Abstract
Background and Purpose
Evidence suggests the use of stimulation to increase corticomotor excitability improves hand function in persons with cervical spinal cord injury (SCI). We assessed effects of multi-day application of 10Hz repetitive transcranial magnetic stimulation (rTMS) applied to the corticomotor hand area combined with repetitive task practice (RTP) in participants with tetraplegia and neurologically healthy participants.
Methods
Using a double-blind randomized crossover design, 11 participants with chronic tetraplegia and 10 neurologically healthy participants received 3 sessions of 10Hz rTMS+RTP and 3 sessions of sham-rTMS+RTP to the corticomotor hand region controlling the weaker hand. RTMS was interleaved with RTP of a skilled motor task between pulse trains. Hand function (Jebsen-Taylor Hand Function Test [JTT], pinch, and grasp strength) and corticomotor excitability (amplitude of motor-evoked potential) were assessed prior to and following the rTMS+RTP and sham-rTMS+RTP phases. We assessed significance using paired t-tests on pre-post differences and effect sizes using standardized response mean (SRM).
Results
RTMS+RTP was associated with larger effect sizes compared to sham-rTMS+RTP for improvement in JTT for both the trained hand (SRM=0.85 and 0.42, respectively), non-trained hand (0.55, 0.31, respectively), and for grasp strength of the trained hand in the SCI group (0.67, 0.39, respectively) alone. Effect sizes for all other measures were small and there were no statistical between-condition differences in the outcomes assessed.
Discussion and Conclusions
RTMS may be a valuable adjunct to RTP for improving hand function in persons with tetraplegia. Higher stimulation dose (frequency, intensity, number of sessions) may be associated with larger effects. Video Abstract available (See Supplemental Digital Conent 1) for more insights from the authors.
Keywords: spinal cord injury, upper extremity, repetitive transcranial magnetic stimulation, function
Introduction
In the United States alone, there are approximately 123,000 individuals living with the consequences of incomplete tetraplegia, the most common manifestation of traumatic spinal cord injury (SCI).1 Improvement of arm and hand function is ranked highest of all rehabilitation goals in this population.2-4 Deficits in muscle activation after SCI have been associated with decreased firing rate along the neural fibers of the corticospinal tract, both in animal models5 and in humans with chronic SCI.6 Prior studies in persons with tetraplegia have used indirect approaches to increase corticomotor excitability via somatosensory stimulation combined with repetitive task practice (RTP).7-10 These studies indicate that stimulation augments the effects of RTP, resulting in greater gains in hand function.
Repetitive high-frequency (ie, 5Hz or greater)11 transcranial magnetic stimulation (rTMS) is an approach that can be used to directly stimulate the cortex and promote corticomotor excitation (as indicated by increased amplitude of motor evoked potentials [MEPs]).11 Two prior studies have assessed the use of different rTMS stimulation protocols in persons with tetraplegia; results have been mixed. Belci et al12 performed a sham-controlled crossover (order not randomized) study with four participants using 10 Hz rTMS (100 ms interpulse interval [ie, 10 Hz] with 2 pulses every 10s, resulting in 720 total pulses applied in doublets, delivered while at rest), a paired pulse protocol intended to decrease intracortical inhibition and thereby increase cortical activation to the spared corticospinal neurons. The authors reported improvements in hand motor and sensory function. Kuppuswamy et al13 proposed a different approach, using 5Hz rTMS intended to directly facilitate corticospinal output in 15 participants with tetraplegia in a sham-controlled, randomized cross-over study, and found no difference between stimulation and sham conditions with regard to change in hand motor performance.
Our study differs from the study by Belci by employing the use of 10Hz rTMS in a protocol aimed at facilitating corticospinal output interleaved with the performance of a fine motor task, which would provide an opportunity to direct the increased cortical excitability to a relevant functional context. In addition, we included a group of neurologically healthy participants, to gain further insight into the effects of this protocol in individuals with no neurologic impairment. We hypothesized that a 3-day approach of high-frequency rTMS interleaved with repetitive task practice would be associated with greater improvements in hand function and cortical excitability compared with sham-rTMS and repetitive task practice in isolation in persons with tetraplegia and a group of neurologically healthy participants. In addition, we hypothesized that improvements would be seen at the domains of both “body functions and structure” and “activity” of the International Classification of Functioning, Disability and Health (ICF) framework.14
Methods
Participants in the SCI group were recruited through the research volunteer registry of the Miami Project to Cure Paralysis and a non-matched group of neurologically healthy participants were recruited from the University of Miami community via verbal recruitment. To be included participants with SCI had to be between the ages of 18-65 years old with chronic SCI (at least one year post-injury), neurological level of injury C7 or above,15,16 and sufficient voluntary activation to elicit a visible twitch of the thenar muscles in at least one hand. Exclusion criteria were: history of head injury, history or family history of seizures, metal elements in the head (eg aneurysm clip), and presence of any other neurologic, orthopedic, or cognitive condition. Participants in the SCI group were advised to not make changes to usual level of physical activity or medication regimen. All participants gave written informed consent to participate in the study, which had been approved by the Human Subjects Research Office of the University of Miami Miller School of Medicine, Miami, FL.
This double-blind, crossover study was performed over two weeks comprising two conditions: rTMS+RTP and sham-rTMS+RTP. Sample size was estimated based a prior study of rTMS in persons with SCI in which statistically significant pre-post differences in fine motor performance measured by the nine-hole peg test were detected with 4 participants,12 and increased to compensate for possible attrition. Randomization was performed, based on a random number generator, by a member of the laboratory staff who was not otherwise involved with the study. The investigator involved in the outcomes assessment was blinded to the intervention order.
