Abstract
Aims
To determine the impact of HIV infection on mortality over time among persons who inject drugs (PWID) in settings with free HIV/AIDS care.
Design and Setting
Prospective cohort study of PWID in Vancouver, Canada, recruited between May 1996 and December 2011. We ascertained morality rates and causes of death through a confidential linkage with the provincial vital statistics registry.
Participants
2283 individuals were followed for a median of 60.9 months (Interquartile range: 34.4 – 113.1) among whom 622 (27.2%) individuals were HIV-positive at baseline, and 179 (7.8%) seroconverted during follow-up.
Measurements
The primary and secondary outcomes of interests were all-cause mortality and cause of death, respectively. The main independent variable of interest was HIV serostatus (positive vs. negative). We used Cox proportional hazards regression to determine factors associated with mortality, including socio-demographic variables, drug use behaviors and other risk behaviors.
Findings
Over the study period, 491 (21.5%) individuals died. In multivariate analyses, HIV infection remained independently associated with all-cause mortality (adjusted hazard ratio = 3.15; 95% CI: 2.59 – 3.82). While all-cause mortality rates declined markedly during the study period (p < 0.001, the independent effect of HIV infection on mortality remained unchanged over time (p = 0.640). Among HIV-positive individuals, significant changes in causes of death from infectious and AIDS-related causes to non-AIDS-related etiologies were observed.
Conclusions
HIV infection continues to have a persistent impact on mortality rates among persons who inject drugs in settings with free HIV/AIDS care, though causes of death have shifted markedly from infectious and AIDS-related causes to non-AIDS-related etiologies.
Keywords: illicit drug use, mortality, HIV/AIDS, Vancouver
INTRODUCTION
Injection drug use is a well-established risk factor for morbidity and pre-mature mortality due to various causes, including HIV infection (1–3). A recent systematic review and meta-analysis that included over 60 cohorts of persons who inject drugs (PWID) demonstrated a pooled crude mortality rate of 2.4 per 100 person-years among this population, and found a pooled standardized mortality ratio to be almost 15 times higher among those who inject drugs compared to the general population (1). Premature mortality among this population results primarily from potentially preventable causes such as accidental overdose, infectious diseases including HIV infection, suicide and injuries (1–5).
Globally, it is estimated that one in three new HIV infections outside of Sub-Saharan Africa occur among PWID (6, 7) and HIV infection has consistently been a risk factor for premature mortality among this population (8). However, with advancements in and increased availability of combination antiretroviral therapy (ART), the natural history of HIV disease has changed such that people infected with HIV have enjoyed substantial reductions in HIV/AIDS-associated morbidity and mortality (9–11).
Previous studies have shown that PWID with optimal access and adherence to highly-active antiretroviral therapy (HAART) can benefit from HIV treatment to the same degree as other HIV-infected populations (12, 13). It is important to note, however, that PWID are often less likely to be prescribed ART, frequently because of clinicians’ fears of noncompliance and the subsequent development of antiretroviral resistance (14–16). Furthermore, in many settings PWID face numerous structural barriers to optimal HIV/AIDS care, not the least of which are financial barriers to HIV/AIDS treatment (14, 17, 18). As a result of these barriers, the improved survival rates seen in HIV populations overall have been less pronounced among HIV-positive PWID (1, 19–22).
Although there have been various past assessments of mortality among PWID (1, 22), there are few recent studies to examine the long term impacts of ART use and HIV infection on mortality, especially in settings with universal no-cost HIV/AIDS care and treatment. We therefore conducted the present study to determine the impact of HIV infection on mortality among PWID in a Canadian setting where access to all HIV/AIDS care and treatment is offered free of charge through a universal health care system.
METHODS
Study Sample
The Vancouver Injection Drug Users Study (VIDUS) and AIDS Care Cohort to Evaluate Access to Survival Services (ACCESS) are open prospective cohorts of persons who use drugs in Vancouver, Canada. To allow for combined analyses, the recruitment and follow-up procedures for the two studies are identical with the only substantive difference being that HIV positive individuals who use illicit drugs other than cannabis are followed in ACCESS, whereas HIV negative individuals who injected drugs in the month prior to the enrollment are followed in VIDUS. In both studies the primary modes of enrollment were self-referral, word of mouth, and street outreach. The shared sampling and recruitment procedures for these two cohorts have been described previously (23, 24).
Participants who were 18 years of age or older and resided in the greater Vancouver region were eligible to be enrolled in the two cohorts. All participants provided written informed consent. Participants were given a stipend ($20 CDN) at each study visit for their time and transportation. The study was approved by the University of British Columbia/Providence Healthcare Research Ethics Board.
Outcome Measures
At baseline and at semianual follow-up visits, participants completed an interviewer-administered questionnaire that elicited a range of data, including demographic characteristics, information regarding injection and non-injection drug use, and sexual risk behaviors. In addition, venous blood samples were drawn to test for HIV and hepatitis C virus (HCV) at baseline and at each follow-up visit for individuals whose test results were negative at the previous assessment. All participants had private interviews and were offered both pre- and post-test counseling with trained nurses. Referral for free healthcare was provided to those who tested positive for HIV, and these individuals were subsequently followed in the ACCESS cohort, rather than VIDUS.
We ascertained all-cause mortality rates and underlying causes of death among participants through a confidential record linkage with the British Columbia Vital Statistics Agency, the centralized mortality registry for the province of British Columbia. The provincial Vital Statistics database recorded causes of death according to the International Classification of Diseases, 10th edition (ICD-10).
