Abstract
The critical roles of integrins in thrombosis have enabled the successful development and clinical use of the first generation of integrin antagonists as represented by abciximab (Reopro), eptifibatide (Integrilin), and tirofiban (Aggrastat). These integrin αIIb β3 antagonists are potent anti-thrombotics, but also have significant side effects. In particular, their induction of ligand-induced integrin conformational changes is associated with thrombocytopenia. Increased bleeding risk prevents integrin antagonists from being used at higher doses and in patients at risk for bleeding. To address the ligand-induced conformational changes caused by current integrin antagonists, compounds that minimally induce conformational changes in integrin αIIb β3 have been developed. Recent studies on the mechanisms of integrin signaling suggest that selectively targeting integrin outside-in signaling mechanisms allows for potent inhibition of thrombosis while maintaining hemostasis in animal models.
Keywords: platelets, integrins, thrombosis, outside-in signaling, Platelet inhibitors
Introduction
Integrins, a family of cell adhesion receptors, play important roles in cell adhesion, spreading, retraction, migration, anchorage-dependent survival and proliferation. Integrins exist as an α:β heterodimeric complex of transmembrane proteins. In blood platelets, the most abundant integrin is integrin αIIb β3. Integrin αIIb β3 binds to fibrinogen through the HHLGGAKQAGV sequence in the C-terminus of the fibrinogen γ chain and RGD sequences in the α chain. RGD-like sequences are also present in several other integrin-binding adhesive proteins including vitronectin, fibronectin and von Willebrand factor. In addition, platelets express integrins αV β3, α2 β1, α6 β1, and α5 β1, among which α5 β1 and αV β3 also recognize the RGD sequence. Integrin α2 β1 and α6 β1 bind to collagen and laminin1. By binding to adhesive proteins, the integrins mediate platelet adhesion to injured vascular wall and platelet aggregation, which is important for the maintenance of hemostasis, preventing excessive bleeding. The importance of integrin αIIb β3 in hemostasis is exemplified in patients suffering from Glanzmann’s thrombasthenia, in which genetic deficiencies in integrin αIIb β3 causes bleeding diathesis2. Integrin αIIb β3 is critical for arterial thrombosis3, which is evident by the protective effects seen in experimental models of thrombosis using either pharmacologic inhibition or genetic deletion/mutation of integrin αIIb β34, 5; and by the clinical efficacy of αIIb β3 antagonists6–8. However, despite successful clinical use of integrin antagonists as potent anti-thrombotics, their use is primarily limited to patients undergoing percutaneous coronary intervention, mainly due to significant bleeding risk. In fact, increased bleeding risks are a major problem shared by all currently available anti-thrombotic drugs. In this review, we briefly discuss the major problems associated with the currently used integrin antagonists, and new advances in developing the next generation of integrin antagonists.
Current αIIb β3 Integrin Antagonists
The three current FDA-approved platelet integrin antagonists are designed to block the ligand binding function of integrin αIIb β3. Among these drugs, abciximab (Reopro) is a ~48 kilodalton mouse/human chimeric antibody fragment that binds to an epitope near the ligand binding site of β34, 9–12; eptifibatide (Integrilin), is a 832 dalton synthetic disulfide-linked cyclic heptapeptide ligand-mimetic, containing an integrin binding sequence, KGD, based on a snake venom peptide, barbourin9, 12–14; tirofiban (Aggrastat) is a 495 dalton synthetic compound, engineered to mimic RGD sequence9, 12, 14–16. Both eptifibatide and tirofiban are integrin ligand mimetics, which interact with the ligand-binding site of integrin αIIb β312. Tirofiban appears to be specific for αIIb β3. Eptifibatide inhibits αIIb β3 and αV β3, and abciximab inhibits αIIb β3, αV β3 and αM β212, 17, 18. All three integrin antagonists are administered intravenously. Orally active integrin antagonists were also developed. However, clinical trials of oral integrin antagonists suggested increased mortality instead of beneficial effects19, 20.
The current integrin antagonists have each demonstrated clear therapeutic benefits in high-risk patients undergoing percutaneous coronary intervention (PCI), as indicated by significant reductions in death and reoccurrence of myocardial infarction6, 7, 9, 14. There have also been clinical trials studying the effect of integrin antagonist treatment on patients suffering from acute ischemic stroke. Although, these trials so far have been mainly designed for the purpose of determining safety, and thus the therapeutic efficacy in stroke patients is yet to be conclusively established. In these trials, αIIb β3 antagonist treatment alone showed no beneficial impact on mortality or debilitating stroke-related outcomes21, 22, but increased the incidence of symptomatic or fatal intracranial hemorrhage21, 23, with the exception of a trial of tirofiban24. In the tirofiban trial, no significant difference in hemorrhage was found between placebo and tirofiban groups, although the placebo group had significantly more patients also treated with aspirin, which may influence the outcome. Some clinical trials tested a combination of fibrinolytic therapy, using recombinant tissue plasminogen activator (r-tPA), and integrin antagonists, and suggested that integrin αIIb β3 antagonists may have a beneficial effect by reducing adverse outcome due to stroke24–26; although, there is increased risk of hemorrhage, especially with abciximab25. In other clinical trials, fibrinolytic therapy, a reduced dose of r-tPA (<0.6 mcg/kg), together with eptifibatide-treatment shows similar bleeding profiles as the normal dose of r-tPA (0.9 mcg/kg) alone26–28. Treatment of patients with reduced r-tPA doses in combination with an integrin antagonist implicate the investigators’ consideration of potential hemorrhagic risk of the combination therapy.
The benefit of current integrin antagonists over other anti-platelet agents for general antithrombotic therapy is their rapid onset of action, potency, and low inter-patient variability7, 9, 14. By contrast, there is significant interpatient variability in response to aspirin (irreversible COX-1 inhibitor) or clopidogrel (P2Y12 inhibitor), mainly due to drug resistance7, 12, 16. However, the potent effects of current integrin antagonists are associated with increased bleeding risk29–31, which can be potentially life-threatening. Bleeding risk limits the use and dose of integrin antagonists, and thus also limits their effectiveness11, 31. Abciximab, tirofiban and eptifibatide all cause thrombocytopenia, which may be associated with conformational changes of integrins following the binding of these drugs32.