Upon completing informed consent, participants were randomized one intervention condition on week 1, followed by the alternate condition on week 2. On the first and last weekday (ie. Monday, Friday) of each of the 2 study weeks, participants performed the assessments, and on the intervening weekdays (ie, Tuesday, Wednesday, Thursday), participants received rTMS+RTP or sham-rTMS+RTP. The testing order was maintained constant to avoid interference between clinical outcome measures and neurophysiologic outcome measures (and vice-versa), and was as follows: motor threshold, MEP recruitment curves, Jebsen-Taylor hand function test, pinch strength, and grasp strength. Because training effects are known to transfer to the non-trained hand, performance measures were assessed in both hands.17-19
According to safety guidelines for TMS studies, participants wore earplugs during the intervention, and completed a subjective symptom and safety assessment prior to and following rTMS or sham-rTMS.20 RTMS was delivered to the representation area of the thenar muscles representation area in the hemisphere contralateral to the weaker hand (the non-dominant hand in the neurologically healthy subjects) using an rTMS stimulator (Magstim Rapid 2; Magstim Co, UK). A total of 800 pulses of 10 Hz rTMS with intensity set at 80% of the biceps RMT were delivered in 2-sec trains of 40 pulses, with an inter-train interval of 30 seconds, during which participants practiced a fine motor task (the Nine-hole Peg Test [NHPT]) in both the rTMS and sham-rTMS phases.21 Other investigators studying rTMS for persons with SCI have also standardized the rTMS stimulation intensity in this manner.22 Sham-rTMS was performed using a previously validated approach that mimics the experience of the real rTMS by using electrical stimulation to create a sensation of stimulation; the surface stimulation electrodes were also in place during the real rTMS so that both conditions were the same from the perspective of the participant.23
Corticomotor excitability was assessed with transcranial magnetic stimulation (TMS). MEPs were recorded from the thenar and biceps muscles via surface electromyography (EMG) using Ag Ag/Cl electrodes (3.2×2.2 cm2). MEPs were elicited under two conditions: 1) while the subject was at rest (resting MEPs), and 2) while the subject performed minimal voluntary contraction of 10 – 15% of their maximum voluntary contraction (active MEPs).24 For the measurement of active MEPs of the thenar muscles, one electrode was placed at the distal one-third of the thenar eminence, and the other electrode was placed 2 cm caudally; the ground electrode was placed on the styloid process of the ulna. The thenar muscles were chosen because control of the opposing thumb is necessary for prehensile function that comprises many hand activities, and to enable comparisons with previous studies.7-10 Measurement of MEPs of the biceps muscle was performed using one electrode placed centrally on the muscle belly of the biceps, another electrode placed 2 cm caudally, and the ground electrode placed on the olecranon.
EMG signals were split between two computers, one for data acquisition, storage, and off-line analysis (Signal, CED, UK) and the other for biofeedback of the EMG activity, with a target corresponding to 10-15% of maximum voluntary contraction of the thenar muscles (Spike, CED, UK). For data acquisition, EMG was amplified (×1k), band-pass filtered (10-2kHz) (Grass model P511AC, Grass-Telefactor, USA) and converted from analogue to digital (CED model 1401; CED, UK) at a sampling rate of 2 kHz.25,26 EMG activity was captured prior to and following the stimulation artifact, to capture both baseline and post-stimulus activity. TMS was delivered using a figure-of-eight coil (maximum field intensity 2 Tesla; Magstim Rapid 2, Magstim Co, UK) placed tangential to the scalp and in a postero-lateral direction an angle of 45° with the midsagital line on the hemisphere contralateral to the weaker hand approximately 5 cm lateral to the vertex. With an initial intensity set at 50% maximum stimulator output, the hotspot was sought24 and documented using a coordinate system27 that measured the distance of the hotspot from the vertex.
Motor threshold (MT) was defined as the stimulation intensity wherein it was possible to obtain responses greater than 50 μV peak-to-peak in amplitude either at rest28 or above baseline EMG during a voluntary contraction of 10-15% of maximum voluntary contraction.24 The relationship between increases in stimulation intensity and MEP amplitude was assessed using input-output curves. Beginning at the intensity corresponding to 80% of the thenar active MT, five stimuli were delivered at each stimulator intensity with inter-stimulus interval of 4-6 seconds, in 20% increments of stimulator output, until reaching the maximum stimulator output.23,29 To assess changes in cortical excitability, data from the input-output curves was analyzed based on the area under the curve (AUC).30
Change in the Jebsen-Taylor hand function test (JTT) scores31 from pre-intervention (i.e., the day prior to start of the 3-day rTMS+RTP and sham-rTMS+RTP phases) to post-intervention (i.e., the day following the intervention phases) was the primary outcome measure. The JTT is designed to measure skilled use of the hand to assess activity limitations (i.e., ICF activity domain), and has been shown to be sufficiently sensitive to capture training-related change in functional hand use in other studies of persons with tetraplegia.7-10 The time taken to complete the tasks associated with the JTT (e.g., turning cards, feeding, and manipulating small, light and heavy objects) was recorded. As in other studies,9,32 we omitted the writing task.
Change in pinch and grasp strength from pre-intervention to post-intervention for each intervention phase was measured to assess body function impairment (i.e., ICF “body structure and function” domain) using a handheld dynamometer (Microfet4; Hoggan Health Industries, Utah). The average force in 3 maximal voluntary contractions was recorded. Immediate, within-session effects of rTMS+RTP versus sham-rTMS+RTP on fine motor performance (i.e., ICF “activity” domain) were measured using the NHPT.12,13 For each session, the average number of pegs successfully placed and removed during each inter-train interval was calculated.