The present study included individuals who reported injection drug use in the previous six months at baseline and completed at least one follow-up visit between May 1996 and December 2011. To avoid potential bias relating to long durations between the last study visit where HIV status and behavioural information were assessed, and the date of death (i.e., loss to regular follow-up), individuals who were identified as deceased more than 24 months after the last follow-up visit were treated as censored on the date of the last follow-up and only considered in sub-analyses.
The primary endpoint in this analysis was all-cause mortality. The primary explanatory variable of interest was HIV serostatus (positive vs. negative), and this was treated as a time-updated covariate based on semi-annual HIV testing. Potential confounders that were considered included: gender (male vs. female), age (per 10 years older), ethnicity (Caucasian vs. other), homelessness (yes vs. no), daily heroin injection (yes vs. no), daily cocaine injection (yes vs. no), daily non-injection cocaine use (yes vs. no), years since first injection (per year longer), HCV serostatus (positive vs. negative), sex work involvement (yes vs. no), enrollment in methadone maintenance therapy (yes vs. no) and calendar year (four-year intervals). With the exception of gender and ethnicity, all other variables were measured at each semiannual follow-up visit and were treated as time-updated. All behavioural variables referred to the participant’s behavior in the six months prior to the interview. Sex work involvement was defined as exchanging sex for money, gifts, food, shelter, clothes, etc. as in a previous study (25).
Statistical Analyses
First, we compared the baseline characteristics of participants using the Chi-square test (for binary measures) and the t-test (for continuous measures). All-cause mortality rate and 95% confidence interval (CI) were then calculated using the Poisson distribution. Survival probabilities for baseline HIV negative and HIV positive participants were estimated using the Kaplan-Meier product limit method, and compared using the two-sample log-rank test.
We then used Cox proportional hazards regression to examine bivariate associations between each explanatory variable and the time to all-cause mortality. We subsequently used Cox proportional hazards regression to develop a fixed multivariate model which included HIV serostatus and all of the covariates to account for potential confounding.
To determine if the independent effect of HIV serostatus on all-cause mortality has changed over time, we subsequently tested for significant interactions between HIV serostatus and calender year. We graphically presented the adjusted hazard ratios and 95% CIs for HIV serostatus over time with the follow up period divided into four-year intervals.
ART Adherence
In order to determine the potential effect of ART adherence on mortality among those who were HIV positive, we calculated mortality rates for those who were HIV positive and had ≥ 95% ART adherence, and compared them with the mortality rates of the persistently HIV negative participants. We selected ≥ 95% adherence as previous studies have shown that ART adherence rates of ≥ 95% are an important predictor of survival (9) and slower progression of disease (26), and are also associated with improved virologic and immunologic outcomes (27–29). Data on HIV treatment was obtained from the British Columbia Centre for Excellence in HIV/AIDS Drug Treatment program as described elsewhere (13). In brief, information from a province-wide centralized ART pharmacy provides complete information on all antiretroviral medications dispensed to all participants during the study period. We measured adherence to therapy using pharmacy refill data. We have previously demonstrated the clinical validity of this pharmacy refill data and have shown that it reliably predicts virologic suppression (27, 30) and survival (9, 13).
Causes of Death
A sub-analysis was conducted where we examined the cause of death according to ICD-10 classification, among those patients who were HIV-positive in order to specifically examine trends in mortality over time. Causes of death were classified into three groups: HIV-related causes of death (ICD-10 codes: B20–24 and R99 with descriptions “HIV” or “HIV related” in the community follow-up record), accidental causes of death (including overdoses, suicides and homicides), and non-AIDS-related causes. In order to examine if there has been a change in the number of deaths related to infectious etiologies compared to non-infectious causes over time, we also classified deaths in the non-AIDS-related group as either infectious or non-infectious and examined trends over time.
All statistical analyses were performed using SAS software version 9.3 (SAS, Cary, NC). All p-values were two-sided.
RESULTS
Study sample
A total of 2597 eligible individuals were recruited between May 1996 and December 2011, among whom 314 (12.1%) were ineligible due to the lack of follow-up visit information to ascertain behavioral or biological information. In comparing the study sample to those that were excluded for this reason, the excluded sample was younger, and was more likely to be homeless, to be HCV-positive, and had shorter time since first injection (all p < 0.05), but there was no difference by HIV status (p = 0.28). The mortality rate for the excluded sample was 0.44 (95% CI: 0.25 – 0.77) deaths per 100 person-years.
In total, 2283 individuals were included in the present analyses and were followed for a median of 60.9 months (interquartile range [IQR]: 34.4 – 113.1). Table 1 shows the characteristics of the cohort stratified by HIV serostatus at baseline. At baseline, 622 (27.2%) were HIV-positive, and 1925 (84.3%) were HCV-positive. Compared to HIV-negative individuals, those who were HIV-positive at baseline were more likely to be older, to participate in a methadone program, to have a longer time since first injection and to be co-infected with HCV. They were less likely to be Caucasian and to inject heroin daily.
TABLE 1.