New Inhibitors That Minimally Induce Conformational Changes
Integrin structure and conformations
Both α and β chains of the αIIb β3 complex contain a long extracellular region, a single-pass transmembrane region, and a short cytoplasmic tail. The amino terminal region of the α and β chains interact to form what is known as the ‘head’, which contains the ligand binding pocket where a conserved structural motif, known as the metal ion-dependent adhesion site (MIDAS) is critical33. In αIIb β3 the MIDAS is on the β3 -subunit and thought to stabilize ligand-binding by coordinating a metal ion with the aspartic acid on RGD-containing ligands34. Some other integrin α-subunits contain an additional domain called the interactive domain (I-domain), which also contain a MIDAS12. Below the head region are two long ‘legs’: in αIIb, two calf domains and a thigh domain constitute a leg; whereas in β3, four integrin epidermal growth factor-like domains, two hybrid domains, and a plexin semaphorin integrin domain form the other leg.
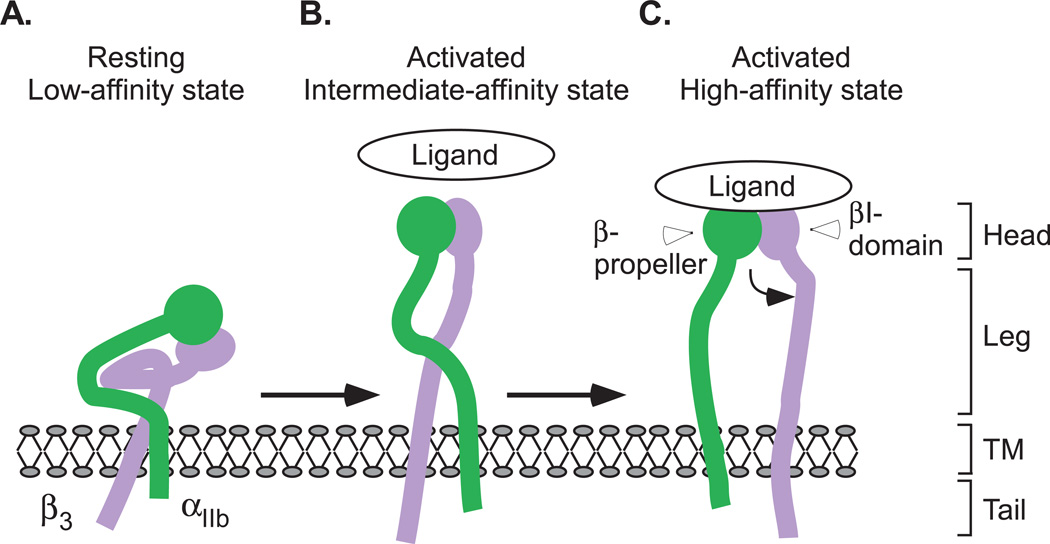
Integrin molecules undergo conformational changes upon receptor activation and ligand-binding34–36. Integrin αIIb β3 is kept in a resting (low-affinity) state in normal circulation, preventing undesirable thrombus formation. This state is maintained by interactions between the α and β chains within the transmembrane and membrane proximal cytoplasmic domains which constrain the ectodomain37. The resting state has been suggested to correspond to the ‘bent’ conformation as revealed in crystal structure and electron microscopy (EM)33, 38, 39,40. Integrin activation induces the separation of α and β transmembrane and cytoplasmic domains and “un-bending” of the ectodomain, resulting in an ‘extended’ active conformation41,42. The extended conformation with a ‘closed’ configuration, wherein the β3 head and hybrid domain form an acute angle, represents an active intermediate affinity state, which is recognized by the RGD sequence or HHLGGAKQAGV sequence in fibrinogen. Binding of ligand recognition sequences induces further conformational changes, resulting in an 'open' head piece conformation, which is the high affinity state35, 38, 39, 41–44 (Fig. 1A–C). Between these major conformational states, six different intermediate states have also been suggested, based on crystal structures of the ectodomain of αIIb β343. EM studies using intact αIIb β3 in a nanodisc suggest different pictures of ‘bent’ and ‘extended’ conformations40,45. Different from models obtained from crystal structures of integrin ectodomains, electron microscopy analyses of intact αIIb β3 show that the resting integrin headpiece points away from the membrane and that the intermediate extended integrin conformation contains crossed legs40, 45. The differences in models of resting and activated integrin structure are possibly due to the contribution of the transmembrane/cytoplasmic domains to integrin conformation39. The ligand-induced conformational changes are physiologically important because they: (1) expose new epitopes and binding sites on integrins (ligand-induced binding sites, LIBS)36, 46; (2) enable the initial interaction of ‘resting’ integrins with the exposed RGD-like sequence in certain ligands (such as immobilized fibrinogen) to transform integrins into a high affinity form (ligand-induced integrin activation), bypassing the need for inside-out signaling47; and (3) are important for integrin clustering and “outside-in” signaling44, 48.
Figure 1. Conformational states during integrin activation and ligand binding.
(A) The resting (low-affinity) state is maintained by interactions between the α and β chains within the transmembrane and membrane proximal cytoplasmic domains which constrain the ectodomain. (B) The activated (intermediate affinity) state has an extended conformation with a ’closed’ configuration. (C) Binding of ligand recognition sequences induces further conformational changes, resulting in an ’open’ high affinity state. TM, transmembrane domain. Curved black arrow indicates the swing-out motion of the β3 subunit hybrid domain upon full integrin activation.