Data Analysis
Statistical testing was performed using SAS software (SAS Institute Inc., Cary, North Carolina) and built-in functions in Excel 2010 (Microsoft Corporation, USA). We assessed order effects using a published protocol33 and did not find order effects for Jebsen-Taylor hand function test, pinch strength and grasp strength, motor threshold, recruitment curves and area under the curve, (p>0.05 for all comparisons). Therefore, data from within each condition (rTMS+RTP, sham-rTMS+RTP) regardless of intervention order, were pooled within the two participant groups (SCI, neurologically healthy), and paired t-tests on the difference in score (posttest-pretest) were used to make comparisons between conditions separately for the two groups (SCI participants and neurologically healthy participants). Descriptive statistics, including means, standard deviations and 95% confidence intervals (CI) of the pre-post change for each condition were calculated for all measures. In addition, the median pre-post change was calculated for the JTT. Analyses with p ≤ 0.05 were considered to have achieved statistical significance.
Order effects were identified in the outcomes related to performance on the NHPT. In addition, evidence suggests that non-invasive brain stimulation may influence the rate of learning rather than the magnitude of the learning effect.34,35 Therefore, we assessed within-condition change for each group using trend analysis with simple linear regression wherein the independent variable was represented by the training day (1, 2, 3) and the dependent variable was the number of peg transfers successfully completed on each day. Then, comparisons were made between weeks (week 1 and week 2) for each condition (rTMS+RTP and sham-rTMS+RTP) by comparing the slopes of the performance curves for each week. An exploratory analysis was carried out to assess the within-session change in NHPT performance. We performed comparisons between the first 5 bouts and last 5 bouts of NHPT on each training day (1,2,3,4,5,6, irrespective of condition) using paired t-tests.
In the presence of large variability and small sample sizes (such as is true of many intervention studies directed at improving function in clinical populations interpreting the clinical meaningfulness of results based on the effect size36,37 and minimal clinically important differences may be more useful.38,39 Therefore, we calculated effect sizes using the standardized response mean (SRM) dividing the change in score from pretest to posttest by the standard deviation of the change.17 Prior studies have used this measure to assess the size of training effects in persons with spinal cord injury.40 As in previous studies, the effect sizes were interpreted as: < 0.2 trivial effect, 0.2 – 0.5 small effect, 0.5 – 0.8 moderate effect, > 0.8 large effect.41 For participants with SCI, the minimal clinically important difference (MCID) for the change in time to perform the JTT was determined by a distribution method using the one standard error of measurement (SEM) approach,38,39 estimated by multiplying the standard deviation of the measurement by the square root of one minus the reliability coefficient of the outcome measure.
Results
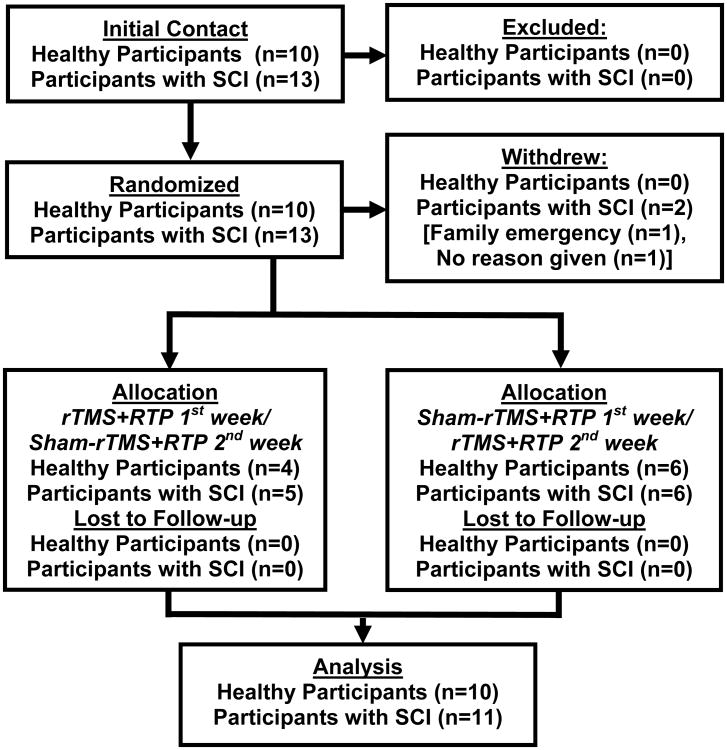
Twenty-three participants, 13 individuals with SCI (3 females, mean age 46.7±12 years) and 10 individuals without disability (4 females, mean age 33±7 years), enrolled in the study. Two individuals with SCI discontinued participation prior to the end of the study. One participant reported needing to tend to a family emergency, and the other participant did not share a reason. The remaining 11 participants with SCI and 10 neurologically healthy participants completed the study, and were included in the analysis. A flowchart illustrating the recruitment and study design is given in Figure 1. With the exception of a transient headache (reported by 3 participants), no adverse effects were observed. Descriptive characteristics and baseline characteristics for all outcome measures are given in Table 1.
Figure 1.
Participant recruitment flow diagram.
Table 1.