Baseline characteristics of the study sample stratified by HIV serostatus at baseline (n = 2283).
| HIV serostatus | |||||
|---|---|---|---|---|---|
| Characteristic | Total n (%) |
HIV-positive 622 (27.2) |
HIV-negative 1661 (72.8) |
Odds Ratio£ (95% CI) |
p-value |
| Gender | |||||
| Male | 1522 (66.7) | 402 (64.6) | 1120 (67.4) | 0.88 (0.73–1.07) | 0.213 |
| Female | 761 (33.3) | 220 (35.4) | 541 (32.6) | ||
| Age, in years (mean, SD‡) | 36.9 (9.7) | 38.9 (9.1) | 36.2 (9.8) | 2.7 (1.9–3.6)§ | <0.001 |
| Ethnicity | |||||
| Caucasian | 1395 (61.1) | 359 (57.7) | 1036 (62.4) | 0.82 (0.68–0.99) | 0.043 |
| Other | 888 (38.9) | 263 (42.3) | 625 (37.6) | ||
| Homelessness* | |||||
| Yes | 510 (22.3) | 137 (22.0) | 373 (22.5) | 0.98 (0.79–1.23) | 0.910 |
| No | 1769 (77.5) | 481 (77.3) | 1288 (77.5) | ||
| Sex work involvement* | |||||
| Yes | 538 (23.6) | 164 (26.4) | 374 (22.5) | 1.23 (1.00–1.52) | 0.059 |
| No | 1737 (76.1) | 456 (73.3) | 1281 (77.1) | ||
| Daily heroin injection* | |||||
| Yes | 882 (38.6) | 164 (26.4) | 718 (43.2) | 0.47 (0.38–0.57) | <0.001 |
| No | 1396 (61.2) | 458 (73.6) | 938 (56.5) | ||
| Daily cocaine injection* | |||||
| Yes | 711 (31.1) | 204 (32.8) | 507 (30.5) | 1.11 (0.91–1.35) | 0.335 |
| No | 1558 (68.2) | 416 (66.9) | 1142 (68.8) | ||
| Daily non-injection cocaine use* | |||||
| Yes | 546 (23.9) | 161 (25.8) | 385 (23.2) | 1.16 (0.94–1.43) | 0.186 |
| No | 1734 (76.0) | 460 (74.0) | 1274 (76.7) | ||
| Enrollment in a methadone program* | |||||
| Yes | 514 (22.5) | 189 (30.4) | 325 (19.6) | 1.80 (1.46–2.22) | <0.001 |
| No | 1759 (77.1) | 430 (69.1) | 1329 (80.0) | ||
| Years since first injection, in years | |||||
| 15.6 (10.7) | 17.2 (10.3) | 15.0 (10.8) | 2.2 (1.2–3.2)§ | <0.001 | |
| (mean, SD‡) | |||||
| HCV serostatus | |||||
| Positive | 1925 (84.3) | 591 (95.0) | 1334 (80.3) | 4.67 (3.19–6.84) | <0.001 |
| Negative | 358 (15.7) | 31 (5.0) | 327 (19.7) | ||
Refers to behaviors in the last six months.
Refers to difference between means.
SD = standard deviation.
Comparisons were made as HIV-positive vs. HIV-negative.
Mortality rates
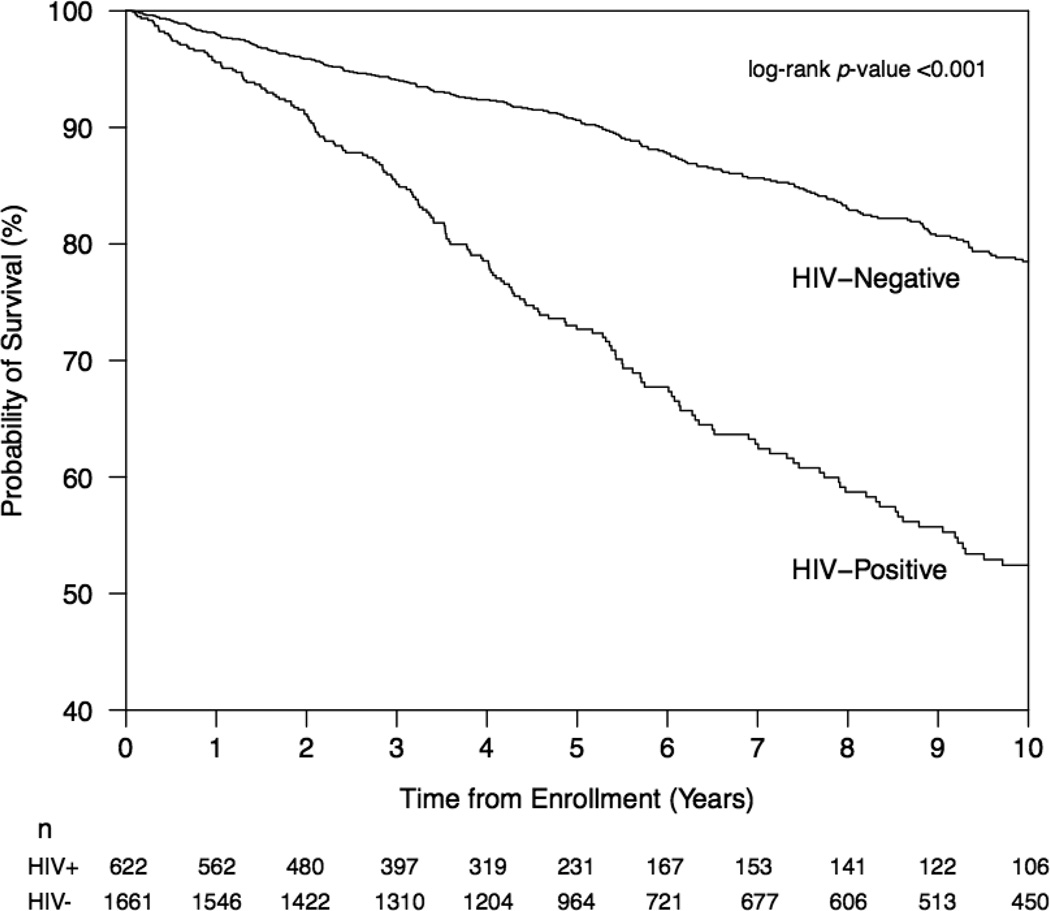
During the study period, 179 (7.8%) individuals seroconverted to HIV, and 491 (21.5%) individuals died for an incidence density of mortality of 3.23 (95% CI: 2.96 – 3.52) deaths per 100 person-years. Figure 1 shows the results of the Kaplan-Meier analysis of time to all-cause mortality stratified by HIV serostatus at baseline. As shown, HIV-positive individuals were significantly more likely to die during follow-up than HIV-negative individuals (p < 0.001).