Integrin antagonists that minimally affect integrin conformation
Tirofiban and eptifibatide are RGD mimetics, and thus cause “ligand-induced conformational changes”49, 50, resulting in exposure of LIBS and ligand-induced integrin activation51, although these monomeric RGD-like peptides or compounds in general do not appear to directly induce integrin outside-in signaling47, 49, 52. The conformational changes induced by ligand mimetic antagonists are thought to be important for the adverse effect of thrombocytopenia32. Abciximab also induces LIBS and thrombocytopenia5354. The ability of these antagonists to induce an active conformation of integrins carries the risk of possible pro-thrombotic effects after antagonist dissociation50,20. There were some reports of such antagonist-induced prothrombotic effects50, 52, 55. Recently, new small molecule integrin antagonists have been developed that exhibit increased specificity and potency without exposing β3 LIBS epitopes56, 57. RUC-1 and its more potent derivative RUC-2, inhibit the ligand binding function of integrins, platelet aggregation and in vivo thrombus formation, and importantly they do not induce integrin activation56, 57. RUC-1 interacts with αIIb whereas RUC-2 appears to interact with β3 Mg2+ coordinating sites. Interestingly, unlike RUC-1 and current integrin antagonists, RUC-2 competes with Mg2+ for binding to the β3 -subunit, and its inhibitory effects are attenuated by adding exogenous Mg2+ 57.
Targeting Integrin Signaling
Inside-out signaling
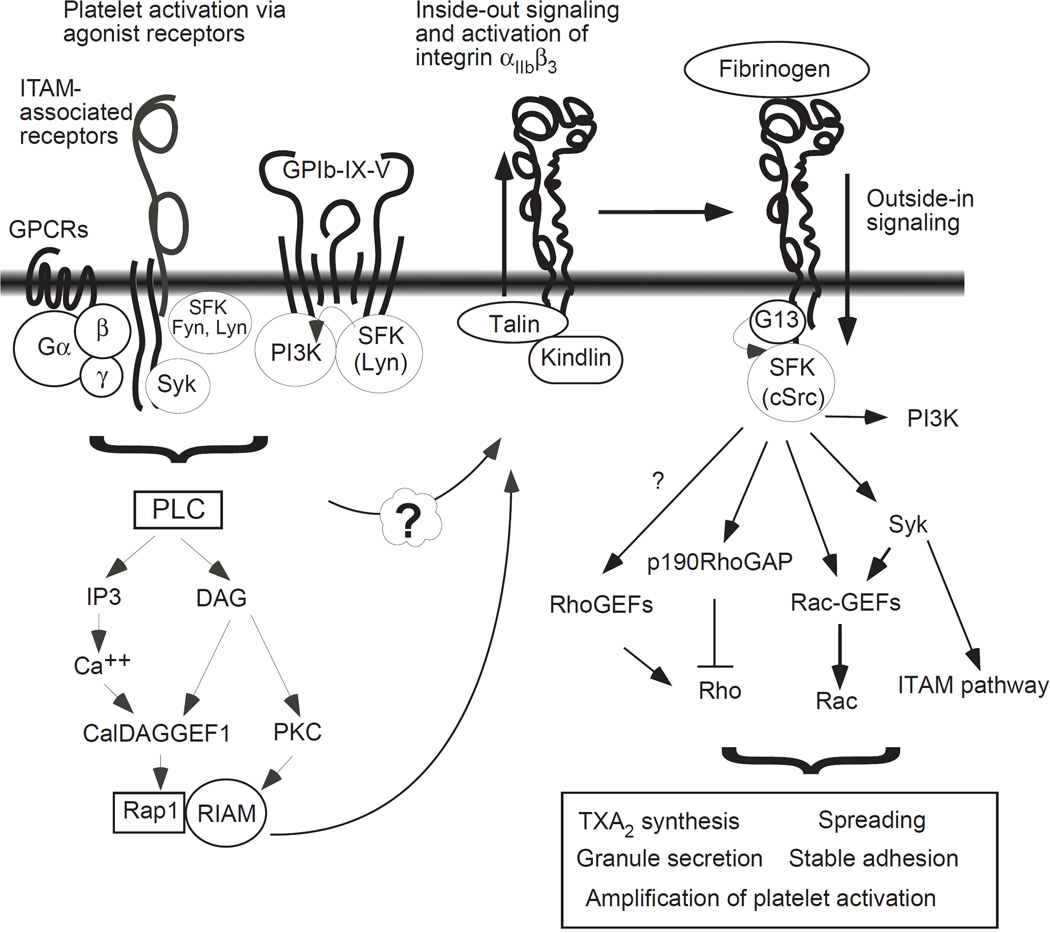
Platelets circulating in blood vessels are normally in a resting state and become activated and adherent only when exposed to the site of vascular injury or platelet agonists. Platelet agonists elicit platelet activation signals via various receptor-mediated intracellular signaling pathways58. These intracellular signals converge to transform αIIb β3 from a ‘resting’ state to an ‘activated’ state1, 58. This process is called inside-out signaling (Fig. 2). A key requirement for integrin inside-out signaling is the induction of the binding of talin to the membrane proximal half of the β3 cytoplasmic domain which includes an important NPXY motif. Talin-binding induces unclasping of the transmembrane and cytoplasmic domains of αIIb and β3, and thus integrin activation59–62. This talin-dependent integrin activation is facilitated by kindlin, which interacts with the C-terminal region of the β3 cytoplasmic domain62, 63. It is conceivable that disruption of talin/kindlin binding to integrin αIIb β3 or disruption of the signal responsible for the induction of talin/kindlin binding would also inhibit integrin activation and thus thrombus formation, as evidenced by talin1 gene deletion or mutations63, 64.
Figure 2. Inside-out and Outside-in signaling of the platelet integrin αIIb β3.
For a detailed description of individual agonist-induced platelet activation pathways, please see ref58. PLC, phospholipase C; PI3K, phosphoinositide 3-kinase; GPCRs, G-protein coupled receptors; IP3, inositol trisphosphate; DAG, diacylglycerol; PKC, protein kinase C; Syk, spleen tyrosine kinase; SFK, src family kinase, CalDAG-GEF1, calcium and diacylglycerol-regulated guanine nucleotide exchange factor 1; RIAM, Rap1-GTP interacting adapter molecule; G13, guanosine nucleotide-binding protein alpha subunit 13; GEF, Guanine nucleotide exchange factor; GAP, GTPase-activating protein; ITAM, immunoreceptor tyrosine-based activation motif; TXA2, thromboxane A2.