Demographic and baseline characteristics of the participants (SCI Group and Neurologically Healthy Adults).
| Subject Characteristics | SCI Group | Healthy adults |
|---|---|---|
| Age (years) | 46.7±12.0 | 33.7±7 |
| Gender | 1 female, 10 males | 4 females, 6 males |
| Post-injury (years) | 6.6±8.2 | N/A |
| Injury Level | C6 (median) | N/A |
| AIS | D(6), C(5) | N/A |
| JTT-t (time) | 267±216 | 31±4 |
| JTT-nt (time) | 177±202 | 29±3 |
| Pinch-t (kg) | 1.7±1.5 | 8.6±1.7 |
| Pinch-nt (kg) | 4.4±2.8 | 8.7±1.9 |
| Grasp-t (kg) | 2.3±2.1 | 29.1±10.4 |
| Grasp-nt (kg) | 4.8±2.3 | 34.0±10.8 |
| Resting Motor Threshold (%MSO) | 0.62±0.1 | 0.57±0.1 |
| Active Motor Threshold (%MSO) | 0.50±0.1 | 0.39±0.09 |
| AUC | 1.3±1.7 | 4.6±2.6 |
AIS=American Spinal Cord Injury Impairment Scale; JTT-t=Jebsen-Taylor Hand Function Test, trained hand; JTT-nt=Jebsen-Taylor Hand Function Test, non-trained hand; Pinch-t=Pinch force, trained hand; Pinch-nt=Pinch force, non-trained hand; Grasp-t=Grasp force, trained hand; Grasp-nt=Grasp force, non-trained hand; MSO=maximum stimulator output of TMS device (2Tesla); AUC=area under the curve of input-output curves acquired with transcranial magnetic stimulation.
Changes in score from pretest to posttest for each condition for all performance-based measures are presented in Table 2. The time to complete the JTT was improved in both conditions (rTMS+RTP and rTMS-sham+RTP), and no between-condition differences in the change in JTT time were found. However, the effect size for the improvement in JTT was large for rTMS+RTP (SRM=0.85) while sham-rTMS+RTP was associated with a small effect size (SRM=0.42). A transfer of training effect to the non-trained hand was observed, with improvement in JTT associated with a moderate effect size rTMS+RTP (SRM=0.55), while sham-rTMS+RTP was associated with a small effect size (SRM=0.31) in the non-trained hand. Between-condition comparisons approached significance in the non-trained hand (p=0.06).
Table 2.
Descriptive statistics for the change in score from pre-test to post-test for each performance measures (SCI group). The reduction in JTT time was associated with a greater effect size in the rTMS+RTP group.
| Outcome Measure | rTMS+RTP | Sham-rTMS+RTP | Between- condition difference (Paired t-test) | ||||
|---|---|---|---|---|---|---|---|
| Difference Scores | Effect Size | Difference scores | Effect Size | ||||
| Mean±SD | (95%CI) | Mean±SD | (95%CI) | ||||
| JTT-t (time) | -45±52 | (-80,-9) | 0.85 | -29±68 | (-75.2,16.7) | 0.42 | 0.4 |
| JTT-nt (time) | -31.3±56 | (-69,6) | 0.55 | -13±41 | (-40,14) | 0.31 | 0.06 |
| Pinch-t (kg) | 0.1±0.5 | (-0.1,0.5) | 0.38 | 0.24±0.8 | (-0.3,0.7) | 0.30 | 0.8 |
| Pinch-nt (kg) | 0.2±0.9 | (-0.4,0.8) | 0.22 | -0.05±0.6 | (-0.5,0.4) | 0.07 | 0.5 |
| Grasp-t (kg) | 0.5±0.8 | (0.003,1.0) | 0.67 | 0.4±1.1 | (-0.3,1.2) | 0.39 | 0.7 |
| Grasp-nt (kg) | 0.11±1.2 | (-0.7,0.9) | 0.08 | 0.2±1.2 | (-0.7,0.9) | 0.08 | 0.6 |
JTT-t=Jebsen-Taylor Hand Function Test, trained hand; JTT-nt=Jebsen-Taylor Hand Function Test, non-trained hand; Pinch-t=Pinch force, trained hand; Pinch-nt=Pinch force, non-trained hand; Grasp-t=Grasp force, trained hand; Grasp-nt=Grasp force, non-trained hand; kg=kilogram.
The median pre-post changes in JTT time (± lower and upper quartiles) for both rTMS+RTP and sham-rTMS+RTP are illustrated in Figure 2. The distribution-based estimates of the MCID for the main outcome measure (JTT) used in the present study determined that a decrease in time of 20.8 seconds (indicating faster performance) would be considered a clinically meaningful change (represented by a horizontal dotted line on the Figure 2). While there were 2 outliers, Figure 2 shows that the median change in JTT time exhibited by the rTMS+RTP condition (21.2 seconds) met the MCID, indicating that most participants made clinically meaningful improvements in hand function when they engaged in rTMS+RTP. In counterpart, the median decrease in time associated with the sham-rTMS+RTP condition (6.2 seconds) did not meet the criteria for MCID.
Figure 2.
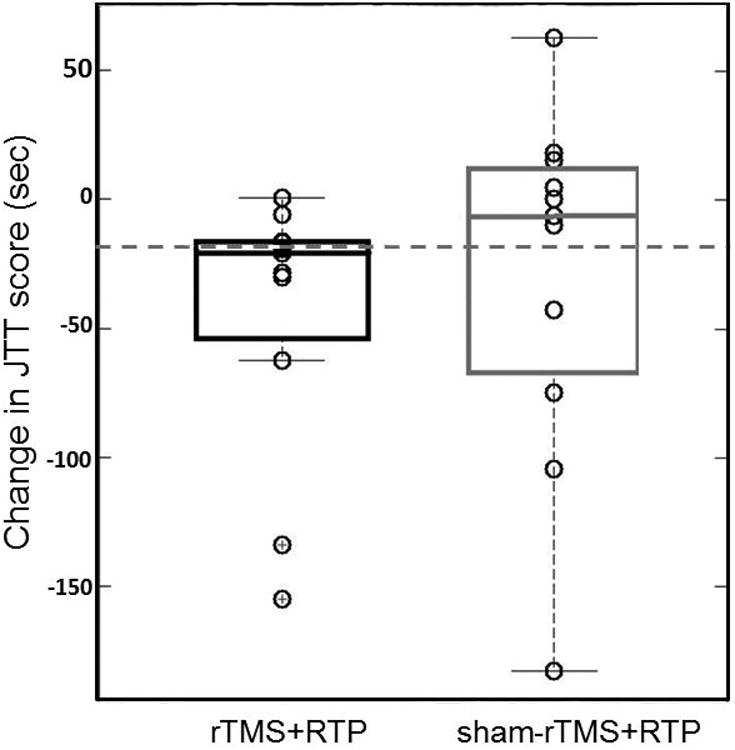
Boxplots indicating median pre-post change in JTT time (± lower and upper quartiles) per condition (rTMS+RTP=black box) and sham-rTMS+RTP (grey box) and the minimal clinically important change (MCID) (dotted horizontal line). Note that the median pre-post change for the rTMS condition met the MCID.