Figure 1.
Kaplan-Meier Survival Curve showing cumulative survival probability from all-cause mortality, stratified by baseline HIV seropositivity
Table 2 shows results of the bivariate and multivariate Cox regression analyses of all-cause mortality. In the multivariate analysis, after adjustment for potential confounders, HIV seropositivity remained independently and positively associated with time to all-cause death (adjusted hazard ratio [AHR] = 3.15, 95% CI: 2.59 – 3.82). Other variables that were independently and positively associated with time to all-cause mortality included age (AHR = 1.43, 95% CI: 1.23 – 1.66, per 10 years older), longer time since first injection (AHR = 1.01, 95% CI: 1.00 – 1.03, per year longer) and homelessness (AHR = 1.16, 95% CI: 0.90 – 1.50), while participation in a methadone maintenance program (AHR = 0.78, 95% CI: 0.65 – 0.95) was negatively associated with time to all cause mortality.
TABLE 2.
Bivariate and multivariate Cox proportional hazard regression analyses of the time to all-cause mortality among persons who inject drugs in Vancouver, Canada (n = 2283).
| Unadjusted Hazard Ratio (HR) | Adjusted Hazard Ratio (AHR)† | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P value | AHR | 95% CI | P value |
|
HIV serostatus* (positive vs. negative) |
2.88 | 2.41–3.45 | <0.001 | 3.15 | 2.59–3.82 | <0.001 |
|
Gender (male vs. female) |
1.10 | 0.91–1.32 | 0.339 | 0.96 | 0.78–1.18 | 0.683 |
|
Ethnicity (Caucasian vs. other) |
1.06 | 0.89–1.27 | 0.499 | 1.10 | 0.91–1.34 | 0.330 |
|
Sex work involvement* (yes vs. no) |
0.77 | 0.57–1.04 | 0.091 | 0.94 | 0.67–1.31 | 0.699 |
|
Daily heroin injection* (yes vs. no) |
0.80 | 0.64–1.01 | 0.057 | 0.91 | 0.72–1.15 | 0.420 |
|
Daily cocaine injection* (yes vs. no) |
1.43 | 1.14–1.79 | 0.002 | 1.21 | 0.96–1.53 | 0.104 |
|
Daily non-injection cocaine use* (yes vs. no) |
0.89 | 0.73–1.08 | 0.235 | 1.05 | 0.84–1.30 | 0.690 |
|
Years since first injection (per year longer) |
1.02 | 1.01–1.03 | <0.001 | 1.01 | 1.00–1.03 | 0.050 |
|
HCV serostatus* (Positive vs. Negative) |
1.94 | 1.26–3.00 | 0.003 | 0.92 | 0.57–1.46 | 0.711 |
|
Calendar year (1996–1999) |
1.00 | 1.00 | ||||
| (2000–2003) | 0.54 | 0.38–0.79 | 0.001 | 0.56 | 0.38–0.82 | 0.003 |
| (2004–2007) | 0.58 | 0.39–0.85 | 0.006 | 0.61 | 0.40–0.94 | 0.023 |
| (2008–2011) | 0.33 | 0.22–0.48 | <0.001 | 0.28 | 0.19–0.40 | <0.001 |
Refers to behaviors in the last six months.
Also adjusted for age, homelessness, and enrollment in methadone.