Inhibitors of inside-out signaling
Current platelet inhibitors including ADP receptor antagonists (e.g. clopidogrel), cyclooxygenase inhibitors (e.g. aspirin), and thrombin receptor inhibitors (e.g. vorapaxar), primarily exert their effects by inhibiting early receptor signaling pathways that initiate inside-out signaling and integrin activation16. Pharmacological inhibition of inside-out signaling was demonstrated with cell-permeable peptides containing talin binding sequences65, 66. A cell permeable peptide corresponding to αIIb residues 1000–1008 important in talin binding and β3 interaction, were also used to inhibit integrin activation67. Because inhibition of inside-out signaling results in the loss of the activation of the ligand binding function of integrins, it is expected that the inhibitors of inside-out signaling should show characteristics similar to that of integrin antagonists, which inhibit both thrombosis and hemostasis. Indeed, talin1 deletion or mutational disruption of talin-binding site (β3 L746A) protected mice from thrombosis, but they still displayed impaired hemostasis as shown by prolonged tail bleeding times in these mice63,64. However, one report suggests that partial inhibition of talin binding to the integrin β3 NPXY motif caused defective thrombus formation, with only minor bleeding side effect68. It remains to be investigated whether partial inhibition of αIIb β3 activation may also result in less potent anti-thrombotic effects or whether finding the right balance between potent anti-thrombotic effects and hemorrhagic side effects may allow anti-thrombotic therapy with proper control of bleeding risk.
Outside-in signaling
Ligand binding to integrins not only mediates platelet adhesion and primary aggregation but also induces signal transduction into cells that triggers the activation of vast intracellular signaling networks and cytoskeleton reorganization58, 69. This process is known as outside-in signaling. Outside-in signaling leads to a series of cellular responses including platelet spreading, stable adhesion, granule secretion and clot retraction, which greatly amplify platelet aggregation and thrombus size69, 70 (Fig. 2).
Several protein tyrosine kinases have been shown to be important in outside-in signaling including focal adhesion kinase, ILK, and Syk, Src family kinases (SFK)58, 69. In particular, integrin β3 -bound c-Src71, is now recognized as a key early signaling molecule. Following integrin ligation, c-Src has been shown to phosphorylate two NXXY motifs in the β3 tail58, 69; and induce activation of the phosphoinositide 3-kinase (PI3K) pathway72, inhibition of RhoA73, and activation of the Syk-ITAM pathway74. Src-dependent transient inhibition of RhoA and activation of PI3K is necessary for platelet spreading on integrin ligands72. The PI3K pathway and the Syk-ITAM pathway stimulate granule secretion72, 74. Tyrosine phosphorylation in β3 may also help recruit phosphotyrosine-binding proteins, such as SHC and myosin heavy chain58, 69. Phosphorylation at Y747 also regulates talin binding and thus the direction and dynamics of integrin signaling64, and phosphorylation at Y759 protects β3 from calpain cleavage75. These events are important for controlling the switch between platelet spreading and retraction73. Interestingly, the role of c-Src requires its interaction with the β3 cytoplasmic domain. Deletion of the c-Src binding RGT sequence in the C-terminus of β3 abolished the ability of c-Src to mediate cell spreading even when constitutively active c-Src was expressed76. Thus, it appears that targeting c-Src binding to integrins may selectively inhibit integrin outside-in signaling. This notion is supported by a study using β3 -RGT-deleted integrin-expressing mice, which are defective in platelet responses associated with outside-in signaling, and protected against arterial thrombosis, but display only a mild defect in inside-out signaling76.
The most proximal signaling mediator of outside-in signaling identified so far is Gα13. Gα13 directly interacts with an ExE motif in the cytoplasmic domain of integrin β-subunits, and this binding is required for c-Src activation and Src-dependent outside-in signaling77. Gα13 binding to β3 occurs only during early phase outside-in signaling. The Gα13 binds to an ExE motif located near talin binding sites of β3. However, the ExE motif is not required for talin binding, and Gα13-binding is not involved in integrin activation66. Thus, suppression of Gα13 expression or disruption of the Gα13-binding site in β3 selectively inhibits the early phase of outside-in signaling responsible for stabilization and amplification of a thrombus, but does not affect inside-out signaling nor the ligand binding function of integrins.
Selective inhibitors of outside-in signaling
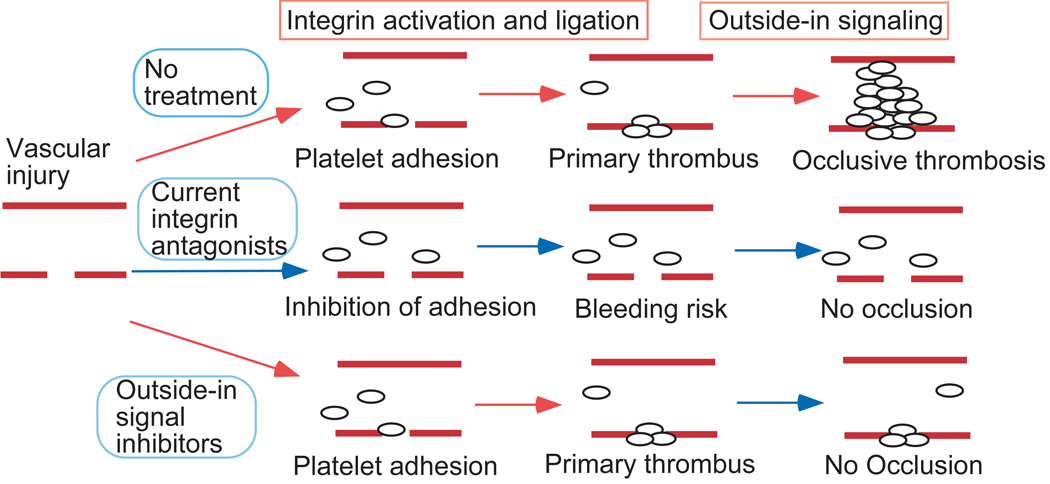
Recent conceptual advances in integrin outside-in signaling reveal the potential in developing selective inhibitors of integrin outside-in signaling as new anti-thrombotic drugs. A major advantage for targeting outside-in signaling is that inhibition of outside-in signaling should not affect primary platelet adhesion and aggregation, which is critical for hemostasis, but should limit the size of a thrombus to prevent vessel occlusion (Fig. 3). Our laboratory has recently demonstrated the potential of such an approach with a myristoylated ExE motif peptide that selectively inhibits Gα13-integrin interaction66. This inhibitor selectively inhibits outside-in signaling, platelet spreading and the second wave of platelet aggregation without affecting primary platelet aggregation. Importantly, this inhibitor potently inhibits occlusive thrombosis in mouse models in vivo without affecting bleeding time, unlike eptifibatide, which dramatically prolongs bleeding time66. Thus, selective inhibitors of outside-in signaling as a new antithrombotic strategy have the potential to selectively inhibit arterial thrombosis without causing excessive hemorrhage. Although, it is still important to consider and investigate potential off-target effects caused by selective targeting of Gα13.