There were no significant between-condition differences in grasp or pinch force in the trained hand or the untrained hand. However, change in grasp force in the trained hand in the rTMS+RTP condition was associated with a moderate effect size (SRM=0.67), while sham-rTMS+RTP was associated with a small effect size (SRM=0.39). All other performance-based and neurophysiologic outcomes were associated with small effect sizes (Table 2).
Changes in score from pretest to posttest for all performance-based measures in the neurologically healthy group for each condition are presented in Table 3. There were no between-condition effects in any performance measures, and all effect sizes for both conditions were small.
Table 3.
Descriptive statistics for the change in score from pre-test to post-test for each performance measures (Healthy Adults). There were no significant between-condition differences, and effect sizes were small for all outcome measures assessed.
| Outcome Measure | rTMS+RTP | Sham-rTMS+RTP | Between-condition difference (Paired t-test) | ||||
|---|---|---|---|---|---|---|---|
| Difference Scores | Effect Size | Difference scores | Effect Size | ||||
| Mean±SD | (95%CI) | Mean±SD | (95%CI) | ||||
| JTT-t (time) | -0.5±3.4 | (-3.2,2.1) | 0.16 | -0.2±4.7 | (-3.9,3.3) | 0.05 | 0.3 |
| JTT-nt (time) | -0.3±1.3 | (-1.3,0.6) | 0.27 | -0.07±1.2 | (-0.9,0.8) | 0.05 | 0.4 |
| Pinch-t (kg) | 0.2±2.5 | (-1.5,2.1) | 0.11 | -0.03±2.1 | (-1.5,1.5) | 0.33 | 0.7 |
| Pinch-nt (kg) | 0.2±0.9 | (-0.4,0.8) | 0.22 | -0.05±0.6 | (-0.5,0.4) | 0.07 | 0.5 |
| Grasp-t (kg) | 0.1±6.0 | (-4.2,4.4) | 0.23 | 2.3±5.3 | (-1.4,6.1) | 0.19 | 0.3 |
| Grasp-nt (kg) | 1.26±4.2 | (-1.7,4.2) | 0.29 | 0.6±2.7 | (-1.3,2.6) | 0.24 | 0.7 |
JTT-t=Jebsen-Taylor Hand Function Test, trained hand; JTT-nt=Jebsen-Taylor Hand Function Test, non-trained hand; Pinch-t=Pinch force, trained hand; Pinch-nt=Pinch force, non-trained hand; Grasp-t=Grasp force, trained hand; Grasp-nt=Grasp force, non-trained hand; kg=kilogram.
Pre-post changes in motor threshold at rest and under active condition, and in the AUC are presented for both groups (SCI and neurologically healthy) are presented in Table 4. There were no significant between-condition differences in either group. In the SCI group the rTMS+RTP condition was associated with a change in area under the curve that approached a moderate effect size (SRM=0.48). All other effects sizes were small.
Table 4.
Descriptive statistics for the change in score from pre-test to post-test for neurophysiologic outcome measures (Healthy Adults and SCI participants). There were no significant between-condition differences, and effect sizes were small for all outcome measures assessed.
| Outcome Measure | rTMS+RTP | Sham-rTMS+RTP | Between-condition difference (Paired t-test) | ||||
|---|---|---|---|---|---|---|---|
| Difference scores | Effect size | Difference scores | Effect size | ||||
| Mean±SD | (95%CI) | Mean±SD | (95%CI) | ||||
| SCI Group | 0.02±0.03 | (-0.09,0.13) | 0.11 | 0.01±0.19 | (-0.12,0.14) | 0.05 | 0.9 |
| Resting Motor Threshold (%MSO) | |||||||
| Active Motor Threshold (%MSO) | -0.04±0.09 | (-0.1,0.02) | 0.40 | -0.04±0.1 | (-0.1,0.04) | 0.34 | 0.9 |
| AUC | 0.4±0.9 | (-0.1,1.1) | 0.48 | 0.4±1.5 | (-0.6,1.4) | 0.26 | 0.9 |
| Comparison Group | -0.002±0.1 | (-0.1,0.09) | 0.01 | -0.02±0.1 | (-0.1,0.05) | 0.20 | 0.6 |
| Resting Motor Threshold (%MSO) | |||||||
| Active Motor Threshold (%MSO) | 0.01±0.05 | (-0.02,0.4) | 0.11 | 0.03±0.1 | (-0.1,0.05) | 0.26 | 0.5 |
| (AUC) | -1.1±3.8 | (-3.8,1.6) | 0.28 | -0.2±1.3 | (-1.2,3.5) | 0.21 | 0.5 |
SCI=spinal cord injury; MSO=maximum stimulator output of TMS device (2Tesla); AUC=area under the curve of input-output curves acquired with transcranial magnetic stimulation
Performance on the NHPT during the inter-train interval improved in both conditions and in both groups over the course of the 3-day intervention during week 1. Regardless of condition or group, the slopes were greater on week 1 compared to week 2 (Table 5). Within-session change in performance of NHPT is presented on supplementary table 1. There were significant within-session improvements in the SCI group on training Days 1,2,3,5 and 6. In the neurologically healthy group, there were significant within-session improvements on training Days 1 and 5. The mean within-session change (collapsed across days) achieved significance in both groups.