Mortality over time
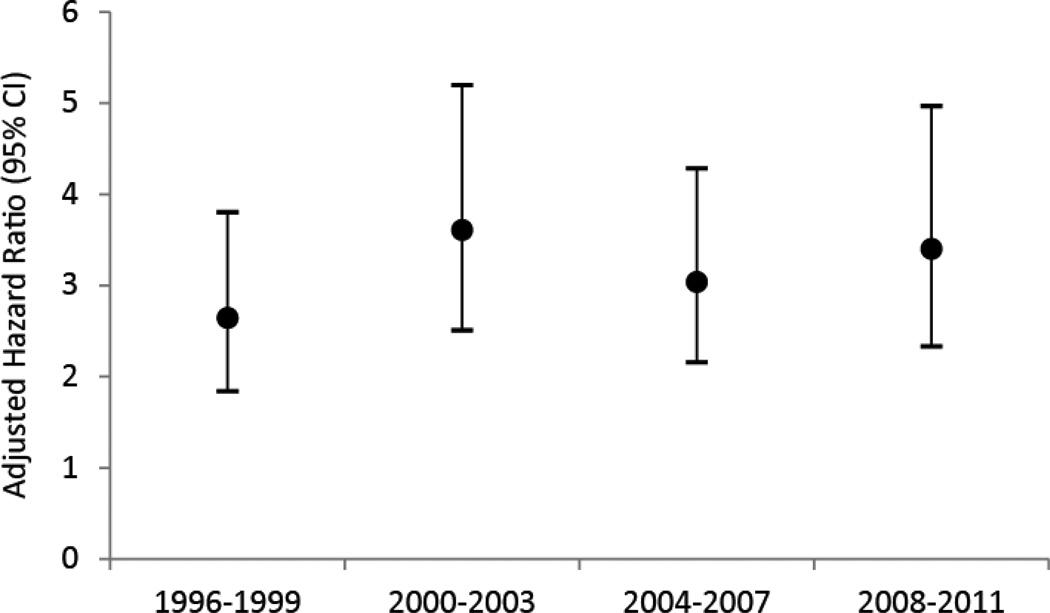
In a multivariate analysis, calendar year was independently and negatively associated with time to all-cause death. Using the calendar year interval of 1996 – 1999 as a reference group, statisticially significant reductions in all-cause mortality were seen in the year interval 2000–2003, in 2004–2007 and in 2008–2011. However, no statistically significant interaction was found with HIV serostatus and calendar year (p = 0.640). As shown in Figure 2, the independent effect of HIV serostatus on mortality did not change significantly when the adjusted hazard ratios were examined graphically over time. In sub-analyses (data available from the corresponding author), we found that adjusting for cohort of recruitment did not confound the effect of other covariates.
Figure 2.
Adjusted hazard ratios for HIV seropositivity to all-cause mortality by four-year intervals.
ART Adherence
The overall mortality rate for all HIV negative participants was 2.11 (95% CI: 1.85 – 2.40) deaths per 100 person-years, while the mortality rates for HIV positive participants with ≥ 95% adherence to ART was 4.60 (95% CI: 3.65 – 5.79) deaths per 100 person-years. Despite having ≥ 95% adherence to ART, the HIV positive participants in this group continued to have an increased risk of death (Hazard Ratio = 2.17, 95% CI: 1.65 – 2.85), compared to those who were HIV negative.
Causes of death
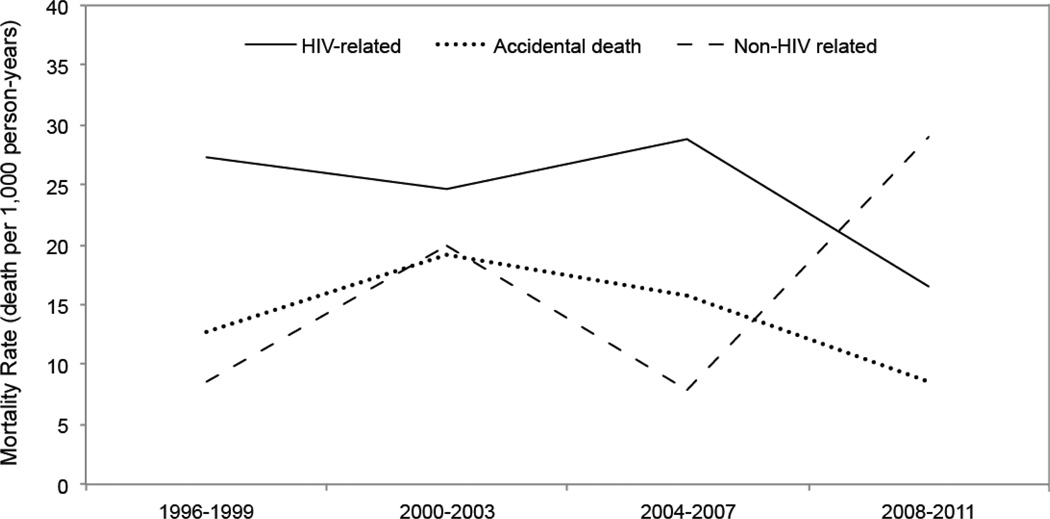
We then grouped causes of death among HIV-positive individuals as defined a priori into three mutually exclusive groups: HIV-related, accidental (including overdoses) and non-AIDS-related causes. As shown in Figure 3, HIV-related mortality rates fell from a high of 27.29 deaths (95% CI: 19.32 – 38.53) per 1,000 person-years in the 1996–1999 period to 16.46 deaths (95% CI: 11.10 – 24.39) per 1,000 person-years in the 2008–2011 period. Rates of accidental deaths fell from 12.79 deaths (95% CI: 7.71 – 21.22) per 1,000 person-years in the 1996–1999 period to 8.56 deaths (95% CI: 4.97 – 14.74) per 1,000 person-years in the 2008–2011 period. Finally, non-AIDS-related mortality rates rose from 8.53 deaths (95% CI: 4.59 – 15.85) per 1,000 person-years in the 1996–1999 period to 28.96 deaths (95% CI: 21.67 – 38.71) per 1,000 person-years in the 2008–2011 period. Although there was a high prevalence of HCV infection among the total cohort (84.3%), we only observed 3 deaths among the HIV-negative group and 5 deaths among the HIV-positive group that were attributable to HCV infection throughout the study period.