Figure 3. A schematic showing the anti-thrombotic effect of selective inhibitors of outside-in signaling without causing hemorrhage.
(Reprinted from Nature, 503: 131-135, 2013).
Since SFK is a required signal downstream of Gα13 in outside-in signaling, inhibitors of SFK could potentially be effective inhibitors. However, SFK play multiple roles in platelets and other cells. For example, SFK is important in the ITAM pathway and GPIb-IX signaling pathways58, and thus is important in inside-out signaling, which limits the value of SFK inhibitors as selective outside-in signaling inhibitors. However, blocking the interaction between SFK and integrins may selectively inhibit outside-in signaling. A myristoylated peptide inhibitor derived from the c-Src-binding sequence of β3, abolished platelet spreading without affecting ADP-induced fibrinogen binding78. However, disruption of the β3 c-Src- binding site in mice provided protection from thrombosis, but also mildly affected hemostasis76.
Conclusions
All current integrin antagonists function by blocking the binding of integrin ligands9, 12, 34. These inhibitors induce conformational changes in integrins, which are associated with thrombocytopenia and possibly other adverse effects. New inhibitors with minimal conformational effects may potentially help resolve this issue. A major problem associated with the current integrin antagonists is that at doses where they exhibit high potency they also increase the risk of hemorrhage. Emerging evidence suggests that selective inhibition of outside-in signaling has the potential to have potent antithrombotic effects without causing bleeding.
Acknowledgments
Sources of funding
This work is supported in part by grants and a contract from NHBLI (HL062350, HL080264, and HHSN268201400007C). BE is also supported by an F31 NIH fellowship (HL123319).
Footnotes
Disclosures
Dr. Xiaoping Du, University of Illinois, Chicago, holds patents relevant to the topic of this review.
References
- 1.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Nurden AT, Fiore M, Nurden P, Pillois X. Glanzmann thrombasthenia: A review of ITGA2B and ITGB3 defects with emphasis on variants, phenotypic variability, and mouse models. Blood. 2011;118:5996–6005. doi: 10.1182/blood-2011-07-365635. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes. New England Journal of Medicine. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 4.Coller B, Scudder L. Inhibition of dog platelet function by in vivo infusion of f(ab')2 fragments of a monoclonal antibody to the platelet glycoprotein IIb/IIIa receptor. Blood. 1985;66:1456–1459. [PubMed] [Google Scholar]
- 5.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman C, xE, Mollie, Ross FP, Coller BS, Teitelbaum S, Hynes RO. β3-integrin–deficient mice are a model for glanzmann thrombasthenia showing placental defects and reduced survival. The Journal of clinical investigation. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lincoff AM, Topol EJ. Platelet glycoprotein IIb/IIIa inhibitors in cardiovascular disease. Springer; 2003. Overview of the glycoprotein iib/iiia interventional trials; pp. 167–199. [Google Scholar]
- 7.Patrono C, Baigent C, Hirsh J, Roth G. Antiplatelet drugs: American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:199s–233s. doi: 10.1378/chest.08-0672. [DOI] [PubMed] [Google Scholar]
- 8.Bosch X, Marrugat J, Sanchis J. Platelet glycoprotein IIb/IIIa blockers during percutaneous coronary intervention and as the initial medical treatment of non-st segment elevation acute coronary syndromes. The Cochrane database of systematic reviews. 2013;11 doi: 10.1002/14651858.CD002130.pub4. Cd002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bledzka K, Smyth SS, Plow EF. Integrin αIIbβ3: From discovery to efficacious therapeutic target. Circ Res. 2013;112:1189–1200. doi: 10.1161/CIRCRESAHA.112.300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coller BS. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. The Journal of clinical investigation. 1985;76:101–108. doi: 10.1172/JCI111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coller BS, Folts JD, Smith SR, Scudder LE, Jordan R. Abolition of in vivo platelet thrombus formation in primates with monoclonal antibodies to the platelet GPIIb/IIIa receptor. Correlation with bleeding time, platelet aggregation, and blockade of gpIIb/IIIa receptors. Circulation. 1989;80:1766–1774. doi: 10.1161/01.cir.80.6.1766. [DOI] [PubMed] [Google Scholar]
- 12.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: Lessons and opportunities. Nature reviews. Drug discovery. 2010;9:804–820. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 13.Scarborough RM. Development of eptifibatide. American Heart Journal. 1999;138:1093–1104. doi: 10.1016/s0002-8703(99)70075-x. [DOI] [PubMed] [Google Scholar]
- 14.Schneider DJ. Anti-platelet therapy: Glycoprotein IIb/IIIa antagonists. Br J Clin Pharmacol. 2011;72:672–682. doi: 10.1111/j.1365-2125.2010.03879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch JJ, Jr, Cook JJ, Sitko GR, Holahan MA, Ramjit DR, Mellott MJ, Stranieri MT, Stabilito II, Zhang G, Lynch RJ, et al. Nonpeptide glycoprotein IIb/IIIa inhibitors. 5. Antithrombotic effects of MK-0383. The Journal of pharmacology and experimental therapeutics. 1995;272:20–32. [PubMed] [Google Scholar]