Table 5.
Regression of nine-hole peg test (NHPT) performance by Intervention Day (1,2,3) for participants in both groups (SCI, Neurologically Healthy) who received Stim 1st and Sham 1st. Regardless of condition, individuals demonstrated greater improvement on their first week.
| Regression of NHPT by Day for SCI Participants | ||
| Week | rTMS+RTP 1st | Sham-rTMS+RTP 1st |
| Slope | Slope | |
| 1 | 32.0 ± 15.8, p=0.06 | 30.5 ± 21.6, p=0.2 |
| 2 | 7.7 ± 16.3, p=0.6 | 15.1 ± 10.1, p=0.1 |
| Regression of NHPT by Day for Neurologically Healthy Participants | ||
| rTMS1st+RTP | Sham-rTMS 1st+RTP | |
| Week | Slope | Slope |
| 1 | 42.3 ± 15.6, p=0.03 | 46.3 ± 19.5, p=0.03 |
| 2 | 9.1 ± 13.7p=0.5 | 10.9 ± 14.5, p=0.4 |
NHPT=Nine-hole Peg Test
Discussion
We assessed the influence of a 3-day intervention consisting of 10 Hz rTMS applied over the hand motor area interleaved with RTP of a fine motor task during the inter-stimulus intervals in both subjects with SCI and a group of neurologically healthy participants. The results indicate that the addition of rTMS to the RTP was associated with an improvement in grasp strength and the ability to perform the JTT tasks beyond the improvements associated with RTP alone, although statistically significant differences between the two conditions were not found. Regardless of stimulus condition during week 1, both the SCI and neurologically healthy group had improved performance on the pegboard task that was performed during the intervention that week. No other meaningful changes in performance-based measures or neurophysiologic measures were found in either group.
The size of the effects for the changes in JTT and grasp strength were nearly twice as large with the rTMS+RTP condition (SRM=0.85 and 0.67, respectively) compared to the sham-rTMS+RTP condition (SRM=0.42 and 0.39, respectively). This finding supports previous evidence8 indicating that improvements in hand function are greater when fine motor practice is performed concurrently with stimulation aimed at increasing cortical excitability. In a study of persons with tetraplegia that used a higher RTP dose than employed in the present study (daily training 2hrs/day 5days/week for 3 weeks), improvements in skilled hand use were greater when RTP was combined with peripheral nerve stimulation (SRM=1.11), compared to RTP alone (SRM=0.59).8 Since our rTMS+RTP intervention was only 3 sessions (a training dose that is lower than most training protocols in the clinical setting), it is possible that an intervention with a higher dose (of stimulation and training) could have led to a greater magnitude of change.
Beyond the measures of effect size, the median pre-post change in JTT time associated with the rTMS+RTP condition met the MCID for this measure, while the sham-rTMS+RTP did not. The JTT includes activities such as picking up small objects, handling light and heavy cans, which one would do routinely in daily life. In addition to the effects observed in the trained hand, change in JTT in the non-trained hand was associated with a moderate effect size (SRM=0.55), suggesting that there was inter-manual transfer of training effects.17-19 This performance improvement in the hand contralateral to the hand used in a training intervention has been observed in healthy subjects,42,43 however this is the first study to demonstrate inter-manual transfer of training effects in individuals with tetraplegia.
The use of rTMS for improving arm and hand function in persons with SCI has led to mixed results. Our study and the two prior studies that assessed the effects of multi-day rTMS approaches in individuals with tetraplegia utilized equivalent daily dosages of stimulation; we used 800 pulses daily while Belci et al12 (using 720 pulses; 100 ms interpulse interval [i.e., 10 Hz], 2 pulses every 10 s, over 5 days) and Kuppuswamy et al13 (using 900 pulses; 2 s trains at 5 Hz separated by 8 s for 15 min, over 5 days). However, all three studies employed different rTMS paradigms with different stimulation parameters. Belci et al12 used a 10 Hz paired pulse protocol for 5 days and found changes in the silent period, AIS motor and pin-prick scores post-treatment, as well as delayed functional improvement measured by the pegboard test at a follow-up period 3 weeks after the last session.12 While Kuppuswamuy et al13 have described the study by Belci et al12 as a study that incorporated both high and low-frequency rTMS, the findings of Belci et al12 could possibly be attributable to the high-frequency of the paired pulse stimulation (10Hz), as others have shown that rTMS approaches at 0.1Hz do not influence cortical excitability.44,45
Another possible explanation for the differences in outcomes of the aforementioned studies and the present study is the use of different inter-train intervals (30 seconds in the present study, 10 seconds in the study by Belci et al12 and 8 seconds in the study by Kuppuswamy et al13). Rothkegel et al46 assessed the effects of a 5Hz rTMS protocol (1200 pulses total), either delivered continuously or with a 60 second inter-train interval, and found that when the protocol was delivered continuously, there was a reversal of the net effect from increased excitability to decreased excitability. Furthermore, the after-effect of theta-burst stimulation (another rTMS method consisting of triplet pulses delivered at 50Hz and repeated every 200ms) depends highly on the interval between the bursts.47 The exact impact of differences in inter-train intervals on the after-effects of rTMS warrants further investigation in future studies. The outcomes of classical statistical testing in our study are consistent with those of the study of Kuppuswamy et al13 that found no statistical between-condition differences in the pre-post change in hand motor performance assessed by the action arm research or corticomotor excitability (resting motor threshold, active motor threshold, silent period) after a 5-day rTMS intervention in a sham-controlled crossover study. We also did not find statistical significant differences in the comparisons between rTMS and sham-rTMS. Bayesian statistics are preferable for identifying the clinical meaningfulness of outcomes36,37 and are now commonly used in studies of clinical populations.40 which often have small sample sizes such as the present study. Unfortunately, Kuppuswamy et al13 did not include sufficient information for estimation of effect sizes, thereby making it difficult to make comparisons between that study and ours difficult. The large variability observed may also represent inter-individual differences in responsiveness to the protocol.48
Our study has limitations. We believe that using the biceps brachii as our reference for calculating the rTMS stimulator frequency could have resulted in insufficient excitation in the corticospinal pathway's projection to the thenar muscles from which we measured the MEPs, likely to represent the lack of changes in both pinch force and thenar MEPs. In addition, allowing individuals to perform self-paced RTP could have resulted in differences in training intensity. However, we were most interested in comparing the within-subject differences. The lack of effects in the neurologically healthy group could suggest that the dose of rTMS stimulation was insufficient to induce changes in cortical excitability in that group. Another possibility is a ceiling effect for performance of routine motor task, such as those included in the JTT, as individuals with intact nervous systems may have a smaller capacity for change in response to a brief intervention.