Figure 3.
Causes of death among HIV positive participants between 1996 and 2011.
Of those classified as non-AIDS-related causes, the primary underlying causes of death included infections (24.7%), non-AIDS defining neoplasms (15.1%), respiratory disease (11.0%), cerebrovascular disease (8.2%) and cardiovascular disease (5.5%). Nineteen of the deaths in this category (26.0%) were specified as “other ill-defined and unspecific cause mortality.” The remaining causes of deaths individually contributed to less than 5% of mortality in this category. Among those classified in the non-AIDS-related group, we saw a decline in the number of deaths from infectious etiologies over the study period, with 5.99 deaths (95% CI: 2.86 – 12.58) per 1,000 person-years in the 1996–1999 calendar year interval to 2.63 (95% CI: 0.99 – 7.03) deaths per 1,000 person-years in the 2008–2011 calendar year interval. There was a steady increase in deaths from non-infectious causes over the study period from 2.57 deaths (95% CI: 0.83 – 7.96) per 1,000 person-years in 1996–1999 calendar year interval to 26.33 deaths (95% CI: 19.43 – 35.67) per 1,000 person-years in the 2008–2011 calendar year interval.
DISCUSSION
The present study demonstrated that among a longstanding sample of PWID, HIV-positive individuals were at a significantly increased risk of death compared to those who were HIV-negative, even after consideration of age, ART adherence and other important risk factors for mortality. Our study also demonstrated that although all-cause mortality rates have declined substantially during the study period, the effect of HIV infection on mortality has been persistent and largely unchanged. This has occurred despite free healthcare services and free ART through universal coverage for all HIV/AIDS care. Among HIV-positive individuals who died during the study period, we observed a marked shift away from deaths from AIDS-related etiologies (i.e., AIDS defining illnesses) and accidental deaths (including overdoses) to deaths from other causes (i.e., non-AIDS defining malignancies, respiratory disease, cerebrovascular disease as well as cardiac disease). While the proportion of deaths related to chronic etiologies appears to be rising among persons with HIV, close to one quarter of the non-AIDS-related deaths in our study were related to infectious causes which may be a reflection of HIV positive PWID being at elevated risk of non-AIDS associated infectious diseases (31).
Our findings showing changing patterns in causes of death among HIV-positive PWID are congruent with the previous literature that has focused primarily on groups other than PWID (32). Specifically, recent evidence suggests that HIV-positive individuals who have demonstrated viral suppression on ART continue to have a higher than expected risk for comorbidities that are commonly seen in an aging HIV-negative population, including cardiovascular disease, renal disease, liver disease and non-AIDS related cancers (32–35). This suggests that HIV infection, even when well managed with ART, likely produces chronic inflammation which contributes to increased atherosclerotic risk (34, 36). Furthermore, cumulative exposure to ART over decades can be associated with metabolic changes that may increase the risk of cerebrovascular disease including body fat redistribution (central lipoaccumulation and peripheral lipoatrophy), as well as insulin resistance, diabetes and hyperlipidemia (37). An important consideration for persons living with HIV with a history of injection drug use is related to other lifestyle issues like tobacco use (38, 39). While past studies have shown that individuals with a history of illicit drug addiction (e.g., heroin) are amenable to smoking cessation strategies (40), this intervention is commonly overlooked in this population (41).
Our findings of declining overdose deaths over the study period may be related to improved harm reduction strategies in the Vancouver area. Previous literature has shown that the opening of Insite, a supervised injection facility in Vancouver’s Downtown Eastside, was associated with a significant decline in the number of fatal overdose deaths in the surrounding neighbourhood (42). Additionally, interaction with the staff and nurses at harm reduction services provides persons who inject drugs an opportunity to seek medical attention, for acute infections before they become life threatening for example (43). Additionally, previous studies among this cohort have observed a pattern in reduced injection drug use (44), in favor of increased crack smoking (45), which may also contribute to the decreased overdose deaths observed in this study. Finally, the number of patients enrolled in methadone maintenance therapy has increased in British Columbia, Canada over time (46), an intervention which has previously been shown to decrease mortality rates (47, 48).
Our study has several limitations. First, as the study was not recruited at random, our findings may not be generalizable to all PWID. Second, the self-reported data may be affected by reporting biases, including recall bias and socially desirable responding. However, we note that our endpoints were based on laboratory (i.e. HIV infection) and mortality data, and self-reported data has been commonly used to control for potential confouding in observational studies involving PWID and found to be valid (49). Third, as with all observational studies, the relationships between the explanatory variables and outcome assessed may be under the influence of unmeasured confounding. While we sought to address this bias with multivariate adjustment of the key demographic and behavioral predictors of survival, there may be residual confounding. For example, we were unable to control for tobacco use in this study, which, as mentioned earlier, contributes to increased morbidity and mortality among PWID who are HIV-positive (39). Fourth, mortality rates may have been underestimated, as participants who died outside of the province were not included in the provincial registry and were thus not accounted for. However, previous studies have shown that migration rates among drug users are relatively low in this setting (50). Finally, when examining the causes of death, over one quarter of patients were specified as dying from “other ill-defined and unspecific cause mortality,” making it difficult to assess true trends in all causes of death.