- 16.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nature reviews. Drug discovery. 2010;9:154–169. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 17.Lele M, Sajid M, Wajih N, Stouffer GA. Eptifibatide and 7E3, but not tirofiban, inhibit αvβ3 integrin-mediated binding of smooth muscle cells to thrombospondin and prothrombin. Circulation. 2001;104:582–587. doi: 10.1161/hc3101.092199. [DOI] [PubMed] [Google Scholar]
- 18.Simon DI, Xu H, Ortlepp S, Rogers C, Rao NK. 7E3 monoclonal antibody directed against the platelet glycoprotein IIb/IIIa cross-reacts with the leukocyte integrin Mac-1 and blocks adhesion to fibrinogen and ICAM-1. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:528–535. doi: 10.1161/01.atv.17.3.528. [DOI] [PubMed] [Google Scholar]
- 19.Cannon CP, McCabe CH, Wilcox RG, Langer A, Caspi A, Berink P, Lopez-Sendon J, Toman J, Charlesworth A, Anders RJ, Alexander JC, Skene A, Braunwald E. Oral glycoprotein IIb/IIIa inhibition with orbofiban in patients with unstable coronary syndromes (OPUS-TIMI16) trial. Circulation. 2000;102:149–156. doi: 10.1161/01.cir.102.2.149. [DOI] [PubMed] [Google Scholar]
- 20.Chew DP, Bhatt DL, Sapp S, Topol EJ. Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: A meta-analysis of phase III multicenter randomized trials. Circulation. 2001;103:201–206. doi: 10.1161/01.cir.103.2.201. [DOI] [PubMed] [Google Scholar]
- 21.Ciccone A, Motto C, Abraha I, Cozzolino F, Santilli I. Glycoprotein IIb/IIIa inhibitors for acute ischaemic stroke. The Cochrane database of systematic reviews. 2014;3 doi: 10.1002/14651858.CD005208.pub3. Cd005208. [DOI] [PubMed] [Google Scholar]
- 22.Mandava P, Thiagarajan P, Kent T. Glycoprotein IIb/IIIa antagonists in acute ischaemic stroke. Drugs. 2008;68:1019–1028. doi: 10.2165/00003495-200868080-00001. [DOI] [PubMed] [Google Scholar]
- 23.Adams HP, Effron MB, Torner J, Dávalos A, Frayne J, Teal P, Leclerc J, Oemar B, Padgett L, Barnathan ES, Hacke W. Investigators ftA-I. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: Results of an international phase III trial: Abciximab in emergency treatment of stroke trial (AbESTT-II) Stroke; a journal of cerebral circulation. 2008;39:87–99. doi: 10.1161/STROKEAHA.106.476648. [DOI] [PubMed] [Google Scholar]
- 24.Siebler M, Hennerici MG, Schneider D, von Reutern GM, Seitz RJ, Rother J, Witte OW, Hamann G, Junghans U, Villringer A, Fiebach JB. Safety of tirofiban in acute ischemic stroke: The satis trial. Stroke; a journal of cerebral circulation. 2011;42:2388–2392. doi: 10.1161/STROKEAHA.110.599662. [DOI] [PubMed] [Google Scholar]
- 25.Eckert B, Koch C, Thomalla G, Kucinski T, Grzyska U, Roether J, Alfke K, Jansen O, Zeumer H. Aggressive therapy with intravenous abciximab and intra-arterial rtpa and additional pta/stenting improves clinical outcome in acute vertebrobasilar occlusion: Combined local fibrinolysis and intravenous abciximab in acute vertebrobasilar stroke treatment (FAST): Results of a multicenter study. Stroke; a journal of cerebral circulation. 2005;36:1160–1165. doi: 10.1161/01.STR.0000165918.80812.1e. [DOI] [PubMed] [Google Scholar]
- 26.Pancioli AM, Adeoye O, Schmit PA, Khoury J, Levine SR, Tomsick TA, Sucharew H, Brooks CE, Crocco TJ, Gutmann L, Hemmen TM, Kasner SE, Kleindorfer D, Knight WA, Martini S, McKinney JS, Meurer WJ, Meyer BC, Schneider A, Scott PA, Starkman S, Warach S, Broderick JP. Combined approach to lysis utilizing eptifibatide and recombinant tissue plasminogen activator in acute ischemic stroke-enhanced regimen stroke trial. Stroke; a journal of cerebral circulation. 2013;44:2381–2387. doi: 10.1161/STROKEAHA.113.001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangiafico S, Cellerini M, Nencini P, Gensini G, Inzitari D. Intravenous glycoprotein IIb/IIIa inhibitor (tirofiban) followed by intra-arterial urokinase and mechanical thrombolysis in stroke. AJNR. American journal of neuroradiology. 2005;26:2595–2601. [PMC free article] [PubMed] [Google Scholar]
- 28.Seitz RJ, Hamzavi M, Junghans U, Ringleb PA, Schranz C, Siebler M. Thrombolysis with recombinant tissue plasminogen activator and tirofiban in stroke: Preliminary observations. Stroke; a journal of cerebral circulation. 2003;34:1932–1935. doi: 10.1161/01.STR.0000080535.61188.A6. [DOI] [PubMed] [Google Scholar]
- 29.Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. The New England journal of medicine. 1997;336:1689–1696. doi: 10.1056/NEJM199706123362401. [DOI] [PubMed] [Google Scholar]
- 30.Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The pursuit trial investigators. Platelet glycoprotein IIb/IIIa in unstable angina: Receptor suppression using integrilin therapy. The New England journal of medicine. 1998;339:436–443. doi: 10.1056/NEJM199808133390704. [DOI] [PubMed] [Google Scholar]
- 31.Kereiakes DJ, Kleiman NS, Ambrose J, Cohen M, Rodriguez S, Palabrica T, Herrmann HC, Sutton JM, Weaver WD, McKee DB, Fitzpatrick V, Sax FL. Randomized, double-blind, placebo-controlled dose-ranging study of tirofiban (MK-383) platelet gpIIb/IIIa blockade in high risk patients undergoing coronary angioplasty. Journal of the American College of Cardiology. 1996;27:536–542. doi: 10.1016/0735-1097(95)00500-5. [DOI] [PubMed] [Google Scholar]