Conclusions
In persons with tetraplegia, 10 Hz rTMS applied over the hand motor area interleaved with RTP of a fine motor task performed during the inter-train intervals was associated with clinically meaningful improvements in skilled hand use and grasp strength. While there were no statistical between-condition differences, our results suggest that the combination of rTMS and repetitive task practice leads to improvements that have a greater magnitude (effect sizes) and clinical relevance (MCID) than is observed with repetitive task practice used in isolation. Further studies are encouraged to identify the rTMS+RTP parameters (frequency, intensity, duration) that are associated with best results.
Supplementary Material
List of Supplemental Digital Content
Supplemental Digital Content 1: Video abstract Gomes-Osmon.mpf
Acknowledgments
This work was funded by National Institutes of Health award R01HD53854 to EFF. The authors would like to thank Alvaro Pasqual-Leone, MD, PhD for his contributions to the study design.
References
- 1.Birmingham A. National spinal cord injury statistical center, facts and figures at a glance. 2013 Mar; doi: 10.1179/1079026813Z.000000000136. Prevalence of SCI (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KD. Targeting recovery: Priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 3.Snoek GJ, IJzerman MJ, Hermens HJ, Maxwell D, Biering-Sorensen F. Survey of the needs of patients with spinal cord injury: Impact and priority for improvement in hand function in tetraplegics. Spinal Cord. 2004;42(9):526–532. doi: 10.1038/sj.sc.3101638. [DOI] [PubMed] [Google Scholar]
- 4.Simpson LA, Eng JJ, Hsieh JT, Wolfe DL. Spinal Cord Injury Rehabilitation Evidence Scire Research Team. The health and life priorities of individuals with spinal cord injury: A systematic review. J Neurotrauma. 2012;29(8):1548–1555. doi: 10.1089/neu.2011.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nashmi R, Fehlings MG. Changes in axonal physiology and morphology after chronic compressive injury of the rat thoracic spinal cord. Neuroscience. 2001;104(1):235–251. doi: 10.1016/s0306-4522(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 6.Davey NJ, Smith HC, Wells E, et al. Responses of thenar muscles to transcranial magnetic stimulation of the motor cortex in patients with incomplete spinal cord injury. J Neurol Neurosurg Psychiatry. 1998;65(1):80–87. doi: 10.1136/jnnp.65.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beekhuizen KS, Field-Fote EC. Sensory stimulation augments the effects of massed practice training in persons with tetraplegia. Arch Phys Med Rehabil. 2008;89(4):602–608. doi: 10.1016/j.apmr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Beekhuizen KS, Field-Fote EC. Massed practice versus massed practice with stimulation: Effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabil Neural Repair. 2005;19(1):33–45. doi: 10.1177/1545968305274517. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman LR, Field-Fote EC. Functional and corticomotor changes in individuals with tetraplegia following unimanual or bimanual massed practice training with somatosensory stimulation: A pilot study. J Neurol Phys Ther. 2010;34(4):193–201. doi: 10.1097/NPT.0b013e3181fbe692. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman LR, Field-Fote EC. Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: A case report. Phys Ther. 2007;87(2):208–223. doi: 10.2522/ptj.20050365. [DOI] [PubMed] [Google Scholar]
- 11.Kandel M, Beis JM, Le Chapelain L, Guesdon H, Paysant J. Non-invasive cerebral stimulation for the upper limb rehabilitation after stroke: A review. Ann Phys Rehabil Med. 2012;55(9-10):657–680. doi: 10.1016/j.rehab.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Belci M, Catley M, Husain M, Frankel HL, Davey NJ. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord. 2004;42(7):417–419. doi: 10.1038/sj.sc.3101613. [DOI] [PubMed] [Google Scholar]
- 13.Kuppuswamy A, Balasubramaniam AV, Maksimovic R, et al. Action of 5 hz repetitive transcranial magnetic stimulation on sensory, motor and autonomic function in human spinal cord injury. Clin Neurophysiol. 2011;122(12):2452–2461. doi: 10.1016/j.clinph.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Rauch A, Cieza A, Stucki G. How to apply the international classification of functioning, disability and health (ICF) for rehabilitation management in clinical practice. Eur J Phys Rehabil Med. 2008;44(3):329–342. [PubMed] [Google Scholar]
- 15.Marino RJ, Barros T, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26(Suppl 1):S50–6. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- 16.Waring WP, 3rd, Biering-Sorensen F, Burns S, et al. 2009 review and revisions of the international standards for the neurological classification of spinal cord injury. J Spinal Cord Med. 2010;33(4):346–352. doi: 10.1080/10790268.2010.11689712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang MH, Fossel AH, Larson MG. Comparisons of five health status instruments for orthopedic evaluation. Med Care. 1990;28(7):632–642. doi: 10.1097/00005650-199007000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Koeneke S, Battista C, Jancke L, Peters M. Transfer effects of practice for simple alternating movements. J Mot Behav. 2009;41(4):347–355. doi: 10.3200/JMBR.41.4.347-356. [DOI] [PubMed] [Google Scholar]