Despite advances in the availability and tolerability of ART, HIV infection continues to play a significant and ongoing role on mortality rates among PWID in this setting. These findings highlight the necessity to continue to promote and implement HIV prevention services among this population. Of those who are HIV-positive, an increasing proportion of deaths was attributable to infections as well as chronic disease. This underscores the importance of encouraging safe injection practices to prevent infections, as well as engaging in chronic disease management and other health promotion (e.g. smoking cessation) activities in these patients. Once patients are established on ART, treatment priorities must shift to a preventative approach for chronic disease while remaining closely attuned to infections and illnesses associated with HIV infection.
Acknowledgements
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff.
The study was supported by the US National Institutes of Health (VIDUS: R01DA011591,). This research was undertaken, in part, thanks to funding for a Tier 1 Canada Research Chair in Inner City Medicine, which supports Dr. Evan Wood. Dr. Milloy is supported by the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research. Dr. Hayashi is supported by the Canadian Institutes of Health Research.
Footnotes
Declaration of interest: The authors declare no other competing interests.
REFERENCES
- 1.Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bulletin of the World Health Organization. 2013;91(2):102–123. doi: 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hickman M, Carnwath Z, Madden P, Farrell M, Rooney C, Ashcroft R, et al. Drug-related mortality and fatal overdose risk: pilot cohort study of heroin users recruited from specialist drug treatment sites in London. Journal of urban health : bulletin of the New York Academy of Medicine. 2003;80(2):274–287. doi: 10.1093/jurban/jtg030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller CL, Kerr T, Strathdee SA, Li K, Wood E. Factors associated with premature mortality among young injection drug users in Vancouver. Harm reduction journal. 2007;4:1. doi: 10.1186/1477-7517-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gossop M, Stewart D, Treacy S, Marsden J. A prospective study of mortality among drug misusers during a 4-year period after seeking treatment. Addiction. 2002;97(1):39–47. doi: 10.1046/j.1360-0443.2002.00079.x. [DOI] [PubMed] [Google Scholar]
- 5.Degenhardt L, Hall W, Warner-Smith M. Using cohort studies to estimate mortality among injecting drug users that is not attributable to AIDS. Sexually transmitted infections. 2006;82(Suppl 3):iii56–iii63. doi: 10.1136/sti.2005.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Report on the global HIV/AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2006. HIV/AIDS JUNPo. [Google Scholar]
- 7.De Cock KM, Jaffe HW, Curran JW. The evolving epidemiology of HIV/AIDS. AIDS. 2012;26(10):1205–1213. doi: 10.1097/QAD.0b013e328354622a. [DOI] [PubMed] [Google Scholar]
- 8.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Annals of internal medicine. 2006;145(6):397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 9.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, Montaner JS. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 × 10(9) cells/L. Annals of internal medicine. 2003;139(10):810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 10.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 11.Collaboration H-C, Ray M, Logan R, Sterne JA, Hernandez-Diaz S, Robins JM, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24(1):123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celentano DD. Mortality among urban drug users and the impact of highly active antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41(6):873–874. doi: 10.1086/432895. [DOI] [PubMed] [Google Scholar]
- 13.Wood E, Hogg RS, Lima VD, Kerr T, Yip B, Marshall BD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA : the Journal of the American Medical Association. 2008;300(5):550–554. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376(9738):355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- 15.Ding L, Landon BE, Wilson IB, Wong MD, Shapiro MF, Cleary PD. Predictors and consequences of negative physician attitudes toward HIV-infected injection drug users. Archives of internal medicine. 2005;165(6):618–623. doi: 10.1001/archinte.165.6.618. [DOI] [PubMed] [Google Scholar]
- 16.Maisels L, Steinberg J, Tobias C. An investigation of why eligible patients do not receive HAART. AIDS patient care and STDs. 2001;15(4):185–191. doi: 10.1089/10872910151133701. [DOI] [PubMed] [Google Scholar]
- 17.Human Rights Watch. Deadly denial: barriers to HIV/AIDS treatment for people who use drugs in Thailand. New York: 2007. [Google Scholar]
- 18.Bobrova N, Sarang A, Stuikyte R, Lezhentsev K. Obstacles in provision of anti-retroviral treatment to drug users in Central and Eastern Europe and Central Asia: a regional overview. The International journal on drug policy. 2007;18(4):313–318. doi: 10.1016/j.drugpo.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Lert F, Kazatchkine MD. Antiretroviral HIV treatment and care for injecting drug users: an evidence-based overview. The International journal on drug policy. 2007;18(4):255–261. doi: 10.1016/j.drugpo.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aceijas C, Oppenheimer E, Stimson GV, Ashcroft RE, Matic S, Hickman M. Antiretroviral treatment for injecting drug users in developing and transitional countries 1 year before the end of the "Treating 3 million by 2005. Making it happen. The WHO strategy" ("3 by 5") Addiction. 2006;101(9):1246–1253. doi: 10.1111/j.1360-0443.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 21.Vlahov D, Celentano DD. Access to highly active antiretroviral therapy for injection drug users: adherence, resistance, and death. Cadernos de saude publica. 2006;22(4):705–718. doi: 10.1590/s0102-311x2006000400002. [DOI] [PubMed] [Google Scholar]