- 32.Bougie DW, Rasmussen M, Zhu J, Aster RH. Antibodies causing thrombocytopenia in patients treated with RGD-mimetic platelet inhibitors recognize ligand-specific conformers of αIIbβ3 integrin. Blood. 2012;119:6317–6325. doi: 10.1182/blood-2012-01-406322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin αvβ3. Science (New York, N Y.) 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–511. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 36.Du X, Gu M, Weisel JW, Nagaswami C, Bennett JS, Bowditch R, Ginsberg MH. Long range propagation of conformational changes in integrin αIIbβ3. The Journal of biological chemistry. 1993;268:23087–23092. [PubMed] [Google Scholar]
- 37.Vinogradova O, Velyvis A, Velyviene A, Hu B, Haas T, Plow E, Qin J. A structural mechanism of integrin αIIbβ3 "inside-out" activation as regulated by its cytoplasmic face. Cell. 2002;110:587–597. doi: 10.1016/s0092-8674(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Zhu J, Negri A, Provasi D, Filizola M, Coller BS, Springer TA. Closed headpiece of integrin αIIbβ3 and its complex with an αIIbβ3-specific antagonist that does not induce opening. Blood. 2010;116:5050–5059. doi: 10.1182/blood-2010-04-281154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim C, Schmidt T, Cho E-G, Ye F, Ulmer TS, Ginsberg MH. Basic amino-acid side chains regulate transmembrane integrin signalling. Nature. 2012;481:209–213. doi: 10.1038/nature10697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi WS, Rice WJ, Stokes DL, Coller BS. Three-dimensional reconstruction of intact human integrin αIIbβ3: New implications for activation-dependent ligand binding. Blood. 2013;122:4165–4171. doi: 10.1182/blood-2013-04-499194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science (New York, N.Y.) 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 42.Lau TL, Kim C, Ginsberg MH, Ulmer TS. The structure of the integrin αIIbβ3 transmembrane complex explains integrin transmembrane signalling. Embo j. 2009;28:1351–1361. doi: 10.1038/emboj.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu J, Zhu J, Springer TA. Complete integrin headpiece opening in eight steps. The Journal of cell biology. 2013;201:1053–1068. doi: 10.1083/jcb.201212037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li R, Mitra N, Gratkowski H, Vilaire G, Litvinov R, Nagasami C, Weisel JW, Lear JD, DeGrado WF, Bennett JS. Activation of integrin αIIbβ3 by modulation of transmembrane helix associations. Science (New York, N Y.) 2003;300:795–798. doi: 10.1126/science.1079441. [DOI] [PubMed] [Google Scholar]
- 45.Eng ET, Smagghe BJ, Walz T, Springer TA. Intact αIIbβ3 integrin is extended after activation as measured by solution x-ray scattering and electron microscopy. The Journal of biological chemistry. 2011;286:35218–35226. doi: 10.1074/jbc.M111.275107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parise LV, Helgerson SL, Steiner B, Nannizzi L, Phillips DR. Synthetic peptides derived from fibrinogen and fibronectin change the conformation of purified platelet glycoprotein IIb/IIIa. Journal of Biological Chemistry. 1987;262:12597–12602. [PubMed] [Google Scholar]
- 47.Du X, Plow EF, Frelinger AL, III, O'Toole TE, Loftus JC, Ginsberg MH. Ligands “activate” integrin αIIbβ3 (platelet gpIIb/IIIa) Cell. 65:409–416. doi: 10.1016/0092-8674(91)90458-b. [DOI] [PubMed] [Google Scholar]
- 48.Hato T, Pampori N, Shattil SJ. Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin αIIbβ3. The Journal of cell biology. 1998;141:1685–1695. doi: 10.1083/jcb.141.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cox D, Smith R, Quinn M, Theroux P, Crean P, Fitzgerald DJ. Evidence of platelet activation during treatment with a gpIIb/IIIa antagonist in patients presenting with acute coronary syndromes. Journal of the American College of Cardiology. 2000;36:1514–1519. doi: 10.1016/s0735-1097(00)00919-0. [DOI] [PubMed] [Google Scholar]
- 50.Hantgan RR, Stahle MC. Integrin priming dynamics: Mechanisms of integrin antagonist-promoted αIIbβ3:PAC-1 molecular recognition. Biochemistry. 2009;48:8355–8365. doi: 10.1021/bi900475k. [DOI] [PubMed] [Google Scholar]
- 51.Jennings LK, Haga JH, Slack SM. Differential expression of a ligand induced binding site (LIBS) by gpIIb/IIIa ligand recognition peptides and parenteral antagonists. Thrombosis and haemostasis. 2000;84:1095–1102. [PubMed] [Google Scholar]
- 52.Bassler N, Loeffler C, Mangin P, Yuan Y, Schwarz M, Hagemeyer CE, Eisenhardt SU, Ahrens I, Bode C, Jackson SP, Peter K. A mechanistic model for paradoxical platelet activation by ligand-mimetic αIIbβ3 (gpIIb/IIIa) antagonists. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:E9–E15. doi: 10.1161/01.ATV.0000255307.65939.59. [DOI] [PubMed] [Google Scholar]
- 53.Berkowitz SD, Harrington RA, Rund MM, Tcheng JE. Acute profound thrombocytopenia after C7E3 fab (abciximab) therapy. Circulation. 1997;95:809–813. doi: 10.1161/01.cir.95.4.809. [DOI] [PubMed] [Google Scholar]
- 54.Peter K, Straub A, Kohler B, Volkmann M, Schwarz M, Kübler W, Bode C. Platelet activation as a potential mechanism of GPIIb/IIIa inhibitor-induced thrombocytopenia. The American Journal of Cardiology. 1999;84:519–524. doi: 10.1016/s0002-9149(99)00370-7. [DOI] [PubMed] [Google Scholar]
- 55.Peter K, Schwarz M, Ylänne J, Kohler B, Moser M, Nordt T, Salbach P, Kübler W, Bode C. Induction of fibrinogen binding and platelet aggregation as a potential intrinsic property of various glycoprotein IIb/IIIa (αIIbβ3) inhibitors. Blood. 1998;92:3240–3249. [PubMed] [Google Scholar]