- 19.Camus M, Ragert P, Vandermeeren Y, Cohen LG. Mechanisms controlling motor output to a transfer hand after learning a sequential pinch force skill with the opposite hand. Clin Neurophysiol. 2009;120(10):1859–1865. doi: 10.1016/j.clinph.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YH, You SH, Ko MH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37(6):1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- 22.Kumru H, Murillo N, Samso JV, et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair. 2010;24(5):435–441. doi: 10.1177/1545968309356095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mennemeier M, Triggs W, Chelette K, Woods A, Kimbrell T, Dornhoffer J. Sham transcranial magnetic stimulation using electrical stimulation of the scalp. Brain Stimul. 2009;2(3):168–173. doi: 10.1016/j.brs.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(2):79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 25.Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS) Clin Neurophysiol. 2002;113(3):376–382. doi: 10.1016/s1388-2457(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 26.Jubeau M, Rupp T, Perrey S, et al. Changes in voluntary activation assessed by transcranial magnetic stimulation during prolonged cycling exercise. PLoS One. 2014;9(2):e89157. doi: 10.1371/journal.pone.0089157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalography and Clinical neurology. 1958:270–375. [Google Scholar]
- 28.Rossini PM, Berardelli A, Deuschl G, et al. Applications of magnetic cortical stimulation. the international federation of clinical neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:171–185. [PubMed] [Google Scholar]
- 29.Godfrey SB, Lum PS, Chan E, Harris-Love ML. Cortical effects of repetitive finger flexion-vs. extension-resisted tracking movements: A TMS study. J Neurophysiol. 2013;109(4):1009–1016. doi: 10.1152/jn.00143.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carson RG, Nelson BD, Buick AR, Carroll TJ, Kennedy NC, Cann RM. Characterizing changes in the excitability of corticospinal projections to proximal muscles of the upper limb. Brain Stimul. 2013;6(5):760–768. doi: 10.1016/j.brs.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50(6):311–319. [PubMed] [Google Scholar]
- 32.Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128(Pt 3):490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 33.Wellek S, Blettner M. On the proper use of the crossover design in clinical trials: Part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2012;109(15):276–281. doi: 10.3238/arztebl.2012.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nitsche MA, Schauenburg A, Lang N, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15(4):619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- 35.Stagg CJ, O'Shea J, Kincses ZT, Woolrich M, Matthews PM, Johansen-Berg H. Modulation of movement-associated cortical activation by transcranial direct current stimulation. Eur J Neurosci. 2009;30(7):1412–1423. doi: 10.1111/j.1460-9568.2009.06937.x. [DOI] [PubMed] [Google Scholar]
- 36.Musselman K. Clinical sugnificance testing in rehabilitation research: What, why and how? Physical Therapy Reviews. 2007;12:287–296. [Google Scholar]
- 37.Ottenbacher KJ. Why rehabilitation research does not work (as well as we think it should) Arch Phys Med Rehabil. 1995;76(2):123–129. doi: 10.1016/s0003-9993(95)80021-2. [DOI] [PubMed] [Google Scholar]
- 38.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52(9):861–873. doi: 10.1016/s0895-4356(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 39.Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care. 1999;37(5):469–478. doi: 10.1097/00005650-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Manella KJ, Roach KE, Field-Fote EC. Operant conditioning to increase ankle control or decrease reflex excitability improves reflex modulation and walking function in chronic spinal cord injury. J Neurophysiol. 2013;109(11):2666–2679. doi: 10.1152/jn.01039.2011. [DOI] [PubMed] [Google Scholar]
- 41.Cohen J. Statistical power analysis for the behavioural sciences. New York: Academic Press; 1977. [Google Scholar]
- 42.Perez MA, Wise SP, Willingham DT, Cohen LG. Neurophysiological mechanisms involved in transfer of procedural knowledge. J Neurosci. 2007;27(5):1045–1053. doi: 10.1523/JNEUROSCI.4128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez MA, Tanaka S, Wise SP, Willingham DT, Cohen LG. Time-specific contribution of the supplementary motor area to intermanual transfer of procedural knowledge. J Neurosci. 2008;28(39):9664–9669. doi: 10.1523/JNEUROSCI.3416-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48(5):1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 45.Delvendahl I, Jung NH, Mainberger F, Kuhnke NG, Cronjaeger M, Mall V. Occlusion of bidirectional plasticity by preceding low-frequency stimulation in the human motor cortex. Clin Neurophysiol. 2010;121(4):594–602. doi: 10.1016/j.clinph.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 46.Rothkegel H, Sommer M, Paulus W. Breaks during 5Hz rTMS are essential for facilitatory after effects. Clin Neurophysiol. 2010;121(3):426–430. doi: 10.1016/j.clinph.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 47.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 48.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133(4):425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of Supplemental Digital Content
Supplemental Digital Content 1: Video abstract Gomes-Osmon.mpf