- 22.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyndall MW, Currie S, Spittal P, Li K, Wood E, O'Shaughnessy MV, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17(6):887–893. doi: 10.1097/00002030-200304110-00014. [DOI] [PubMed] [Google Scholar]
- 24.Strathdee SA, Palepu A, Cornelisse PG, Yip B, O'Shaughnessy MV, Montaner JS, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA : the Journal of the American Medical Association. 1998;280(6):547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 25.Ti LMM, Shannon K, Simo A, Hogg RS, Guillemi S et L et al. Suboptimal plasma HIV-1 RNA suppression and adherence among sex workers who use illicit drugs in a Canadian setting: an observational cohort study. Sexually transmitted infections. 2014 doi: 10.1136/sextrans-2013-051408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 27.Low-Beer S, Yip B, O'Shaughnessy MV, Hogg RS, Montaner JS. Adherence to triple therapy and viral load response. Journal of acquired immune deficiency syndromes. 2000;23(4):360–361. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 28.Nolan S, Milloy MJ, Zhang R, Kerr T, Hogg RS, Montaner JS, et al. Adherence and plasma HIV RNA response to antiretroviral therapy among HIV-seropositive injection drug users in a Canadian setting. AIDS care. 2011;23(8):980–987. doi: 10.1080/09540121.2010.543882. [DOI] [PubMed] [Google Scholar]
- 29.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, Montaner JS. The impact of adherence on CD4 cell count responses among HIV-infected patients. Journal of acquired immune deficiency syndromes. 2004;35(3):261–268. doi: 10.1097/00126334-200403010-00006. [DOI] [PubMed] [Google Scholar]
- 30.Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O'Shaughnessy MV, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2003;169(7):656–661. [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon RJ, Lowy FD. Bacterial infections in drug users. The New England Journal of Medicine. 2005;353(18):1945–1954. doi: 10.1056/NEJMra042823. [DOI] [PubMed] [Google Scholar]
- 32.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. Journal of acquired immune deficiency syndromes. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 33.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA : the Journal of the American Medical Association. 2013;309(13):1397–1405. doi: 10.1001/jama.2013.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(11):1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 36.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine. 2013;173(8):614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotler DP. HIV and antiretroviral therapy: lipid abnormalities and associated cardiovascular risk in HIV-infected patients. Journal of acquired immune deficiency syndromes. 2008;49(Suppl 2):S79–S85. doi: 10.1097/QAI.0b013e318186519c. [DOI] [PubMed] [Google Scholar]
- 38.Petoumenos K, Worm S, Reiss P, de Wit S, d'Arminio Monforte A, Sabin C, et al. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study(*) HIV medicine. 2011;12(7):412–421. doi: 10.1111/j.1468-1293.2010.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall MM, Kirk GD, Caporaso NE, McCormack MC, Merlo CA, Hague JC, et al. Tobacco use and nicotine dependence among HIV-infected and uninfected injection drug users. Addictive behaviors. 2011;36(1–2):61–67. doi: 10.1016/j.addbeh.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of consulting and clinical psychology. 2004;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 41.Khara M, Okoli CT. The tobacco-dependence clinic: intensive tobacco-dependence treatment in an addiction services outpatient setting. The American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2011;20(1):45–55. doi: 10.1111/j.1521-0391.2010.00096.x. [DOI] [PubMed] [Google Scholar]
- 42.Marshall BD, Milloy MJ, Wood E, Montaner JS, Kerr T. Reduction in overdose mortality after the opening of North America's first medically supervised safer injecting facility: a retrospective population-based study. Lancet. 2011;377(9775):1429–1437. doi: 10.1016/S0140-6736(10)62353-7. [DOI] [PubMed] [Google Scholar]
- 43.Wood E, Tyndall MW, Montaner JS, Kerr T. Summary of findings from the evaluation of a pilot medically supervised safer injecting facility. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2006;175(11):1399–1404. doi: 10.1503/cmaj.060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werb D, Kerr T, Buxton J, Shoveller J, Richardson C, Montaner J, et al. Patterns of injection drug use cessation during an expansion of syringe exchange services in a Canadian setting. Drug and alcohol dependence. 2013;132(3):535–540. doi: 10.1016/j.drugalcdep.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werb D, Debeck K, Kerr T, Li K, Montaner J, Wood E. Modelling crack cocaine use trends over 10 years in a Canadian setting. Drug and alcohol review. 2010;29(3):271–277. doi: 10.1111/j.1465-3362.2009.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nosyk B, Marsh DC, Sun H, Schechter MT, Anis AH. Trends in methadone maintenance treatment participation, retention, and compliance to dosing guidelines in British Columbia, Canada: 1996–2006. Journal of substance abuse treatment. 2010;39(1):22–31. doi: 10.1016/j.jsat.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Fugelstad A, Stenbacka M, Leifman A, Nylander M, Thiblin I. Methadone maintenance treatment: the balance between life-saving treatment and fatal poisonings. Addiction. 2007;102(3):406–412. doi: 10.1111/j.1360-0443.2006.01714.x. [DOI] [PubMed] [Google Scholar]
- 48.Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O'Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462–448. doi: 10.1111/j.1360-0443.2007.02090.x. [DOI] [PubMed] [Google Scholar]
- 49.Darke S. Self-report among injecting drug users: a review. Drug and alcohol dependence. 1998;51(3):253–263. doi: 10.1016/s0376-8716(98)00028-3. discussion 67-8. [DOI] [PubMed] [Google Scholar]
- 50.Rachlis BS, Wood E, Li K, Hogg RS, Kerr T. Drug and HIV-related risk behaviors after geographic migration among a cohort of injection drug users. AIDS and behavior. 2010;14(4):854–861. doi: 10.1007/s10461-008-9397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]