- 56.Blue R, Murcia M, Karan C, Jirouskova M, Coller BS. Application of high-throughput screening to identify a novel αIIb-specific small- molecule inhibitor of αIIbβ3-mediated platelet interaction with fibrinogen. Blood. 2008;111:1248–1256. doi: 10.1182/blood-2007-08-105544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu J, Choi W-S, McCoy JG, Negri A, Zhu J, Naini S, Li J, Shen M, Huang W, Bougie D, Rasmussen M, Aster R, Thomas CJ, Filizola M, Springer TA, Coller BS. Structure-guided design of a high-affinity platelet integrin αIIbβ3 receptor antagonist that disrupts Mg2+ binding to the MIDAS. Science Translational Medicine. 2012;4:125ra132. doi: 10.1126/scitranslmed.3003576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Z, Delaney MK, O'Brien KA, Du X. Signaling during platelet adhesion and activation. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): A co-activator of β3 integrins. The Journal of cell biology. 2008;181:439–446. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 61.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: A final common step in integrin activation. Science (New York, N.Y.) 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 62.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nature medicine. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 63.Nieswandt B, Moser M, Pleines I, Varga-Szabo D, Monkley S, Critchley D, Fassler R. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med. 2007;204:3113–3118. doi: 10.1084/jem.20071827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petrich BG, Fogelstrand P, Partridge AW, Yousefi N, Ablooglu AJ, Shattil SJ, Ginsberg MH. The antithrombotic potential of selective blockade of talin-dependent integrin αIIbβ3(platelet gpIIb/IIIa) activation. The Journal of clinical investigation. 2007;117:2250–2259. doi: 10.1172/JCI31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu XY, Timmons S, Lin YZ, Hawiger J. Identification of a functionally important sequence in the cytoplasmic tail of integrin β3 by using cell-permeable peptide analogs. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11819–11824. doi: 10.1073/pnas.93.21.11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen B, Zhao X, O'Brien KA, Stojanovic-Terpo A, Delaney MK, Kim K, Cho J, Lam SC, Du X. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature. 2013;503:131–135. doi: 10.1038/nature12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koloka V, Christofidou ED, Vaxevanelis S, Dimitriou AA, Tsikaris V, Tselepis AD, Panou-Pomonis E, Sakarellos-Daitsiotis M, Tsoukatos DC. A palmitoylated peptide, derived from the acidic carboxyl-terminal segment of the integrin αIIb cytoplasmic domain, inhibits platelet activation. Platelets. 2008;19:502–511. doi: 10.1080/09537100802266875. [DOI] [PubMed] [Google Scholar]
- 68.Stefanini L, Ye F, Snider AK, Sarabakhsh K, Piatt R, Paul DS, Bergmeier W, Petrich BG. A talin mutant that impairs talin-integrin binding in platelets decelerates αIIbβ3 activation without pathological bleeding. Blood. 2014;123:2722–2731. doi: 10.1182/blood-2013-12-543363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shattil SJ, Newman PJ. Integrins: Dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 70.Shen B, Delaney MK, Du X. Inside-out, outside-in, and inside-outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr Opin Cell Biol. 2012;24:600–606. doi: 10.1016/j.ceb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Brien KA, Gartner TK, Hay N, Du X. ADP-stimulated activation of AKT during integrin outside-in signaling promotes platelet spreading by inhibiting glycogen synthase kinase-3β. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2232–2240. doi: 10.1161/ATVBAHA.112.254680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flevaris P, Stojanovic A, Gong H, Chishti A, Welch E, Du X. A molecular switch that controls cell spreading and retraction. The Journal of cell biology. 2007;179:553–565. doi: 10.1083/jcb.200703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boylan B, Gao C, Rathore V, Gill JC, Newman DK, Newman PJ. Identification of fcgammariia as the itam-bearing receptor mediating αIIbβ3 outside-in integrin signaling in human platelets. Blood. 2008;112:2780–2786. doi: 10.1182/blood-2008-02-142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xi X, Flevaris P, Stojanovic A, Chishti A, Phillips DR, Lam SC, Du X. Tyrosine phosphorylation of the integrin β3 subunit regulates β3 cleavage by calpain. The Journal of biological chemistry. 2006;281:29426–29430. doi: 10.1074/jbc.C600039200. [DOI] [PubMed] [Google Scholar]
- 76.Ablooglu AJ, Kang J, Petrich BG, Ginsberg MH, Shattil SJ. Antithrombotic effects of targeting αIIbβ3 signaling in platelets. Blood. 2009;113:3585–3592. doi: 10.1182/blood-2008-09-180687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong H, Shen B, Flevaris P, Chow C, Lam SC, Voyno-Yasenetskaya TA, Kozasa T, Du X. G protein subunit Gα13 binds to integrin αIIbβ3 and mediates integrin "outside-in" signaling. Science (New York, N.Y.) 2010;327:340–343. doi: 10.1126/science.1174779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su X, Mi J, Yan J, Flevaris P, Lu Y, Liu H, Ruan Z, Wang X, Kieffer N, Chen S, Du X, Xi X. RGT, a synthetic peptide corresponding to the integrin β3 cytoplasmic C-terminal sequence, selectively inhibits outside-in signaling in human platelets by disrupting the interaction of integrin αIIbβ3 with Src kinase. Blood. 2008;112:592–602. doi: 10.1182/blood-2007-09-110437. [DOI] [PMC free article] [PubMed] [Google Scholar]