. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Yaar R, Ladd D, Cataldo LM, Ravid K. Overexpression of A3 adenosine receptors in smooth, cardiac, and skeletal muscle is lethal to embryos. Microvasc. Res. 2002;63:61–69. doi: 10.1006/mvre.2001.2366. [DOI] [PubMed] [Google Scholar]